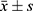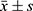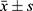Back to Journals » International Journal of General Medicine » Volume 17
Application Value of Serum Cardiac Troponin T, Brain Natriuretic Peptide Levels and Electrocardiogram Changes in the Treatment and Prognosis Evaluation of Severe Pneumonia in Children
Received 14 November 2023
Accepted for publication 5 March 2024
Published 11 March 2024 Volume 2024:17 Pages 925—934
DOI https://doi.org/10.2147/IJGM.S448548
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sandul Yasobant
Liang Deng, Xiaojun Liu, Wei Liu
Departmental of Pediatric Intensive Care Unit, The First Affiliated Hospital of Shaoyang University, Shaoyang city, Hunan Province, 422000, People’s Republic of China
Correspondence: Liang Deng, Department of Pediatric intensive care unit, The First Affiliated Hospital of Shaoyang University, 39 Tongheng Street, Shuangqing District, Shaoyang city, Hunan Province, People’s Republic of China, Email [email protected]
Objective: To explore the application of serum cardiac troponin T (cTnT), brain natriuretic peptide (BNP) levels, and electrocardiogram changes in the treatment and prognosis evaluation of severe pneumonia in children.
Methods: 120 children with severe pneumonia (severe group) admitted to our hospital from June 2020 to December 2022 were selected as the study subjects with prospective study. They were divided into survival group (n=78) and death group (n=42) based on their survival status; 120 children with mild pneumonia were selected as the control group. Compare the levels of serum cTnT and BNP, as well as the changes in electrocardiogram, to analyze their predictive value for the prognosis of pediatric patients and the influencing factors of prognosis.
Results: The proportion of children with cTnT, BNP, and abnormal electrocardiogram after treatment was lower than before treatment (P< 0.05). The proportion of children with cTnT, BNP, and abnormal electrocardiogram in the severe group was higher than that in the mild group (P< 0.05). The proportion of children with serum cTnT, BNP levels, and abnormal electrocardiogram in the death group after treatment was higher than that in the survival group (P< 0.05). Bundle branch block, low or inverted T waves, cTnT, and BNP are prognostic factors for children with severe pneumonia (P< 0.05). The combined prediction of serum cTnT and BNP for the prognosis of severe pneumonia in children is better than that of single prediction (Z combined detection - cTnT=2.474, Z combined detection - BNP=2.494, P=0.013, 0.013).
Conclusion: The proportion of abnormal cTnT, BNP, and electrocardiogram is increased in patients with severe pneumonia, and those with high expression and abnormalities have poor prognosis. cTnT and BNP have high predictive value for the prognosis of children with severe pneumonia.
Keywords: cardiac troponin T, brain natriuretic peptide, severe pneumonia, changes in electrocardiogram, prognosis
Introduction
Pneumonia is the leading cause of morbidity and mortality in children under the age of five worldwide, accounting for 12% of the deaths in pediatric intensive care units, with an estimated 900,000 children dying each year.1 Pediatric pneumonia is a common respiratory disease that, without timely and appropriate treatment, can develop into severe pneumonia. It can easily induce complications such as shock, sepsis, septicemia, and multiple organ failure, seriously endangering the lives of the children affected.2 Severe pneumonia activates the host’s innate and adaptive immune responses, causing an inflammatory reaction. Since the respiratory system of the child cannot function independently from the cardiovascular system, it can easily induce myocardial damage, thereby affecting the child’s cardiovascular system.3 Related studies have shown that mycoplasma pneumonia can not only involve the skin and joint extrapulmonary symptoms, but also cause the heart, gastrointestinal system, central nervous system and other organs involved; Streptococcus pneumoniae may cause suppurative pericarditis.4,5 Electrocardiography, as a non-invasive tool, is a simple, feasible, and rapid diagnostic method in clinical practice with good diagnostic efficacy for myocardial injury. The inflammatory response of pneumonia patients may lead to myocardial damage, which in turn can cause abnormal cardiac activity, such as sinus tachycardia.6 Therefore, it is important to find appropriate serological biomarkers and assess the patient’s level of indicators and prognosis in a timely manner based on the characteristics of the changes in their electrocardiograms. Cardiac troponin T (cTnT) is the most sensitive index for determining myocardial cell necrosis, and its levels are positively correlated with the degree of myocardial cell necrosis.7 Brain Natriuretic Peptide (BNP) is a peptide of 32 amino acids primarily synthesized and secreted by the myocardial cells of the left ventricle and is an important biomarker for cardiovascular diseases such as heart failure, hypertension, and myocardial hypertrophy.8 However, the expression levels of serum cTnT and BNP in children with severe pneumonia, as well as the characteristics of electrocardiographic changes in these children, are not yet clear. Therefore, this study aims to explore the application value of detecting the expression of cTnT and BNP in the serum and the changes in the electrocardiograms of children with severe pneumonia in the treatment and prognosis of the disease, providing a reference for the improvement of patient prognosis.
Study Subjects and Methods
Study Subjects
A total of 120 cases of children with severe pneumonia (severe group) treated in our hospital from June 2020 to December 2022 were selected as the research subjects with prospective study. According to the 30-day survival status, they were divided into the survival group (n=78) and the death group (n=42). Inclusion criteria: (1) Conformed to the relevant diagnostic criteria for severe pneumonia;9 (2) Aged 3 months to 12 years; (3) The research subjects were fully informed about the content of this study and voluntarily signed the consent form. Exclusion criteria: (1) Children with other pulmonary diseases such as pulmonary fibrosis, asthma, pulmonary embolism; (2) Children with congenital heart disease, hypoxic-ischemic encephalopathy, and other cardiovascular and cerebrovascular diseases; (3) Children with autoimmune dysfunction; (4) Children with incomplete clinical data. This study was reviewed and approved by the Medical Ethics Committee of the First Affiliated Hospital of Shaoyang University(Approval number:2020013, approval date: March 30, 2020) and followed the Declaration of Helsinki by the World Medical Association. Written informed consent to participate in this study was provided by the patient’s parents/participants’ legal guardian. Additionally, 120 cases of children with mild pneumonia (mild group) with clinical data consistent with the severe pneumonia cases were selected as the control group. See Figure 1 for the case flow chart.
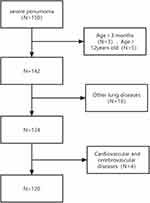 |
Figure 1 Case Flow Chart. |
Methods
Sample Collection
A reasonable and effective treatment plan was developed and implemented for children with mild and severe pneumonia, including oxygen therapy and anti-infection treatment. Venous blood was drawn the next morning after the child’s admission and on the morning of the 8th day after hospitalization treatment, approximately 3–5 mL, and centrifuged with a radius of 12 cm for 10 minutes. The samples were then stored in a −80°C freezer for testing.
Electrocardiogram Examination
The children were examined using a standard 12-lead electrocardiogram recorded by a cardiovascular specialist doctor who analyzed the electrocardiogram paper. Electrocardiogram abnormality criteria:10 The electrocardiogram showed sinus tachycardia, sinus bradycardia, paroxysmal supraventricular tachycardia, bundle branch block, peaked P waves, prolonged PR or QT interval, elevated or depressed ST segment, flattened or inverted T waves, atrial premature beats, ventricular premature beats, ventricular tachycardia, etc.
Serum cTnT and BNP Level Detection
Using the Varioskan LUX multifunctional microplate reader, the serum cTnT and BNP levels of all pneumonia children were detected using a cTnT enzyme-linked immunosorbent assay kit (item number: ml023537, Shanghai Enzyme-linked Biotechnology Co., Ltd.) and a BNP enzyme-linked immunosorbent assay kit (item number: ml060253, Shanghai Enzyme-linked Biotechnology Co., Ltd.).
Statistical Methods
Data were statistically analyzed using SPSS 25.0 software. Measurement data such as cTnT and BNP were tested for normality and conformed to a normal distribution, described with((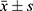 )), and compared between different groups using the independent sample t-test, and within-group comparisons before and after treatment using paired t-test; counting data on electrocardiogram change characteristics were expressed in cases (n) and percentage (%), and compared between groups using the
)), and compared between different groups using the independent sample t-test, and within-group comparisons before and after treatment using paired t-test; counting data on electrocardiogram change characteristics were expressed in cases (n) and percentage (%), and compared between groups using the 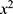 test; multifactorial Logistic regression analysis was used to analyze the influencing factors on the prognosis of children with severe pneumonia; ROC curve analysis was used to evaluate the predictive value of serum cTnT and BNP levels after treatment for the prognosis of children with severe pneumonia, with P<0.05 indicating statistical significance.
test; multifactorial Logistic regression analysis was used to analyze the influencing factors on the prognosis of children with severe pneumonia; ROC curve analysis was used to evaluate the predictive value of serum cTnT and BNP levels after treatment for the prognosis of children with severe pneumonia, with P<0.05 indicating statistical significance.
Results
Comparison of General Data Between the Two Groups
The serum CRP levels in the severe group were significantly higher than those in the mild group (P<0.05); There was no significant difference in age, gender, pathogen category, K+, and TC between the two groups. See Table 1.
 |
Table 1 Comparison of Serum cTnT and BNP Levels Before and After Treatment Between Two Groups |
Comparison of Serum cTnT and BNP Levels Before and After Treatment Between the Two Groups
Prior to and following treatment, the severe group exhibited significantly higher levels of serum cTnT and BNP than the mild group (P<0.05); after treatment, both the mild and severe groups showed significant reductions in serum cTnT and BNP levels compared to before treatment (P<0.05). See Table 2.
 |
Table 2 Comparison of Serum cTnT and BNP Levels Between the Two Groups Before and After Treatment |
Comparison of ECG Changes Before and After Treatment Between the Two Groups of Patients
Before and after treatment, the proportion of patients with ECG abnormalities in the severe group was significantly higher than in the mild group. After treatment, the rates of ECG abnormalities in both groups were significantly lower than before (P<0.05). Following treatment, the mild group showed a significant decrease in the incidence of sinus tachycardia and atrial premature beats compared to before treatment, and the severe group showed a significant decrease in sinus tachycardia, peaked P-waves, and atrial premature beats (P<0.05). Before treatment, the incidence of bundle branch block and peaked P-waves was significantly higher in the severe group compared to the mild group, and after treatment, the incidence of bundle branch block remained significantly higher in the severe group (P<0.05). See Table 3.
 |
Table 3 Comparison of ECG Changes Before and After Treatment Between the Two Groups [n (%)] |
Comparison of Serum cTnT and BNP Levels Before and After Treatment in Patients with Different Prognoses of Severe Pneumonia
After treatment, both the survival and death groups showed significant reductions in serum cTnT and BNP levels compared to before treatment (P<0.05); post-treatment, the death group had significantly higher levels of serum cTnT and BNP than the survival group (P<0.05); there were no significant differences in serum cTnT and BNP levels between the survival and death groups before treatment (P>0.05). See Table 4.
 |
Table 4 Comparison of Serum cTnT and BNP Levels in Patients with Severe Pneumonia with Different Prognosis Before and After Treatment |
Comparison of ECG Change Characteristics Before and After Treatment in Patients with Different Prognoses of Severe Pneumonia
After treatment, the proportion of patients with ECG abnormalities in both the survival and death groups was significantly lower than before treatment (P<0.05); post-treatment, the death group had a significantly higher rate of ECG abnormalities than the survival group, while there was no significant difference between the groups before treatment (P>0.05). After treatment, the survival group showed a significant reduction in the rates of sinus tachycardia, bundle branch block, peaked P-waves, and atrial premature beats compared to before treatment (P<0.05). There were no statistically significant differences in the comparison of ECG abnormality indicators before and after treatment in the death group (P<0.05); after treatment, the death group had significantly higher rates of bundle branch block and flattened or inverted T-waves compared to the survival group (P<0.05). See Table 5.
 |
Table 5 Comparison of ECG Changes in Patients with Severe Pneumonia with Different Prognosis Before and After Treatment [n (%)] |
Multifactorial Logistic Regre6Multifactorial Logistic Regression Analysis of Factors Affecting the Prognosis of Children with Severe Pneumonia
Using the outcome of children with severe pneumonia as the dependent variable (death=1, survival=0), and bundle branch block (yes=1, no=0), flattened or inverted T-waves (yes=1, no=0), cTnT (continuous variable), BNP (continuous variable) as independent variables for multifactorial logistic regression analysis, the results indicate that bundle branch block, flattened or inverted T-waves, cTnT, and BNP are factors affecting the prognosis of children with severe pneumonia (P<0.05). See Table 6.
 |
Table 6 Multivariate Logistic Regression Analysis of Prognostic Factors in Children with Pneumonia |
Predictive Value of Serum cTnT and BNP Levels After Treatment for the Prognosis of Children with Severe Pneumonia
The area under the curve (AUC) for serum cTnT after treatment in predicting the prognosis of children with severe pneumonia was 0.819, and for serum BNP it was 0.774; the combined prediction AUC for both indicators was 0.884. The combined prediction was superior to individual predictions by cTnT and BNP alone (Z combined test-cTnT=2.474, Z combined test-BNP=2.494, P=0.013, 0.013). See Table 7 and Figure 2.
 |
Table 7 Prognostic Value of Serum cTnT and BNP Levels in Children with Severe Pneumonia After Treatment |
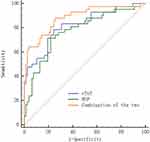 |
Figure 2 ROC Curves for Predicting the Prognosis of Patients with Severe Pneumonia Using Serum cTnT and BNP Levels After Treatment. |
Discussion
During pneumonia infection, pathogens induce inflammatory pathways, activate the sympathetic nervous system, causing an inflammatory response. Excessive inflammation can lead to vasculitis, cardiac damage, plaque instability, hypercoagulable state, myocardial suppression, resulting in a series of electrocardiogram (ECG) abnormalities and further leading to cardiac complications.11 With advancements in medical care, the treatment level for severe pneumonia has improved substantially, but the prognosis for severe pneumonia is still poor. The long-term survival, re-hospitalization, and post-discharge mortality rates at 1 year and 5 years remain high, especially in patients with myocardial injury.12 Studies have shown that in the absence of any other pathological signs, the intensity of heart sounds in viral diseases of the upper respiratory tract is significantly reduced.13 Therefore, there is an urgent need to find serum biomarkers related to the occurrence and prognosis of severe pneumonia and to timely detect changes in ECG characteristics in order to adjust treatment strategies and improve patient outcomes.
cTnT is one of the subtypes of troponin, usually present in a bound state within cardiomyocytes. Under normal circumstances, the level of cTnT in peripheral blood is extremely low. When cardiomyocytes are damaged, the cell membrane is disrupted, and a large amount of cTnT is released into the peripheral blood circulation. Due to its high specificity and sensitivity, cTnT is currently considered the gold standard for determining myocardial necrosis.14 Moridi et al15 found that cTnT was highly expressed in the bodies of patients with acute myocardial infarction and that the level of cTnT expression during autopsy was higher than that in samples taken after arrival at the morgue, which could serve as a diagnostic marker for autopsies of acute myocardial infarction. Xu et al16 discovered that serum cTnT was significantly overexpressed in patients with pulmonary hypertension, and the mortality rate in the cTnT-positive group was higher than in the cTnT-negative group, making it an independent risk factor for increased mortality in pulmonary hypertension. Chezel et al17 found that serum cTnT was significantly overexpressed in patients with systemic lupus erythematosus (SLE), reflecting the potential ongoing myocardial cell damage in patients and leading to atherosclerosis in asymptomatic SLE patients, making it a serum biomarker for predicting cardiovascular events in patients with SLE. Solaro et al18 observed that serum cTnT was significantly overexpressed in COVID-19 patients, with cTnT levels reflecting myocardial cell injury in COVID-19 patients, making it an important cause of cardiovascular complications and increased mortality in these patients. This study found that before and after treatment, the serum cTnT levels in the severe group were significantly higher than in the mild group; after treatment, the serum cTnT levels in both the mild and severe groups were significantly lower than before treatment; after treatment, both the survival and death groups showed significantly lower serum cTnT levels than before treatment; after treatment, the death group had significantly higher serum cTnT levels than the survival group, suggesting that high expression of cTnT may reflect myocardial injury in patients with severe pneumonia and is an important reason for the aggravation of pneumonia and poor prognosis in children.
BNP is a cardiac neurohormone, primarily synthesized by ventricular myocytes, closely related to the functional state of the heart. It responds to various physiological stimuli such as myocardial stretch, inflammation, myocardial ischemia, and other neuroendocrine stimulations. It can reflect early functional changes caused by overall or local structural changes in the heart.19 Xiang et al20 found that serum BNP is significantly overexpressed in patients with chronic heart failure, significantly correlated with the severity of the patient’s heart function classification, and is an independent risk factor for chronic heart failure, serving as a serum biomarker for the disease. Singh et al21 discovered that in patients undergoing non-cardiac surgery with hypertension and diabetes, preoperative and postoperative serum BNP levels are significantly elevated, making BNP an important predictor for perioperative and postoperative complications, hospital stay, and mortality rates in these patients. Zheng et al22 found that BNP is significantly overexpressed in the serum of patients with acute pulmonary embolism combined with pulmonary hypertension, and its expression increases with the severity of pulmonary hypertension, having high diagnostic value for the disease, and correlates positively with disease severity, serving as a predictive marker for the severity of the condition. Our study found that the serum levels of BNP in the severe group before and after treatment were significantly higher than those in the mild group; the expression levels in the serum after treatment were significantly lower than before treatment; and the expression levels in the serum of the death group after treatment were significantly higher than those in the survival group, suggesting that BNP reflects cardiac functional changes due to stress in patients and is one of the reasons for worsening conditions, poor therapeutic effect, and poor prognosis in pneumonia patients. Ueno et al23 found that serum cTnT and BNP levels are significantly elevated in patients with heart failure, and their overexpression is associated with reduced physical function in patients. Our study’s ROC curve shows that the combined prediction of severe pneumonia prognosis in children by serum cTnT and BNP is superior to individual predictions alone, suggesting that both cTnT and BNP may reflect the severity of myocardial injury and the prognosis of patients with severe pneumonia, and clinically, the combination of both can improve the diagnostic efficiency for the prognosis of severe pneumonia patients. Drugs can be developed to inhibit the expression of cTnT and BNP to improve the prognosis of patients. Wang et al24 found that patients with chronic heart failure and arrhythmias are accompanied by abnormal electrocardiogram indicators and elevated serum BNP levels. Our study found that the proportion of children with abnormal ECG after treatment was significantly lower than before treatment, and the proportion of children with severe ECG abnormalities was significantly higher than in the mild group, and the proportion of children with ECG abnormalities in the death group was significantly higher than in the survival group, suggesting that changes in ECG indicators have a high assessment effect on the disease progression, therapeutic effect, and prognosis of children with pneumonia. Multivariate Logistic regression analysis results indicate that bundle branch block, T-wave flattening or inversion, cTnT, and BNP are factors affecting the prognosis of children with severe pneumonia, indicating that clinical attention should be paid to the above indicators to timely improve the prognosis of children with severe pneumonia.
Children with severe pneumonia have significantly higher serum cTnT, BNP levels, and abnormal ECG proportions than those in the mild group; and those with high expression and abnormalities have poor prognoses. Serum cTnT and BNP levels have high predictive value for the prognosis of children with severe pneumonia. However, this study has issues such as a single sample source, small sample size, and unclear mechanisms of cTnT and BNP involvement in severe pneumonia in children. Further experiments will be redesigned for further research.
Data Sharing Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study involving human participants was in accordance with the ethical standards of the Medical Ethics Committee of the First Affiliated Hospital of Shaoyang University(2020013) and with the 1964 Helsinki Declaration. Written informed consent to participate in this study was provided by the patient’s parents/participants’ legal guardian.
Consent for Publication
All authors give consent for publication.
Funding
Scientific research project of Hunan Provincial Health and Family Planning Commission (No.20201520) and Project Supported by Science and Technology Department of Hunan Province (No. 2021SK4039).
Disclosure
The authors declared no conflicts of interest in this work.
References
1. Mvalo T, Smith AG, Eckerle M, et al. Antibiotic treatment failure in children aged 1 to 59 months with World Health Organization-defined severe pneumonia in Malawi: a CPAP IMPACT trial secondary analysis. PLoS One. 2022;17(12):e0278938. doi:10.1371/journal.pone.0278938
2. Lu Y, Song L. Clinical significance of procalcitonin, lactic acid, and endotoxin testing for children with severe pneumonia and sepsis. Altern Ther Health Med. 2023;29(3):218–223.
3. Mol MBA, Strous MTA, van Osch FHM, et al. Heart-rate-variability (HRV), predicts outcomes in COVID-19. PLoS One. 2021;16(10):e0258841. doi:10.1371/journal.pone.0258841
4. Yubbu P, Kaur J, Jamaluddin JA. Constrictive pericarditis following necrotising pneumococcal pneumonia in an immunocompetent child. Cardiol Young. 2019;29(8):1101–1103. doi:10.1017/S1047951119001458
5. Poddighe D, Marseglia GL. Is there any relationship between extra-pulmonary manifestations of mycoplasma pneumoniae infection and atopy/respiratory allergy in children? Pediatr Rep. 2016;8(1):6395. doi:10.4081/pr.2016.6395
6. Liu Y, Ping J, Qiu L, Sun C, Chen M. Comparative analysis of ischemic changes in electrocardiogram and coronary angiography results: a retrospective study. Medicine. 2021;100:24.
7. Wang Q, An Y, Wang H, Zhang N, Deng S. The clinical significance of changes in cTnT, CRP and NT-proBNP levels in patients with heart failure. Am J Transl Res. 2021;13(4):2947–2954.
8. Okamoto R, Ali Y, Hashizume R, Suzuki N, Ito M. BNP as a major player in the heart-kidney connection. Int J Mol Sci. 2019;20(14):3581. doi:10.3390/ijms20143581
9. Bradley JS, Byington CL, Shah SS, et al. Executive summary: the management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):617–630. doi:10.1093/cid/cir625
10. Su Y, Yin L, Lin J, et al. Electrocardiographic findings over time and their prognostic value in patients with COVID-19. Ann Palliat Med. 2021;10(12):12280–12290. doi:10.21037/apm-21-3188
11. Long B, Brady WJ, Bridwell RE, et al. Electrocardiographic manifestations of COVID-19. Am J Emerg Med. 2021;2:41.
12. Meduri GU, Shih M-C, Bridges L, et al. Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia. Intensive Care Med. 2022;48(8):1009–1023. doi:10.1007/s00134-022-06684-3
13. Poddighe D, Boggini T, Savasta S, Marseglia GL. Unrecognised diaphragmatic hernia in a refugee child: an incidental diagnosis. BMJ Case Rep. 2017;2017:2.
14. Chaulin A. Cardiac troponins: contemporary biological data and new methods of determination. Vasc Health Risk Manag. 2021;17:299–316. doi:10.2147/VHRM.S300002
15. Moridi M, Magnusson C, Zilg B. Cardiac troponin T as a postmortem biomarker for acute myocardial infarction. Forensic Sci Int. 2022;341:111506. doi:10.1016/j.forsciint.2022.111506
16. S-L X, Yang J, Zhang C-F, et al. Serum cardiac troponin elevation predicts mortality in patients with pulmonary hypertension: a meta-analysis of eight cohort studies. Clin Respir J. 2019;13(2):82–91. doi:10.1111/crj.12991
17. Chezel J, Costedoat-Chalumeau N, Laouénan C, et al. Highly sensitive serum cardiac troponin T and cardiovascular events in patients with systemic lupus erythematosus (TROPOPLUS study). Rheumatology. 2021;60(3):1210–1215. doi:10.1093/rheumatology/keaa434
18. Solaro RJ, Rosas PC, Langa P, Warren CM, Wolska BM, Goldspink PH. Mechanisms of troponin release into serum in cardiac injury associated with COVID-19 patients. Int J Cardiol Cardiovasc Dis. 2021;1(2):41–47. doi:10.46439/cardiology.1.006
19. Cao Z, Jia Y, Zhu B. BNP and NT-proBNP as diagnostic biomarkers for cardiac dysfunction in both clinical and forensic medicine. Int J Mol Sci. 2019;20(8):1820. doi:10.3390/ijms20081820
20. Xiang Y, Zhang Z, Xie C, et al. Serum Cat S, TSP-1, IL-11, BNP and sST2 diagnostic and prognostic value in chronic heart failure. Altern Ther Health Med. 2022;28(4):55–59.
21. Singh A, Kumar A, Hai AA, Masihullah M, Tripathy N, Singh PK. Serum B-type natriuretic peptide levels (BNP) can be used as a predictor of complications in patients undergoing non-cardiac surgery: a prospective observational study. Open Heart. 2023;10(1):e002256. doi:10.1136/openhrt-2023-002256
22. Zheng Q, Zhang B, Lu N, Li X, Jin B, Jin P. Diagnostic values of serum BNP, PTX3, and VEGF in acute pulmonary embolism complicated by pulmonary artery hypertension and their correlations with severity of pulmonary artery hypertension. Immun Inflamm Dis. 2023;11(9):e986. doi:10.1002/iid3.986
23. Ueno K, Kamiya K, Hamazaki N, et al. Relationship between high-sensitivity cardiac troponin T, B-type natriuretic peptide, and physical function in patients with heart failure. ESC Heart Fail. 2021;8(6):5092–5101. doi:10.1002/ehf2.13577
24. Wang Y, Ma X. Relationship between changes of electrocardiogram indexes in chronic heart failure with arrhythmia and serum PIIINP and BNP. Exp Ther Med. 2020;19(1):591–596. doi:10.3892/etm.2019.8269
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.