Back to Journals » Infection and Drug Resistance » Volume 16
Appropriateness of Empirical Antibiotic Therapy in Hospitalized Patients with Bacterial Infection: A Retrospective Cohort Study
Authors Luo Y, Guo Z, Li Y , Ouyang H, Huang S, Chen Y, Li K, Ji Y, Zhu H, Luo W, Liu X, Li X, Xia J, Liu X
Received 28 January 2023
Accepted for publication 22 June 2023
Published 12 July 2023 Volume 2023:16 Pages 4555—4568
DOI https://doi.org/10.2147/IDR.S402172
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yuting Luo,1,* Zhaowang Guo,2,* Ying Li,1,* Hui Ouyang,1 Shanfeng Huang,1 Yuanli Chen,3 Kenan Li,1 Yuxin Ji,1 Hongqiong Zhu,1 Wentao Luo,1 Xu Liu,1,4 Xinghua Li,1 Jinyu Xia,1 Xi Liu1
1Department of Infectious Diseases, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, People’s Republic of China; 2Clinical Laboratory, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, People’s Republic of China; 3Department of Hospital Infection Control, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, People’s Republic of China; 4Guangdong Provincial Key Laboratory of Biomedical Imaging and Guangdong Provincial Engineering Research Center of Molecular Imaging, Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xi Liu; Xinghua Li, Department of Infectious Diseases, The Fifth Affiliated Hospital, Sun Yat-sen University, 52 Meihua East Road, Xiangzhou District, Zhuhai, 519000, People’s Republic of China, Tel/Fax +86-756-252-8592, Email [email protected]; [email protected]
Objective: The incidence of inappropriate and excessive empirical antibiotic therapy is unclear. The aim of this study was to determine the prevalence of different empirical antibiotic therapy prescriptions, related factors, and outcomes in hospitalized patients with bacterial infection.
Methods: A retrospective cohort study was performed and patients with bacterial infection who were admitted between October 1, 2019, and September 30, 2020, were included. Multivariable analysis was performed by the logistic regression model.
Results: A total of 536 (42.6%) of the 1257 included patients received inappropriate empirical antibiotic therapy (IEAT), and 368 (29.3%) patients received appropriate but unnecessarily broad-spectrum empirical antibiotic therapy (AUEAT). MDRO (adjusted OR 2.932 [95% CI 2.201~3.905]; p < 0.001) and fever on admission (adjusted OR 0.592 [95% CI 0.415~0.844]; p = 0.004) were correlates of IEAT; sepsis (adjusted OR 2.342 [95% CI 1.371~3.999]; p = 0.002), age (adjusted OR 1.019 [95% CI 1.008~1.030]; p < 0.001), MDRO (adjusted OR 0.664 [95% CI 0.469~0.941]; p = 0.021), and urinary tract infection (adjusted OR 0.352 [95% CI 0.203~0.611]; p < 0.001) were correlates of AUEAT. Patients who received AUEAT were more likely to have a poor prognosis (63 [17.8%] vs 101 [27.4%]; p = 0.002). Both IEAT (median [IQR], 24,971 [13,135– 70,155] vs 31,489 [14,894– 101,082] CNY; p = 0.007) and AUEAT (median [IQR], 24,971 [13,135– 70,155] vs 30,960 [16,475– 90,881] CNY; p = 0.002) increased hospital costs. 45.3% (570/1257) of patients were infected with MDRO and 62.9% of them received IEAT.
Conclusion: Inappropriate and excessive empirical antibiotic use was widely prevalent among hospitalized patients. Either inappropriate or excessive use of antibiotics may increase the burden of healthcare costs, the latter of which may be associated with poor prognosis. Clinicians need to be more judicious in choosing antibiotic(s). The MDRO epidemic was severe, especially in patients who received IEAT. It is imperative to take effective measures to improve the current situation of antibiotic abuse and antimicrobial resistance.
Keywords: antimicrobial resistance, bacterial culture-positive, empirical antibiotic therapy, patient outcome, multidrug-resistant organism
Introduction
Inappropriate antibiotic therapy, which is defined as well-recognized, is being closely associated with patients’ adverse outcomes including morbidity and mortality.1 It often occurs when empirical use of antibiotics is required. Empirical antibiotic therapy (EAT) is unavoidable, especially for the first 24 to 48 hours, because of the absence of evidence (or laboratory test results) on the causative pathogen or its susceptibilities.2–4 EAT consists of appropriate empirical antibiotic therapy (AEAT) and inappropriate empirical antibiotic therapy (IEAT). IEAT means that the pathogen isolated in the clinical culture site is not sensitive to the antibiotics used.5,6 AEAT means that the antibiotic regimen was active against the identified pathogen based on susceptibility testing.7 Among patients who received AEAT, a proportion of antibiotic regimens were appropriate but unnecessary (AUEAT, which could also be considered excessive empirical therapy), ie, patients were treated with broad-spectrum antibiotics against resistant organisms, but no resistant organisms were isolated.6 A previous systematic review indicates that IEAT prevalence ranged from 14% to 79%, with nearly half of the included studies describing an incidence of 50% or more.8 In addition, unnecessarily broad-spectrum empirical antibiotic therapy, though appropriate, has been also widely identified in global research. A review points out that half of the intensive care unit (ICU) patients received broad-spectrum empirical antibiotic therapy without definitively confirmed infection.9 IEAT and AUEAT would cause serious adverse events, such as increasing the generation of antimicrobial resistance, the prevalence of Clostridioides difficile infection, antibiotic-related toxicities, health care costs, and mortality.5,10,11
In China, inappropriate antibiotic prescribing for outpatients was prevalent, and over half of them occurred in secondary and tertiary hospitals.12 However, there is a lack of data on IEAT and AUEAT in hospitalized patients. In our study, we investigated the prevalence, relative factors, and outcomes of IEAT and AUEAT of infected inpatients, to describe a current scenario of EAT in a tertiary hospital in China and inform further strategies to improve the appropriateness of EAT.
Methods
Study Design and Participants
This retrospective cohort study was conducted at the Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China. The Fifth Affiliated Hospital, Sun Yat-sen University is a large tertiary-level general hospital that integrates medical care, teaching, research, and preventive care. The hospital has a total of 2300 open beds and more than 70,000 admissions per year. Clinical health records of 76,264 patients admitted to the hospital between October 1, 2019, to September 30, 2020, were retrieved for further inclusion in the analysis against the inclusion and exclusion criteria. All patients aged ≥16 years old, who had at least one clinical specimen collected for in vitro bacterial culture during hospitalization and had a positive result, and who received empirical antibiotic therapy before or within 24 hours after pathogenic specimen collection as prescribed by the physician were included in the analysis. Patients were excluded if they lacked critical hospitalization information, such as diagnosis, discharge pattern, and length of stay, if they lacked antimicrobial susceptibility test results, or if the infecting bacteria were identified on admission. Each patient was included only once, at the time of the first positive culture. Finally, 1257 patients were included for analysis. The study inclusion flowchart is shown in Figure 1.
 |
Figure 1 Case-selection flowchart. Note: Data from multiple hospitalizations were collected only once during the study period. |
Data Source
We extracted demographic, clinical, and laboratory data from an electronic health record system. Clinical medical records were documented using paperless records, which were recorded by medical professionals at the time of patient admission. Clinical data included comorbidities, previous bloodstream infection, fever on admission, suspected site of infection, the severity of infection, antibiotic strategies, and patient outcome. The suspected site of infection defined by admission diagnosis or specimen culture results, eg, positive sputum culture was considered a respiratory tract infection. The severity of infection was assessed by the clinician based on the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score, which was divided into sepsis, septic shock, and no sepsis. Sepsis was defined according to Sepsis-3 criteria13 as life-threatening organ dysfunction, which can be represented by an increase in the SOFA score of 2 points or more, caused by a dysregulated host response to infection. Septic shock was defined as a subset of sepsis, which used vasopressor requirement to maintain a mean arterial pressure of 65 mmHg or greater and serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia.13 Patient outcomes in the medical record system included improvement, death, and discharge against medical advice. It has been noted that discharge against medical advice increases the risk of readmission of patients, as well as morbidity and mortality.14 Therefore, both death and discharge against medical advice were considered as poor prognoses in this study. Laboratory data included microbiological data. Microbiology data contained bacterial isolation and antimicrobial susceptibility testing reports. We collected the empirical use of antibiotics during the hospitalization of each included patient and evaluated the rationality of the antibiotic strategy according to the antimicrobial susceptibility testing report, of which intermediate susceptibilities were treated as resistant. Multidrug-resistant organism (MDRO) is defined as non-susceptibility to at least one agent in three or more antimicrobial categories,15 including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and certain Gram-negative bacilli.16 Extensively drug-resistant (XDR) is defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (ie, bacterial isolates remain susceptible to only one or two categories).15 Pandrug resistance (PDR) is defined as non-susceptibility to all agents in all antimicrobial categories (ie, no agents tested as susceptible for that organism).15 MDRO included XDR and PDR in our analysis. Many bacteria have intrinsic antibiotic resistance (eg, ceftriaxone and Pseudomonas aeruginosa) and were considered insensitive. When the susceptibility of an antibiotic received was not reported, in-vitro susceptibility or resistance was inferred according to the susceptibility results for other antibiotics in the same antibiotic category. For example, a Gram-negative organism susceptible to ceftriaxone may not have susceptibilities reported to all higher-generation cephalosporins (ie, cefepime), but these agents can be safely used.6
Definitions
EAT was defined as an antibiotic treatment before the drug sensitivity test report was obtained (at least 24 hours after sampling).17 IEAT was defined as the patient being treated with empiric antibiotics, but at least one pathogen isolated from any clinical culture site was not sensitive to all antibiotics used.5,6 Appropriate empirical antibiotic therapy (AEAT) was defined as the patient being treated with empiric antibiotics, and the antibiotic regimen was active against the identified pathogen based on susceptibility testing.7 Among patients who received AEAT, a proportion of them received appropriate but unnecessarily broad-spectrum empirical antibiotic therapy (AUEAT), which was defined as the patient received empiric antibiotics and anti-MRSA antibiotics, anti-VRE antibiotics, anti-Pseudomonas β-lactam, or carbapenem, but none of these drug-resistant bacteria (MRSA, VRE, ceftriaxone-resistant Gram-negative organisms (CTX-RO), or extended-spectrum β-lactamase (ESBL) Gram-negative organism) was cultured from the specimen of any infected site;6 the rest received appropriate and necessary empirical antibiotic therapy (ANEAT).
Data Analysis
Continuous variables were compared by using the Student’s t-test for normally distributed variables and the Mann–Whitney U-test for non-normally distributed variables. Categorical variables were assessed by using the Pearson chi-square test or two-tailed Fisher exact test. Univariate analysis was used to describe patient demographics, comorbidities, infection characteristics, site of infection, laboratory data (including antimicrobial susceptibility testing reports), and patient outcomes. Variables of clinical characteristics with P <0.1 in the univariate analysis and a priori variable were included with an enter method of multivariable logistic regression model, and the statistical strategy was developed based on some previous studies.6,18 A priori we chose the following variable: severity of infection. This variable was included in the final model irrespective of its statistical significance since it was considered to have an important impact on outcome indicators of the outcome. We also performed separate subgroup analyses on patients non-admitted to the ICU and patients admitted to ICU. The missing values for the baseline characteristic data were <1% (Table S1). All tests of significance used a 2-sided P < 0.05. Analyses were conducted using IBM SPSS Statistic version 25.0.
Result
Patient Characteristics
We finally identified 1257 patients with a positive bacterial culture who received EAT (Figure 1). The demographic and clinical data are described in Table 1. The laboratory indicators data on admission are presented in Table S2. The median age of 1257 patients was 61 years old (interquartile range [IQR] age, [48–71] years). Seven hundred and twenty-three (57.5%) patients were male. One hundred and ninety-one (15.2%) patients with fever on admission. One hundred and seventy-seven (14.1%) patients with sepsis. The respiratory tract was the most common infection site (409/1257, 32.5%), followed by urinary tract infection (338/1257, 26.9%). Nearly half of the patients were infected with MDRO (570/1257, 45.3%).
 |
Table 1 Characteristics of 1257 Patients with Empiric Antibiotic Therapy |
Prevalence of MDRO
The most common culture-positive pathogen was Escherichia coli (315/1257, 25.1%), followed by Klebsiella species (208/1257, 16.5%), S. aureus (204/1257, 16.2%), and Pseudomonas species (167/1257, 13.3%) (Figure 2). More than two-thirds of the E. coli strains were MDRO. In patients non-admitted to the ICU, the most common isolation strain was E. coli (276/998, 27.7%), which also had the highest incidence of drug resistance (44.4%) (Figure S1). Among patients admitted to the ICU, the most common isolation strains were Klebsiella species (60/259, 27.8%), but E. coli had a higher rate of drug resistance (26.9%) (Figure S2).
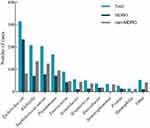 |
Figure 2 Pathogen distribution and antimicrobial susceptibility testing reports. |
Characteristics and Relative Factors Associated with IEAT
The characteristics of patients received appropriate vs inappropriate empirical antibiotic therapy (721 [57.4%] vs 536 [42.6%]) are shown in Table 1. Patients treated with IEAT were less likely to have comorbid chronic respiratory disease (175 [24.3%] vs 94 [17.5%]; p = 0.004) or congestive heart failure (60 [8.3%] vs 29 [5.4%]; p = 0.047), to have a fever on admission (131 [18.2%] vs 60 [11.2%]; p = 0.001), to require vasopressor (104 [14.4%] vs 50 [9.3%]; p = 0.006), to have respiratory tract infections (265 [36.8%] vs 144 [26.9%]; p < 0.001) or bloodstream infections (96 [13.3%] vs 50 [9.3%]; p = 0.029), and more likely to have urinary tract infections (152 [21.1%] vs 186 [34.7%]; p < 0.001) and infection with MDRO (233 [32.3%] vs 337 [62.9%]; p < 0.001). Similar differences were found in the subgroup analysis of non-admitted ICU patients (Table S3). The prevalence of MDRO infection was lower in patients received AEAT than in those received IEAT in both non-ICU-admitted (152 [36.1%] vs 269 [63.9%]; p < 0.001) and ICU-admitted (40 [27.8%] vs 68 [59.1%]; p < 0.001) patients (Table S4). The laboratory data for patients (non-admitted to the ICU and admitted to the ICU) received AEAT and IEAT were shown in Tables S5 and S6, respectively. Multivariable analysis showed that MDRO (adjusted OR 3.421 [95% CI 2.683~4.362]; p < 0.001) and fever on admission (adjusted OR 0.592 [95% CI 0.415~0.844]; p = 0.004) were correlates of IEAT (Table 2).
 |
Table 2 Univariate and Multivariable Logistic Regression Analysis of IEAT-Related Factors |
Pathogen Distribution and Antibiotic Use Analysis in IEAT
Of the patients who received IEAT, 68.3% (366/536) were infected with Gram-negative bacteria, predominantly Escherichia coli and Pseudomonas aeruginosa; 31.7% (170/536) were infected with Gram-positive bacteria, mainly Staphylococcaceae (Figure 3). 10.2% (128/536) of patients were infected with ESBL-producing Gram-negative organisms. Forty-one percent (221/536) of patients received antibiotic therapy with the correct antimicrobial spectrum but failed to cover MDRO, and 62% (136/221) of them had 1 or 2 risk factors for MDRO infection (Figure 4).
 |
Figure 4 Antibiotic use and risk factors of MDRO infection in patients received IEAT. Notes: “Correct antimicrobial spectrum but failed to cover MDRO” referred to the use of antibiotics that could cover the bacterial species, but not the isolated MDRO. “Intrinsic antibiotic resistance” meant that bacteria have intrinsic antibiotic resistance to the antibiotic used. “Correct antimicrobial spectrum but resistant” meant that the antibiotic used could cover the bacterial species, but the culture-positive strain (not MDRO) was resistant to it. “Covering MDRO but resistant” referred to using broad-spectrum antibiotics that can cover resistant bacteria, but the isolated bacteria were resistant to it. MDRO risk factors included:19 1) age ≥65 years; 2) immunocompromised (including patients with diabetes, chronic obstructive pulmonary disease, liver cirrhosis, uremia, patients suffering from cancer treated with immunosuppressive drugs, radiation therapy or chemotherapy); 3) central venous catheter; 4) mechanical ventilation; 5) invasive operations such as indwelling catheter; 6) received 3 or more antimicrobials within 90 days; 7) previous multiple or prolonged hospitalizations (≥2 times); 8) prior MDRO colonization or infection. |
Characteristics and Relative Factors Associated with AUEAT
Compared with 353 (49.0%) patients who received ANEAT, 368 (51.0%) patients who received AUEAT were older (median [IQR] age, 58 [48–68] vs 64 [53–72] years; p < 0.001), and were more likely to have comorbid chronic respiratory disease (64 [18.1%] vs 111 [30.2%]; p < 0.001), to have a previous bloodstream infection (6 [1.7%] vs 16 [4.3%]; p = 0.039), to have a fever on admission (52 [14.7%] vs 79 [21.5%]; p = 0.019) and sepsis (sepsis without shock: 23 [6.5%] vs 50 [13.6%]; p = 0.001; septic shock: 9 [2.5%] vs 34 [9.2%]; p < 0.001), to receive vasopressors (34 [9.6%] vs 70 [19.0%]; p < 0.001), colloids (69 [19.5%] vs 100 [27.2%]; p = 0.016), and mechanical ventilation (40 [11.3%] vs 86 [23.4%]; p < 0.001), and to have respiratory infections (97 [27.5%] vs 168 [45.7%]; p < 0.001) and bloodstream infections (35 [9.9%] vs 61 [16.9%]; p = 0.008); there were fewer urinary tract infections (111 [31.4%] vs 41 [11.1%]; p < 0.001) and skin, soft tissue, bone, or joint infections (60 [17.0%] vs 35 [9.5%]; p = 0.003), and lower rates of MDRO infections (132 [37.4%] vs 101 [27.4%]; p = 0.004) (Table 3). The laboratory data for patients received ANEAT vs AUEAT were shown in Table S7. Similar differences were found in the subgroup analysis of non-admitted ICU patients (Table S8). In the subgroup analysis of patients admitted to the ICU, patients received AUEAT were older than those received ANEAT (Table S9). The laboratory data for patients (non-admitted to the ICU and admitted to the ICU) received ANEAT and AUEAT were shown in Tables S10 and S11, respectively.
 |
Table 3 Patients’ Characteristics of Receiving Appropriate Empiric Antibiotic Therapy |
Four variables were identified as correlates of AUEAT in multivariable logistic regression analysis (Table 4): sepsis (adjusted OR 2.342 [95% CI 1.371~3.999]; p = 0.002), age (adjusted OR 1.019 [95% CI 1.008~1.030]; p < 0.001), MDRO (adjusted OR 0.664 [95% CI 0.469~0.941]; p = 0.021), and urinary tract infection (adjusted OR 0.352 [95% CI 0.203~0.611]; p < 0.001). Sepsis was the strongest correlate of AUEAT.
 |
Table 4 Univariate and Multivariable Logistic Regression Analysis of AUEAT-Related Factors |
Outcome and EAT Prescriptions
Two hundred and fifty-nine (20.6%) patients were admitted to ICU and the median ICU LOS was 8.0 days ([IQR], [3.0–16.0] days) (Table 5). The median hospital LOS was 14 days ([IQR], [8–29] days). Two hundred and sixty-one (20.8%) patients had a poor prognosis. Compared with patients received ANEAT, patients received AUEAT were more likely to be admitted to ICU (54 [15.3%] vs 91 [24.7%]; p = 0.001), to have higher hospital costs (median [IQR], 24,971 [13,135–70,155] vs 30,960 [16,475–90,881] CNY; p = 0.002), and to have a worse prognosis (63 [17.8%] vs 101 [27.4%]; p = 0.002) (Tables 5 and S12). Patients who received IEAT, although not found to be associated with worse prognosis, resulted in longer hospital LOS (median [IQR], 13 [7–25] vs 17 [8–31] days; p = 0.007) and increased hospital costs (median [IQR], 24,971 [13,135–70,155] vs 31,489 [14,894–101,082] CNY; p = 0.007). In the subgroup analysis, a worse prognosis was found in patients admitted to the ICU who received AUEAT compared to those who received ANEAT (19 [35.2%] vs 48 [53.3%]; p = 0.035) (Table S9).
 |
Table 5 Outcome Associated with IEAT and AUEAT |
Discussion
In our study, we found that nearly half of the infected inpatients received IEAT, and in addition, more than half of the patients who received AEAT had been treated with unnecessarily broad-spectrum antibiotics. Inappropriate and unnecessarily broad-spectrum antibiotics were widely prevalent among hospitalized patients.20–22 Investigating the prevalence of EAT prescriptions in different countries and regions is helpful to grasp the burden of unreasonable use of antibiotics in the world and improve antimicrobial stewardship.
We found that infection with MDRO was a relative factor associated with IEAT and AUEAT. Nearly half of the included patients were infected with MDRO. Almost half of the patients who received IEAT failed to cover MDRO, and most of these patients had at least one high-risk factor for MDRO infection. This suggests that clinicians were not properly assessing whether patients may be infected with MDRO. The global dissemination of antimicrobial resistance further complicates empirical antibiotic decisions and is an independent risk factor for IEAT and AUEAT.23,24 Antimicrobial resistance represents a major threat to human health with significant global and security implications and the Global antimicrobial resistance surveillance system (GLASS) report showed disturbing high rates of resistance among antimicrobials frequently used to treat common bacterial infections.25 The spread of resistance results in serious clinical and economic adverse outcomes, threatening the enormous gains made by the availability of antibiotic therapy.26 In China, a range of policies have been introduced to curb antibiotic overuse and have achieved some success,27,28 but MDROs are still prevalent nationwide. The China Antimicrobial Surveillance Network (CHINET) had been set up to monitor bacterial resistance in China since 2005. According to the annual report of CHINET (2021),29 the situation of antibiotic resistance of bacteria represented by Gram-negative bacilli is severe, with the detection rate of the third-generation cephalosporin-resistant E. coli strains remaining high, and the detection rate of carbapenem-resistant Klebsiella pneumoniae keeps rising. Our findings were similar to it. In the subgroup analysis, the detection rate of Klebsiella pneumoniae was higher in patients admitted to ICU, and the drug resistance situation was more severe. Early identification of pathogens and strengthening of antibiotic stewardship is the direction of efforts to reduce antimicrobial resistance and improve the accuracy of antibiotic prescription.30 There is an urgent need for timely, rapid, and accurate pathogen identification technology to assist clinicians in the formulation of treatment strategies. Hospitals should establish preferred empiric regimens for specific diseases and disease guidelines to reduce the rate of the inappropriateness of antibiotic treatment, taking into consideration local and national guidelines as well as antimicrobial susceptibilities.3,31 And it is appropriate and prudent for hospitals to develop systems in which patients are expeditiously recognized and promptly treated with an antimicrobial regimen that is broad enough to cover all plausibly likely pathogens.11
Sepsis was the strongest correlate of receiving AUEAT, followed by age. Fever on admission was negatively correlated with IEAT. The previous literature has shown that illness severity and age were independent predictors of mortality.32,33 Elderly adults are more likely to have bacterial infections or severe infections and the incidence of sepsis is disproportionately increased for them.33 Clinicians may be more aggressive in their choice of antibiotics for those patients with severe infections, older age, or clinically significant presentations, as physicians want to ensure appropriateness mostly in severely ill patients, but it may result in antibiotic abuse and would not improve patient prognosis.34 In our cohort, we found a worse prognosis in patients who received AUEAT, but not in those who received IEAT. This result was also found in the subgroup analysis of patients admitted to the ICU. These differ slightly from the results of some previous studies,6,35–38 which reported that IEAT or AUEAT was linked to increased mortality. We speculated that it might be because they were focused on severe infection patients such as bloodstream infection, ventilator-associated pneumonia, intra-abdominal infections, or sepsis. These patients were in severe condition and may have had a poor prognosis even without being treated with antibiotics. But they could not represent patients with infections of mild or moderate severity, which are more commonly encountered in clinical practice and therefore of greater importance for the overall selection of antibiotics and identification of resistant pathogens.39 Therefore, clinicians have to be more careful in choosing antibiotic regimens. Clinicians should make empirical antibiotic choices based on a combination of patient factors (age, illness severity, etc.), the suspected site of infection, the antimicrobial susceptibility of the expected pathogens, and local microbial resistance patterns, combined with current international guidelines and consensus.40,41
In terms of economic benefits, we found both IEAT and AUEAT would significantly increase healthcare costs, and IEAT would also prolong hospital LOS. There was an association between antimicrobial resistance and increases in healthcare costs. This association was likely the result of inadequate or delayed therapy, or it may be related to the degree of severity of the underlying disease.42 In short, either inappropriate or excessive use of antibiotics may increase the burden of healthcare costs.
There are some limitations in this study: 1) it was performed at a single center and could not be representative of a larger population. 2) we could not distinguish between pathogenic bacteria and colonization/potential contaminants bacteria based on existing data, which may have had a certain impact on the results. However, according to our exclusion criteria, we have excluded patients who are bacterial culture-positive but not treated with antibiotics, which means that clinicians have identified part of the patients with colonized or potentially contaminated bacteria-positive based on their expertise. 3) Only included patients who received antibiotics within 24 hours, which may have resulted in missing some patients who received empirical antibiotics after 24 hours. But some patients may have had initial culture results or other laboratory test results and adjusted their antibiotic regimen within 24–48 hours. 4) The Surviving Sepsis Campaign 2016 recommends that patients with sepsis and septic shock should be treated with IV antimicrobials within 1 h of recognition.43 It may be more suitable to evaluate the rationality of the antibiotic regimen on an hourly basis. However, it was not possible to analyze the time of antibiotic use on an hourly basis based on our database. Most of the subjects in our study were patients with mild infections. A study showed that in patients with mild-to-moderate disease, a delay of therapy (eg, by 4–8 h) is not associated with worse clinical outcomes.35
Conclusions
In summary, inappropriate and excessive empirical antibiotic use was widely prevalent among hospitalized patients. Either inappropriate or excessive use of antibiotics may increase the burden of healthcare costs. AUEAT may be associated with poor prognosis. The patient’s clinical characteristics can inform empirical treatment options. Clinicians need to be more judicious in choosing antibiotic(s). The MDRO epidemic was severe, especially in patients who received IEAT. It is imperative to take effective measures to improve the current situation of antibiotic abuse and antimicrobial resistance.
Abbreviations
EAT, empirical antibiotic therapy; AEAT, appropriate empirical antibiotic therapy; IEAT, inappropriate empirical antibiotic therapy; AUEAT, appropriate but unnecessarily broad-spectrum empirical antibiotic therapy; ANEAT, appropriate and necessary empirical antibiotic therapy; ICU, intensive care unit; PCT, procalcitonin; LOS, length of stay; MDRO, multidrug-resistant organism; XDR, extensively drug-resistant; PDR, pandrug resistance; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococci; CTX-RO, ceftriaxone-resistant Gram-negative organisms; ESBL, extended-spectrum β-lactamase; CRE, carbapenem-resistant Enterobacteriaceae; CRPA, carbapenem-resistant Pseudomonas aeruginosa; CRAB, carbapenem-resistant Acinetobacter baumannii; MRCNS, methicillin-resistant coagulase negative Staphylococcus; OR, Odds ratios.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article (and its additional information files). The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Institutional Review Board of the Fifth Affiliated Hospital of Sun Yat-sen University (Zhuhai, China) (No. ZDWY [2022] Lunzi No. [K26-1]) with a waiver of informed consent. This was a retrospective study, and the clinical data collected were only for the purpose of scientific research, with no additional burden on patients. The data underlying the findings of this study are retained at the Fifth Affiliated Hospital, Sun Yat-sen University and will not be made openly accessible because of ethical and privacy concerns. We declare that this study complies with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank all study participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by: the National Natural Science Foundation of China (Grant No. 82101843), Guangdong Basic and Applied Basic Research Foundation (2020A1515010254).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Raman G, Avendano E, Berger S, Menon V. Appropriate initial antibiotic therapy in hospitalized patients with gram-negative infections: systematic review and meta-analysis. BMC Infect Dis. 2015;15(1):395. doi:10.1186/s12879-015-1123-5
2. Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010;54(11):4851–4863. doi:10.1128/AAC.00627-10
3. Campion M, Scully G. Antibiotic use in the intensive care unit: optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647–655. doi:10.1177/0885066618762747
4. Gradel KO, Jensen US, Schonheyder HC, et al. Impact of appropriate empirical antibiotic treatment on recurrence and mortality in patients with bacteraemia: a population-based cohort study. BMC Infect Dis. 2017;17(1):122. doi:10.1186/s12879-017-2233-z
5. Kadri SS, Lai YL, Warner S, et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021;21(2):241–251. doi:10.1016/S1473-3099(20)30477-1
6. Rhee C, Kadri SS, Dekker JP, et al. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open. 2020;3(4):e202899. doi:10.1001/jamanetworkopen.2020.2899
7. Babich T, Zusman O, Elbaz M, et al. Empirical antibiotic treatment does not improve outcomes in catheter-associated urinary tract infection: prospective cohort study. Clin Infect Dis. 2017;65(11):1799–1805. doi:10.1093/cid/cix680
8. Kristel Marquet AL, Bergs J, Vleugels A, Claes N. Incidence and outcome of inappropriate in-hospital empiric antibiotics for severe infection: a systematic review and meta-analysis. Crit Care. 2015;19:1–2.
9. Timsit JF, Bassetti M, Cremer O, et al. Rationalizing antimicrobial therapy in the ICU: a narrative review. Intensive Care Med. 2019;45(2):172–189. doi:10.1007/s00134-019-05520-5
10. Cunha CB, Opal SM. Antibiotic stewardship: strategies to minimize antibiotic resistance while maximizing antibiotic effectiveness. Med Clin North Am. 2018;102(5):831–843. doi:10.1016/j.mcna.2018.04.006
11. Strich JR, Heil EL, Masur H. Considerations for empiric antimicrobial therapy in sepsis and septic shock in an era of antimicrobial resistance. J Infect Dis. 2020;222(Suppl 2):S119–S131. doi:10.1093/infdis/jiaa221
12. Zhao H, Wei L, Li H, et al. Appropriateness of antibiotic prescriptions in ambulatory care in China: a nationwide descriptive database study. Lancet Infect Dis. 2021;21(6):847–857. doi:10.1016/S1473-3099(20)30596-X
13. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
14. Spooner KK, Salemi JL, Salihu HM, Zoorob RJ. Discharge against medical advice in the United States, 2002–2011. Mayo Clin Proc. 2017;92(4):525–535. doi:10.1016/j.mayocp.2016.12.022
15. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
16. Jane D, Siegel ER, Jackson M, Chiarello L. Management of multidrug-resistant organisms in healthcare settings, 2006. Am J Infect Control. 2007;35(10):S165–S193.
17. Micek ST, Welch EC, Khan J, et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother. 2010;54(5):1742–1748. doi:10.1128/AAC.01365-09
18. Platteel TN, Leverstein-van Hall MA, Cohen Stuart JW, et al. Predicting carriage with extended-spectrum beta-lactamase-producing bacteria at hospital admission: a cross-sectional study. Clin Microbiol Infect. 2015;21(2):141–146. doi:10.1016/j.cmi.2014.09.014
19. Huang X, Deng ZD, Ni YX, et al. Chinese experts’ consensus on prevention and control of multidrug resistance organism healthcare-associated infection. Chin J Infect Contr. 2015;14(01):1–9.
20. Kariv G, Paul M, Shani V, Muchtar E, Leibovici L. Benchmarking inappropriate empirical antibiotic treatment. Clin Microbiol Infect. 2013;19(7):629–633. doi:10.1111/j.1469-0691.2012.03965.x
21. Braykov NP, Morgan DJ, Schweizer ML, et al. Assessment of empirical antibiotic therapy optimisation in six hospitals: an observational cohort study. Lancet Infect Dis. 2014;14(12):1220–1227. doi:10.1016/S1473-3099(14)70952-1
22. Mettler J, Simcock M, Sendi P, et al. Empirical use of antibiotics and adjustment of empirical antibiotic therapies in a university hospital: a prospective observational study. BMC Infect Dis. 2007;7:21. doi:10.1186/1471-2334-7-21
23. Carrara E, Pfeffer I, Zusman O, Leibovici L, Paul M. Determinants of inappropriate empirical antibiotic treatment: systematic review and meta-analysis. Int J Antimicrob Agents. 2018;51(4):548–553. doi:10.1016/j.ijantimicag.2017.12.013
24. Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18(6):596. doi:10.1186/s13054-014-0596-8
25. World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2020. Geneva: World Health Organization; 2020.
26. Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect. 2016;22(5):416–422. doi:10.1016/j.cmi.2015.12.002
27. National Health and Family Planning Commission of the People’s Republic of China. National action plan to contain antimicrobial resistance (2016–2020); 2016. Available from: http://en.nhc.gov.cn/2016-08/26/c_70276.htm.
28. National Health and Family Planning Commission of the People’s Republic of China. Notice on continuous management of clinical application of antibiotics; 2020. Available from: http://www.gov.cn/zhengce/zhengceku/2020-07/24/content_5529693.htm.
29. China Antimicrobial Surveillance Network (CHINET). China antimicrobial surveillance network annual report (2021); 2021. Available from: http://www.chinets.com/Document.
30. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216–225. doi:10.1016/j.jinf.2014.05.005
31. Wiener-Well Y, Hadeedi M, Schwartz Y, Yinnon AM, Munter G. Prospective audit of empirical antibiotic therapy for septic patients. Isr Med Assoc J. 2020;22(6):378–383.
32. Aryee A, Rockenschaub P, Gill MJ, Hayward A, Shallcross L. The relationship between clinical outcomes and empirical antibiotic therapy in patients with community-onset Gram-negative bloodstream infections: a cohort study from a large teaching hospital. Epidemiol Infect. 2020;148:e225. doi:10.1017/S0950268820002083
33. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis*. Crit Care Med. 2006;34(1):15–21. doi:10.1097/01.CCM.0000194535.82812.BA
34. Schuttevaer R, Alsma J, Brink A, et al. Appropriate empirical antibiotic therapy and mortality: conflicting data explained by residual confounding. PLoS One. 2019;14(11):e0225478. doi:10.1371/journal.pone.0225478
35. Davey PG, Marwick C. Appropriate vs. inappropriate antimicrobial therapy. Clin Microbiol Infect. 2008;14:15–21. doi:10.1111/j.1469-0691.2008.01959.x
36. Chen HC, Lin WL, Lin CC, et al. Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. J Antimicrob Chemother. 2013;68(4):947–953. doi:10.1093/jac/dks475
37. Kuti EL, Patel AA, Coleman CI. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J Crit Care. 2008;23(1):91–100. doi:10.1016/j.jcrc.2007.08.007
38. Paul M, Kariv G, Goldberg E, et al. Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2010;65(12):2658–2665. doi:10.1093/jac/dkq373
39. Naucler P, Huttner A, van Werkhoven CH, et al. Impact of time to antibiotic therapy on clinical outcome in patients with bacterial infections in the emergency department: implications for antimicrobial stewardship. Clin Microbiol Infect. 2021;27(2):175–181. doi:10.1016/j.cmi.2020.02.032
40. Buckman SA, Turnbull IR, Mazuski JE. Empiric antibiotics for sepsis. Surg Infect. 2018;19(2):147–154. doi:10.1089/sur.2017.282
41. van der Velden LB, Tromp M, Bleeker-Rovers CP, et al. Non-adherence to antimicrobial treatment guidelines results in more broad-spectrum but not more appropriate therapy. Eur J Clin Microbiol Infect Dis. 2012;31(7):1561–1568. doi:10.1007/s10096-011-1478-5
42. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42 Suppl 2:S82–S89. doi:10.1086/499406
43. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

