Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
An Open-Label Single-Arm, Monocentric Study Assessing the Efficacy and Safety of Natural Pterostilbene (Pterocarpus marsupium) for Skin Brightening and Antiaging Effects
Authors Majeed M, Majeed S , Jain R, Mundkur L , Rajalakshmi HR, Lad PS, Neupane P
Received 14 November 2019
Accepted for publication 15 January 2020
Published 31 January 2020 Volume 2020:13 Pages 105—116
DOI https://doi.org/10.2147/CCID.S238358
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Muhammed Majeed, 1–4 Shaheen Majeed, 1–4 Renuka Jain, 1, 4 Lakshmi Mundkur, 1 HR Rajalakshmi, 4 Prachi Subhash Lad, 4 Prakriti Neupane 4
1Sami Labs Limited, Bangalore, Karnataka, India; 2Sabinsa Corporation, East Windsor, NJ, USA; 3Sabinsa Corporation, Payson, UT, USA; 4ClinWorld Private Limited, Bangalore, Karnataka, India
Correspondence: Renuka Jain
Sami Labs Limited, 19/1 and 19/2, First Main, Second Phase, Peenya Industrial Area, Bangalore 560 058, Karnataka, India
Email [email protected]
Background: Both intrinsic and environmental factors play a role in premature ageing of the skin. Natural extracts have been extensively used to reduce the signs of ageing in recent years. Pterostilbene is a natural analog of resveratrol with therapeutic properties against numerous diseases mainly due to its ability to reduce reactive oxygen species.
Methods: We studied the anti-aging and skin brightening effect of a 0.4% formulation of natural pterostilbene in healthy volunteers (N=38) in an open-label, single-arm, monocentric study for 8 weeks. The melanogenesis inhibitory, anti-tyrosinase and anti-collagenase and anti-elastase properties of pterostilbene were evaluated in vitro.
Results: Natural pterostilbene showed significant inhibition of melanogenesis in vitro. Pterostilbene cream (0.4%) was highly effective in reducing markers of aging and induces even skin tone. The product was effective in reducing wrinkles and fine lines, improved skin hydration elasticity and showed no adverse effects.
Conclusion: Our results suggest that natural pterostilbene is a safe and effective ingredient for use in cosmetic preparation to reduce the markers of skin ageing and brighten the skin tone.
Trial Registration: Clinical Trial Registration India Identifier CTRI/2019/01/017160 [Registered on 18/01/2019].
Keywords: natural pterostilbene, Pterocarpus marsupium, skin brightening, antiaging
Introduction
Skin is one of the largest organs of the body, it acts as a defensive barrier and protect us from outer physical, chemical, and biological environment.1 It is also the most visible organ that makes one conscious of the aging process. Skin aging is induced by various genetic (intrinsic) and environmental factors (extrinsic).2,3 Chronological aging of the skin is a natural, slow and complex biological process, characterized by the appearance of fine wrinkling, fragility, loss of elasticity and skin tone, crow’s feet, nasolabial folds, and mottled dyspigmentation. Chronic exposure to solar ultraviolet, infrared (UVR, IR) irradiation (termed photoaging), smoking, poor nutrition, and pollution are the major environmental influences which induce extrinsic aging.2–6
Antiaging and skin brightening cosmeceuticals delay or reverse the visible signs of aging to get healthy, smooth, flawless, blemish-free, and resilient skin.2,7
Skin brightening cosmetics lighten skin tone and provide an even skin complexion by the reduction in melanin.8
Plants and plant extracts are a good source of anti-aging and skin brightening active ingredients, and the current research trend is to develop phyto-ingredient referring to their traditional uses.7
Pterostilbene is a natural dimethylated analog of resveratrol which is predominantly found in the heartwood of Pterocarpus marsupium.9
Pterostilbene has gained much attention due to its chemopreventive and chemotherapeutic activities.10,11 Multiple studies have demonstrated the antioxidant activity of pterostilbene in both in vitro and in vivo models demonstrating both preventive and therapeutic benefits such as antioxidant, neuroprotective, anti-cancer, cardioprotective, analgesic, antiaging anti-diabetic, antitussive, anti-inflammatory, anthelmintic, and anti-obesity activities have been studied for pterostilbene.12,13 It is also reported to be used in treating boils, sores, vitiligo, greyness of hair and skin diseases.14
Pterostilbene has been found to protect against UVB induced skin damage and tumorogenesis in hairless mice.15 In human keratinocytes exposed to UVB, pterostilbene was observed to increase the nuclear translocation of NF-E2-related factor-2 (Nrf2), expression of Nrf2-dependent antioxidant enzymes, and DNA repair activity.16 We have earlier observed that Pterostilbene can be used as a natural ingredient in SPF formulations (unpublished observation). Being a natural antioxidant, pterostilbene could be an effective agent for antiageing and sun protection. Pterostilbene also maintains the antioxidant enzymes, inhibits oxidative stress induced by UVB, most probably due to the modulation of Nrf2-dependent antioxidant response.17 Moreover, Resveratrol and pterostilbene, have been reported to have reduced oxidative damage, inflammation, and cell senescence, which are some of the hallmarks of cellular aging.18 The cosmetic formulations of pterostilbene have not been studied in a systematic clinical study for their skin benefits.
In this study, we have evaluated skin brightness and antiaging effect of natural Pterostilbene in healthy volunteers, while the inhibition of melanogenesis, anti-tyrosinase, and anti-collagenase activities of the compound was evaluated in vitro.
Materials and Methods
Test Material
Natural pterostilbene (3,5-dimethoxy-4-hydroxy-trans-stilbene) is an extract derived from the dried heartwood of the Indian Kino tree (Pterocarpus marsupium). The cosmeceutical cream formulation PteroWhite® (CS/118/ML163A) was provided by Sami Labs, Bangalore, India. The topical dermal cream formulation contains 0.4% of natural 90% pterostilbene extract along with Disodium EDTA, Cetostearyl alcohol, Softemul165, Caprylic Capric triglyceride, Butylated Hydroxytoluene, Propylene glycol, Xiameter PMX 3031, Dow Corning 556, Sabilize New (Thyme oil, Monolaurin, Magnolol), Amp 95.
In vitro Skin Lightening Potential
Melanogenesis Inhibitory Activity
Melanogenesis inhibition assay was performed as described earlier with slight modification,19 B16F1 mouse melanoma cells were procured from ATCC and were maintained in Dulbecco’s Minimal Essential Medium (DMEM) containing 10% fetal bovine serum (FBS). The cells were harvested by trypsinization and were cultured for 24 hrs in a 96 well microtiter plate at a seeding density of 5000 cells per well. Melanin production was induced by melanocyte-stimulating hormone (∝-MSH) at 1nM, along with varying non-cytotoxic concentrations of the test sample for 3 days. 1N NaOH extracted the melanin, and the absorbance is read at 405nm in a Microplate reader (TECAN Ltd, Männedorf, Switzerland). The inhibitory effect of the sample is calculated based on the degree of inhibition of melanin formation compared to untreated MSH stimulated cells. Kojic acid was used as a positive control.
The results are expressed as IC50 values using Graphpad Prism software. The percentage of melanin is calculated as follows:
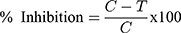
Where C – absorbance due to melanin in untreated cells
T-absorbance due to melanin in sample treated cells,
Anti-Tyrosinase Activity
Tyrosinase acts on L-tyrosine, forming a pink colored complex m the intensity of which is measured at 492 nm. The Tyrosinase activity in the presence and absence of inhibitor was assayed spectrophotometrically at 492 nm18
The assay is performed in 96 well clear microplate. Different concentrations of the samples were pre-incubated with. 6 U/well of mushroom tyrosinase enzyme at 37°C for 10 mins. The reaction was initiated by adding 2.5mM L- Tyrosine disodium, and the absorbance is read immediately every minute until 15 mins using the microplate reader at 492nm (TECAN Ltd, Männedorf, Switzerland). A test blank well was prepared for each sample without the enzyme. Enzyme reaction with vehicle alone served as a control for enzyme activity. Kojic acid was used as a positive control
The results are expressed in IC50 values using Graph pad prism software. The percentage of inhibition is calculated as follows:
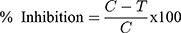
Where C- absorbance in Control, T- Absorbance in test
Anti-Collagenase Activity and Anti-Elastase Activity
The anticollagenase activity was performed using the collagenase kit (Enzchek® collagenase, gelatinase assay kit, Invitrogen, USA). For anti-elastase activity, the elastase kit (Enzchek® Elastase assay kit, Invitrogen, USA) was used. Both the assays were performed as per the manufacturer’s instruction and data was captured using BMG FLUOStar Optima. The results for both the assays are expressed respectively as IC50 values using Graph pad prism software. The percentage of inhibition is calculated as follows:
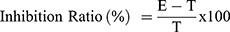
Where E = absorbance due to enzyme activity in the absence of the test sample
T= absorbance due to enzyme activity in the presence of the test sample
Clinical Study
Ethics
The skin brightening and antiaging attributes of Natural pterostilbene were evaluated at MS Clinical Research Pvt. Ltd., Bangalore, Karnataka, India. The study was approved by the local ethics committee (Clinicom Ethics Committee) and was conducted in accordance with good clinical practice guidelines.
Study Design
This was an open-label, single-arm, monocentric efficacy study. It was conducted for 8 weeks for each subject and included a total number of 4 visits, ie, Day1(baseline visit), Visit 2: Week2 (W2), Visit 3:Week4 (W4), Visit 4:Week8 (W8). After obtaining written informed consent, subjects which satisfied the inclusion and exclusion criteria were enrolled. 39 subjects of 38–55 years of age were screened, enrolled, 38 subjects (10 male and 28 female) completed the study. The objective of the study was to assess the efficacy of Natural Pterostilbene for skin brightening benefit as well as improvement in skin hydration in comparison to baseline, to evaluate the efficacy in imparting anti-aging attributes such as the reduction in fine lines, wrinkles & elasticity in comparison to baseline and Skin tolerance monitoring for product safety. Evaluation for the brightening benefit was carried out for 4 weeks and anti-aging attributes for 8 weeks by dermatological assessment, non-invasive instrument assessment, and imaging assessment. The upper inner arm (VB Volar Biceps) of the subjects was used as a control for the brightening assessments. Subjects were provided with the test products to apply one fingertip unit uniformly on complete face twice daily throughout the study (8weeks). They were instructed not to use additional sunscreen. There was no change in the subject’s other skincare regimen for the entire duration of the study which has no direct or indirect effect on our study outcome. The subjects were to carry their allotted test products to the study center on each visit.
Critical Selection criteria were to include healthy male & female adult subjects with the age group mentioned above, with general good health as determined from a recent medical history general physical examination, and dermatological assessment. Subject with Fitzpatrick skin types III to V, having visible fine lines and wrinkles in the periorbital area (Crow’s feet-under eye), forehead, and having skin aging concerns were included in the study.
Participants with following criteria were excluded from the study; Subject with any other signs of significant local irritation or skin disease, currently taking any medication, having chronic illness or had major surgery in the last year, subjects undergoing any treatment of any skin condition on their face/forearm, subjects not willing to discontinue other topical facial products, allergic or sensitive to bar cleansing products, creams/lotions, artificial jewelry or anything else, pregnant or nursing, taking medication including food supplements.
Dermatological Assessment
The dermatological assessment for efficacy was done using a dermatological assessment questionnaire and was recorded at baseline, week 2, week 4, and week 8. Parameters for evaluation include a shade card. Evenness of skin tone, skin texture (was assessed by tactile expert assessment), skin firmness/elasticity, Crow’s feet grading scale in the unanimated face (photo-numerical scale), under eye, frown lines (as per the Galbellear Line Severity Scale (GLSS)) and Nasolabial folds (as per the Wrinkle Severity Rating Scale (WSRS) scale (Table 1). The MSCR scale shade card (Supplementary Figure 1) comprising of 24 different shades designed for the Asian skin was used to assess the skin shade. One being the fairest, while 20 is the darkest skin color.
 |
Table 1 Grading Scales for Dermatological Assessment for Efficacy |
Dermatologist and Subject Self-Assessments
The entire assessment was completely blinded and randomized at two levels. A direct comparative visual assessment was done by the dermatologist between the two Visia images. The dermatologist also asked and assessed seven relative questions to all the subjects at week 4 and week 8 in comparison to baseline.
Subjects were asked to evaluate their skin condition after product application by using the self-assessment questionnaire. The parameter assessed were skin moisture, skin brightness, skin tone, skin firmness, at week 2, week 4 and week 8. Based on the score counts it is represented in percentage.
Instrumental Assessment
VISIA CR 2.2
VISIA CR 2.2 was used for facial imaging to record the visual changes at baseline- Day 0, Week 4, Week 8, Left, Right and front view of the face under CP (Cross-Polarized) and PP (Parallel Polarized), S1(Standard 1) and S2 (Standard 2) condition.
Dermal Assessment by Spectrophotometer CM2600d
For Skin Brightness, assessments were done at the center of the forehead, designated test site on the right and left cheek, and the untreated control site at the upper inner arm by two independent readings on the same location within the measurement area/site. In each reading, skin color components (L*a*and b*) and reflectance over entire spectral wavelength (360 nm to 740 nm) were captured in both SCI (specular component included) and SCE (specular component excluded modes) respectively by Minolta Spectrophotometer CM 2600d with aperture 8mm.
The spectrophotometric data was captured on the test product application site Forehead, Cheek, Upper inner arm (VB), the difference between upper inner arm and forehead ∆L (VB-FH) and the difference between upper inner arm and cheek ∆L (VB - Cheek) at all-time points baseline-day 0, week 2 and week 4.
Dermal Assessment by Cutometer MPA580
Skin Elasticity was measured on the right and left cheek and at baseline -day 0, week 2, and week 4 by Cutometer MPA 580. One independent reading was taken each on the right and left cheek.
Dermal Assessment by Corneometer CM 825
The Corneometer indicates the hydration level of the superficial layers of the skin (stratum corneum) via the measurement of skin dielectric properties. The assessment was done by Corneometer CM 825 on the center of the forehead and right and left cheek at baseline-Day 0, Week2 and Week4. Three independent readings were captured on the same site for all assessments.
Dermal Assessment by Antera3DTM
Antera measurements are based on the width and depth of fine lines, wrinkles, and folds. For fine lines, a 0–1mm filter, medium wrinkles, 0–2mm filter, large wrinkles, 0–3mm filter was selected. Three independent readings were taken by Antera 3DTM for the reduction in fine lines, reduction in wrinkles, skin texture at baseline-day 0, week 4 and week 8 on forehead and right and left cheek at the same site for all assessment.
Assessment for Safety
Localized skin irritation was recorded in the product in use tolerance form. This was done by the dermatological evaluation based on the following parameters erythema, dryness, edema, urticaria, allergic reactions, etc. Any adverse reactions were monitored and documented, determined by the severity of local intolerance/site reactions as determined by the investigator, were documented.
Data Consideration and Statistical Analysis
For Efficacy, the data of all subjects who completed the study were considered for the statistical analysis. A descriptive statistical analysis was carried out, and the normality test was performed using the Shapiro–Wilk test. Based on the output (p-value), the statistical test was decided. If the p-value was more than 0.05, the data was normal and a parametric test was performed to determine the efficacy of the test product. If the p-value was less than 0.05, the data was not normal, and a non-parametric test was performed to determine the efficacy of the test product. In all analyses, a 2-sided significance level of 5% (p-value < 0.05) was used to determine significance levels. Results on continuous measurements were presented on Mean ±SE. The Statistical software, R- ver.3.1.2 was used for the analysis of the data.
Results
Melanogenesis and Tyrosinase Inhibitory Activity
Under in vitro culture conditions, pterostilbene (90%) shows melanogenesis inhibitory activity with an IC50 of 0.55µg/mL in B16F1 mouse melanoma cells. The positive control Kojic acid showed an IC50 of 50 µg/mL. Pterostilbene inhibits the activity of tyrosinase enzyme, which is the most important enzyme catalyzing multiple steps of melanin production, with an IC50 of 7µg/mL in comparison to kojic acid which had an IC50 of 3.5 µg/mL. IC50 for Anti Collagenase and Anti-Elastase activity was found to 125 µg/mL.mL and 50µg/mL, respectively (Table 2).
 |
Table 2 Melanogenesis and Tyrosinase Inhibitory Activity and Anti-Collagenase and Anti-Elastase Activity |
Spectrophotometer - (L-Skin Brightness)
Significant improvement on the forehead was noted in skin luminosity post 4 weeks and for cheeks post 2 and 4 weeks of test product application in comparison to baseline (Figure 1A, 1B). No significant difference was noted in Upper inner arm (Volar Biceps) untreated control noted at all-time points in comparison to baseline (Figure 1C). The upper inner arm is the sun-protected area as it is never exposed to the sun during the study period. ΔL (VB-FH) and ΔB (VB-Cheek) showed a significant decrease in mean value from week 2 onwards till the end of the study week 4 implying that the test product was able to improve the skin luminosity with the continuous application (Figure 1D).
Corneometer - (Skin Hydration)
Significant improvement was noted in skin hydration at all-time points in comparison to baseline, implying that the test product efficacious in improving the hydration level of the skin (Figure 2).
 |
Figure 2 Skin hydration assessment by Corneometer for forehead and cheek at week 2 (day 15), week 4 (day 30) vs baseline all p<0.0001. |
Cutometer - (Skin Elasticity)
Gross Elasticity. Maximum Amplitude of Elasticity, Net Elasticity, Viscoelasticity
Significant improvement in gross elasticity, maximum amplitude, and Net elasticity was noted at all the time points in comparison to baseline. Visco-elasticity exhibited significant improvement at week 8 in comparison to baseline (Figure 3A).
Antera 3D - (Fine Lines and Wrinkles and Skin Texture)
A significant reduction was noted in fine lines, medium, and large wrinkles at weeks 4 and 8 in comparison to baseline, implying that the test product was efficacious in reducing the fine line with continuous application of test product (Figure 3B and Figure 4A). A significant reduction was noted in skin roughness at week 4 and week 8 in comparison to baseline (Figure 3B).
Dermatological Assessment
As per dermatologist visual assessment, significant improvement was noted in skin fairness at forehead and cheeks at week 4, and no significant change was observed in untreated control at all time points in comparison to baseline (Figure 3C). When the forehead and cheek were compared with the untreated control, a significant reduction was observed in mean value at week 4 as compared to baseline (Figure 3C). However, no significant change was observed in the evenness of skin tone, skin elasticity. A significant improvement was noted in skin texture from week 2 onwards, till the end of the study (Figure 5A), a significant change was noted in crow’s feet area post 8 weeks in comparison to baseline (Figure 5B). There was an insignificant change in nasolabial folds. Frown Lines also showed significant change post 8 weeks of product application in comparison to baseline (Figure 5C). Significant improvement was observed in the under-eye fine line from week 2 onwards, till the end of the study (8 weeks) in comparison to baseline (Figure 5D).
Image-Based Comparative Analysis by Dermatologists
There was a significant percentage change in a visual assessment done by the dermatologist based on Visia images analysis week 4 and Week 8 (Figure 4B and Supplementary Table 1) and self- assessment of subjects at week 2, week 4, and week 8 (Supplementary Table 2).
Discussion
Plant extracts have been used as a cosmetic since ancient times. The phenolic compounds and flavonoids present in plants possess antioxidant anti-inflammatory, photoprotection and reducing melanin synthesis, which can be explored in cosmetic formulations. In the past decade, several new plant products have been developed to effectively reduce photo-aging, UV damage, skin brightening, and toning.19 However, scientific validation and characterization with respect to their chemical composition, safety and efficacy are not well explored. There are studies focused on exploring new melanogenic modulators and new mechanisms for skin lightening effects.20
In the present study, we have shown that a cream formulation containing 0.4% pterostilbene is highly effective in reducing markers of aging and induces even skin tone. Pterostilbene showed significant anti-tyrosinase activity and inhibition of melanogenesis in vitro. Further, the in vitro results also showed an anti-collagenase and anti-elastase activity, suggesting that it may prevent premature aging. In many cosmetic products, ingredients like licorice root extract, Chrysanthemum morifolium extract, Vitamin C, Retinol, Abrutine, Kojic acid, Hydroquinone, alpha, and beta hydroxy acids are being used for skin lightening and brightening.21
Pterostilbene was isolated and purified from Pterocarpus marsupium heartwood and formulated as a cream in the present study. Although it shares structural similarities with resveratrol, it is a more powerful antioxidant and anti-inflammatory molecule compared to resveratrol.12,15,22
The presence of methoxy groups in pterostilbene are reported to increase the lipophilicity, membrane permeability resulting in increased activity of the molecule.23 Pterostilbene effectively scavenges several free radicals, attenuates oxidative stress by reducing ROS, increasing cellular antioxidants, via the nuclear factor erythroid 2- related factor 2 (Nrf2) signaling pathway in several cell lines.24,25
It could downregulate the synthesis of proinflammatory cytokines like IL6 and IL18, matrix metalloproteases and NFkB signaling, which can be associated with the anti-aging benefits observed in the present study.26,27
With aging, the skin undergoes many structural and functional changes, the natural moisturizing and water holding capacity of the skin is reduced, which in turn also affects the skin elasticity. For skin rejuvenation, hydration plays an important role.28–31 Phytochemicals like polyphenols, phytoestrogens, and saponosides can improve skin firmness and elasticity. Stilbenes, in general, are known to play an important role in slowing down skin aging by activating cytoplasmic and surface membrane estrogen receptors that maintain skin elasticity and collagen content and reduces wrinkling increases water-holding capacity and glycosaminoglycan content.32
Assessment of skin hydration with corneometer on forehead and cheek showed a positive change at all time points, suggesting that pterostilbene may have acted as a water content modulator, thereby improving skin moisture retention of the stratum corneum. Skin elasticity of study subjects improved with regular usage of the Pterostilbene as analyzed by Cutometer.
The recent development in digital imaging technology viz., Antera 3D, Visia in cosmetic field has simplified and minimized the use of invasive methods by dermatologists for diagnosis, evaluation, and in the therapeutic approach of skin health.33,34 In this study, pterostilbene appears to repair, revitalize, and provides radiance, vibrancy, skin glow in almost all the subjects, probably by increasing cell turn over and impairment of skin, stimulating collagen production thereby reducing the fine lines and wrinkles and restoring skin tone and texture. Significant improvement was observed in skin texture and under-eye fine lines at all-time points and in wrinkles, frown lines, and crow’s feet area at the end of the study as measured by Antera3D and as evaluated and compared at all time points by Dermatologist from Visia images captured under standard light condition.
Melanin production and skin color are determined by several factors apart from melanogenesis. The transfer of melanosomes from melanocytes to keratinocytes and their processing can also affect the skin pigmentation.3435,36 Recently cyclobutane pyrimidine dimer (CPD) was reported to be formed in melanocytes following UVA exposure, which contribute to the uneven pigmentation and dark spots.21 A recent study demonstrated that cytidine could inhibit the melanosome transfer in MelanoDerm™ skin equivalents and reduce skin and was also effective as a skin lightening agent in a clinical study.20 In the present study, the spectrophotometric evaluation showed a significant improvement in skin luminosity after 4 weeks of Pterostilbene application at all-time points in comparison to baseline, suggesting the effectiveness of the product in brightening the skin. These results infer that the Pterostilbene was efficient in imparting brightening benefits and facial anti-aging attributes in healthy male and female subjects. Pterostilbene was able to improve skin luminosity with continuous application. Our in vitro studies showed that Pterostilbene was an effective inhibitor of tyrosinase enzyme activity and melanogenesis in Murine B16 melanocytes. We have observed earlier and it has also been reported that pterostilbene can protect against UV induced photodamage to skin. The product may also have an effect of CPD mediated dark spots, which need to be further explored.
The subjective assessment, dermatological assessment, together with instrumental and image analysis in this study suggests pterostilbene has effectively improved brightness, elasticity, improvement in the appearance of fine lines, wrinkles, and skin texture as compared to baseline in the subjects studied. All subjects agreed that the test product makes their skin moisturized, brighter, younger, firmer, and make their skin look even-toned.
There was no product-related skin irritation, intolerance, adverse events, or Serious adverse event recorded during the study. The study met both primary and secondary objectives of efficacy and safety. The application of pterostilbene cream twice daily for 8 weeks was safe and well-tolerated for consumer application.
Conclusion
The results of the study described herein suggest that Natural pterostilbene is a safe and effective molecule for skin brightening and reducing signs of aging. These findings could potentially be applied in various other formulations of skincare products that help reduce photoaging, UV damage, skin brightening, and even toning. With the increasing demand for the natural cosmetic requirement by the consumers, pterostilbene offers a natural alternative for cosmetic formulations to prevent deteriorative changes during skin aging and keep the skin glowing and healthy.
Abbreviations
GCP, Good Clinical Practice; WSRS, Wrinkle Severity Rating Scale; GLSS, Galbellear Line Severity Scale.
Consent
We have written informed consent of the person for the image for publication that is depicted in Figure 4B.
Data Sharing Statement
All relevant (deidentified participant data) is made available in the manuscript and we do not have a separate repository.
Disclosure
Dr. Muhammed Majeed is the Founder and Managing Director of Sami Labs Limited and Sabinsa Corporation. The authors declare that this study received funding from Sami Labs Limited/Sabinsa Corporation. The funder was involved in conceptualizing the project and providing resources. The funder was not involved in study design, data collection and analysis of results, but was part of reviewing the manuscript and decision to publish. All the authors are affiliated with Sami Labs Limited or Sabinsa Corporation or ClinWorld Private Limited and they report no other conflicts of interest in this work.
References
1. Silva SAME, Michniak-Kohn B, Leonardi GR. An overview about oxidation in clinical practice of skin aging. An Bras Dermatol. 2017;92(3):367–374. doi:10.1590/abd1806-4841.20175481
2. Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermatoendocrinol. 2012;4(3):308–319. doi:10.4161/derm.22804
3. Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi:10.1177/0963689717725755
4. Campa M, Baron E. Anti-aging effects of select botanicals: scientific evidence and current trends. Cosmetics. 2018;5(3):54. doi:10.3390/cosmetics5030054
5. Kohl E, Steinbauer J, Landthaler M, Szeimies RM. Skin ageing. J Eur Acad Dermatol Venereol. 2011;2:873–884. doi:10.1111/j.1468-3083.2010.03963.x
6. Concepcion P, Mercado-Saenz S, Perez-Davo A, Gilaberte Y, Gonzalez S, Juarranz A. Environmental stressors on skin aging. Mechanistic insights front. Pharmacol. 2019;9. doi:10.3389/fphar.2019.00759.
7. Datta HS, Paramesh R. Trends in aging and skincare: ayurvedic concepts. J Ayurveda Integr Med. 2010;1(2):110–113. doi:10.4103/0975-9476.65081
8. Mohiuddin AK. Skin lightening & management of hyperpigmentation. Pharma Sci Anal Res J. 2019;2(2):180020.
9. Maurya R, Singh R, Deepak M, Handa SS, Yadav PP, Mishra PK. Constituents of Pterocarpus marsupium: an ayurvedic crude drug. Phytochemistry. 2004;65:915–920. doi:10.1016/j.phytochem.2004.01.021
10. Tsai HY, Ho CT, Chen YK. Biological actions and molecular effects of resveratrol, pterostilbene, and 3ʹ-hydroxypterostilbene. J Food Drug Anal. 2017;25:134–147. doi:10.1016/j.jfda.2016.07.004
11. McCormack D, McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev. 2013;2013:575482.
12. Estrela JM, Ortega A, Mena S, Rodriguez ML, Asensi M. Pterostilbene: biomedical applications. Crit Rev Clin Lab Sci. 2013;50(3):65–78. doi:10.3109/10408363.2013.805182
13. Chan EWC, Wong CW, Tan YH, Foo JPY, Wong SK, Chan HT. Resveratrol and pterostilbene: a comparative overview of their chemistry, biosynthesis, plant sources, and pharmacological properties. J Appl Pharm Sci. 2019;9(7):124–129. doi:10.7324/JAPS.2019.90717
14. Tiwari M, Sharma M, Khare HN. Chemical constituents and medicinal uses of Pterocarpus marsupium roxb. Flora Fauna. 2015;21(1):5559.
15. Pinto M, Moraes CT. Mechanisms linking mtDNA damage and aging. Free Radic Biol Med. 2015;85:250–258. doi:10.1016/j.freeradbiomed.2015.05.005
16. Li H, Jiang N, Liang B, et al. Pterostilbene protects against UVB-induced photo-damage through a phosphatidylinositol-3-kinase-dependent Nrf2/ARE pathway in human keratinocytes. Redox Report. 2017;22(6):501–507. doi:10.1080/13510002.2017.1329917
17. Sirerol JA, Feddi F, Salvador M, Rodriguez ML. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radical Biol Med. 2015;85:1–11. doi:10.1155/2016/3128951
18. Li YR, Li S, Lin C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors. 2018;44:69–82. doi:10.1002/biof.1400
19. Siegrist W, Eberle AN. In situ melanin assay for MSH using mouse B16 melanoma cells in culture. Anal Biochem. 1986;159(1):191–197. doi:10.1016/0003-2697(86)90327-1
20. Matsuda H, Nakashima S, Oda Y, Nakamura S, Yoshikawa M. Melanogenesis inhibitors from the rhizomes of Alpinia officinarum in B16 melanoma cells. Bioorg Med Chem. 2009;17(16):6048–6053. doi:10.1016/j.bmc.2009.06.057
21. Binic V, Lazarevic V, Ljubenovic M, Mojsa J, Sokolovic D. Skin ageing: natural weapons and strategies. Evidence-Based Complementary Altern Med. 2013;2. doi:10.1155/2013/827248
22. Baswan SM, Leverett J, Pawelek J. Clinical evaluation of the lightening effect of cytidine on hyperpigmented skin. J Cosmet Dermatol. 2018;18:1–8. doi:10.1111/jocd.12784
23. Yim S, Lee J, Jo H, Scholten Willingham J, Nicoll R, Baswan SM. Chrysanthemum morifolium extract andascorbic acid-2-glucoside (AA2G) blend inhibits UVA-induced delayed Cyclobutane PyrimidineDimer (CPD) production in melanocytes. Clin Cosmet Investig Dermatol. 2019;12:823–832. doi:10.2147/CCID.S223802
24. Chiou YS1, Tsai ML, Nagabhushanam K, et al. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM) induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2) mediated antioxidant signaling pathway. J Agric Food Chem. 2011; 23;59(6):2725–2733. doi:10.1021/jf2000103
25. Yeo SC, Ho PC, Lin HS. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: the impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol Nutr Food Res. 2013;57(6):1015–1025. doi:10.1002/mnfr.201200651
26. Yang Y, Fan C, Wang B, et al. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim Biophys Acta. 2017;1863:827–837. doi:10.1016/j.bbadis.2017.01.005
27. Li J, Deng R, Hua ZL, Lu F. Blueberry component pterostilbene protects corneal epithelial cells from inflammation via anti-oxidative pathway. Sci Rep. 2016;6:19408. doi:10.1038/srep19408
28. Choo QY, Yeo SCM, Ho P, Tanaka Y, Lin HS. Pterostilbene surpassed resveratrol for anti-inflammatory application: potencyconsideration and pharmacokinetics perspective. J Funct Foods. 2014;11:352–362. doi:10.1016/j.jff.2014.10.018
29. Xue EX, Lin JP, Zhang Y, et al. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget. 2017;8:41988–42000. doi:10.18632/oncotarget.16716
30. Hwang ESY, Park CS, Yin HT, Kim YM, Yi TH. Anti-aging effects of the mixture of Panax ginseng and Crataegus pinnatifida in human dermal fibroblasts and healthy human skin. J Ginseng Res. 2016;41(1). doi:10.1016/j.jgr.2016.01.001
31. Pena Ferreira MR, Costa PC, Bahia FM. Efficacy of anti-wrinkle products in skin surface appearance: a comparative study using non-invasive methods. Skin Res Technol. 2010;2:189–194. doi:10.1111/j.1600-0846.2010.00458.x
32. Langton AK, Sherratt MJ, Griffiths C, Watson RE. A new wrinkle on old skin: the role of elastic fibers in skin ageing. Int J Cosmet Sci. 2010;32(5):330–339. doi:10.1111/j.1468-2494.2010.00574.x
33. Poljšak B, Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract. 2012;135206. doi:10.1155/2012/135206
34. Verdier-Sevrain S. Effect of estrogens on skin aging and the potential role of selective estrogen receptor modulators. Climacteric. 2007;10:289–297. doi:10.1080/13697130701467157
35. Linming F, Wei H, Anqi L, et al. Comparison of two skin imaging analysis instruments: the VISIA® from canfield vs the ANTERA3D®CS from Miravex. Skin Res Technol. 2018;24:3–8. doi:10.1111/srt.12381
36. Costin GE. Hearing Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi:10.1096/fj.06-6649rev
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.