Back to Journals » International Journal of Women's Health » Volume 15
Application of the Joint Frailty Copula Model for Analyzing Time to Relapse and Time to Death of Women with Cervical Cancer
Authors Shewa Gari F , Fenta Biru T, Endale Gurmu S
Received 30 March 2023
Accepted for publication 29 July 2023
Published 8 August 2023 Volume 2023:15 Pages 1295—1304
DOI https://doi.org/10.2147/IJWH.S414946
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Firomsa Shewa Gari, Tashome Fenta Biru, Selamawit Endale Gurmu
Department of Statistics, Assosa University, Assosa, Ethiopia
Correspondence: Firomsa Shewa Gari, Tel +251912114186, Email [email protected]
Background: Worldwide, there were 12.7 million new cervical cancer cases, of which 5.6 million took place in industrialized nations and 7.1 million in underdeveloped nations. In eastern, western, middle, and southern Africa, it is the main cancer-related cause of death in female patients. In Ethiopia, cancer was responsible for roughly 5.8% of all fatalities. This study makes use of sophisticated statistical models that take into account population heterogeneity in terms of frailty and dependence between two endpoints in terms of copulas.
Methods: Based on hospital registry data, this retrospective study intends to examine the time to relapse and time to death of cervical cancer. This study analyzes 907 cervical cancer-positive women from various parts of Ethiopia. The copula model was used to link time to relapse and time to death of women with cervical cancer. Shared frailty model was used to incorporate unexplained heterogeneity for women with cervical cancer patients.
Results: Of the 907 cervical cancer patients, 275 (30.32%) experienced a relapse, 353 (38.92%) died, and 554 (61.08%) were censored. Age, smoking status, family planning, HIV status, family history, abortion, and stage are the most reliable predictors of both time to relapse and time to death of cervical cancer patients. The estimate of the copula parameter (θ = 1.476, 95% CI: 1.082, 1.870) shows moderate amount of dependence between time to relapse and time to death (Kendall’s rank correlation (τ) = 0.425). The estimate of the variability (heterogeneity) parameter in the population of clusters (region) is η = 0.495, 95% CI: 0.101, 0.889.
Conclusion: Age, smoking status, family planning, HIV status, family history, abortion, and more advanced stage significantly increase the risk of relapse and death of female cervical patients. There was a significant association between the time to relapse and the time to die for women with cervical cancer. There was a significant heterogeneity effect in the Tikur Anbessa Specialized Hospital.
Keywords: Clayton copula, dependence, frailty model, heterogeneity, relapse
Introduction
Worldwide, an estimated 604,127 women were diagnosed with cervical cancer in 2020.1 More than 270,000 women die from it every year, and less developed nations account for more than 85% of these fatalities.2 Cervical cancer is the fourth most common cancer among women globally, with an estimated 604,000 new cases and 342,000 deaths in 2020. About 90% of the new cases and deaths worldwide in 2020 occurred in low- and middle-income countries.3 In 2020, the WHO launched the global Cervical Cancer Elimination Initiative to accelerate the elimination of cervical cancer,1 aiming to reduce incidence below a threshold of four cases per 100 000 women-years in every country and thus narrow international disparities associated with this disease.
Worldwide, there were 12.7 million new cervical cancer cases, of which 5.6 million took place in industrialized nations and 7.1 million in underdeveloped nations. Total cancer fatalities were estimated to be 7.6 million worldwide, 2.8 million in industrialized nations, and 4.8 million in developing nations.4 The impact of cervical cancer on patients, families, and societies as a whole is enormous. Over a million women are thought to have cervical cancer at any given time worldwide; the majority of them have not received a diagnosis and lack access to medical care that could save or extend their lives.5
In eastern, western, middle, and southern Africa, it is the main cancer-related cause of death in female patients.6 Poor pathology services, a lack of facilities for diagnosis and treatment, and a lack of healthcare infrastructure all contribute to the difficulty of treating cervical cancer in Africa. More than 443,000 women a year are predicted to die from cervical cancer by the year 2030, the majority of them in sub-Saharan Africa.7 In Ethiopia, cervical cancer was responsible for roughly 5.8% of all fatalities. An estimated 60,960 cases of cancer are diagnosed there each year, and over 44,000 people pass away from the disease there each year.8,9 According to data collected by the radiotherapy department at Tikur Anbesa Specialized Hospital (TASH), cervical cancer is the second most common form of female cancer among patients who visit the oncology department.10 There are 20.90 million women in Ethiopia who are 15 years of age or older and at risk of acquiring cervical cancer.11 Further, one in four women had their first sexual encounter before turning 15.12 This is thought to raise the risk of contracting human papilloma virus (HPV), the virus that causes cervical cancer. Additionally, investigations have revealed that Ethiopia had a cervical cancer screening rate of less than 10%.13
Two survival outcomes are frequently recorded for each patient in medical investigations. For instance, patient’s medical records include information about the date of relapses and deaths of cancer patients.14 For patients with cervical cancer, there is a direct relationship between time to relapse and time to death. Relapse and death are clearly associated because patients frequently pass away right after relapse progression.15 This implies that after going through recurring progression, the likelihood of dying can significantly increase. In addition, prediction power of time to death improves by taking such dependence structures into account.16 These suggest the requirement for a suitable statistical model for the dependence between the time to relapse and time to death of cervix cancer patients. In spite of the numerous advantages of the modern statistical models, their applications to cancer data are rarely seen in the medical literature.
When considering two survival outcomes, censoring increases the difficulty of analyzing survival data. Survival outcomes may be censored if their follow-up is stopped before endpoints are seen. The occurrence of relapse, for instance, can be censored by an early death. When the time to tumor progression (such as a cancer relapse) is censored by an informative terminal event, the problem of dependent censoring arises (eg, death).17 Dependent censoring is the term used when an endpoint of interest is blocked by a mechanism connected to the endpoint.18 However, due to the dependence between relapse and death, classical statistical methods (eg, Cox regression) on time to relapse may be biased. Analyzing survival statistics for patients who are gathered from various regions (cluster) presents even greater challenges.
The classic Cox regression, which can only handle a single event, a single study, independent censoring, and a limited number of prognostic factors, is insufficient for the analysis of survival data with two events. This study makes use of novel statistical models that take into account population heterogeneity in terms of frailty and dependence between two endpoints in terms of copulas.
Methods
Study Area
The study area was Tikur Anbessa Specialized Hospital. It started an organized oncology service in the 1998 Ethiopian calendar. The cancer unit at the TASH provides chemotherapy, radiation therapy, hormone therapy and other supportive and palliative care. It is the main center for cancer registry, early detection, prevention, standard treatment and palliative care in Addis Ababa.
Study Population and Variables
Based on hospital registry data, this retrospective study intends to examine the time to relapse and time to death of cervical cancer. All women who had been registered at TASH, an oncology center, from September 2011 to September 2015 and had cervical cancer were the study’s population. From the registration log book and patients’ registration cards, the data was carefully retrieved and evaluated; if any counters included insufficient information, they were checked in the file and, if found to be so, were excluded from analysis. This study analyzes 907 cervical cancer-positive women from various parts of Ethiopia. The time to relapse and time to death of cervical cancer patients commencing from the day of the patients’ hospital registration are the response variables in this study. The time is assessed in months. Patients who lost follow-up but are still alive are censored. Age, smoking status, HIV status, family plan, abortion, family history, age at marriage, age at first birth, treatments received, and stage are explanatory variables.
Inclusion and Exclusion Criteria
All cervical cancer patients registered with full information including in the registration log book or in the patient’s identification card were considered to be eligible for the study. Patients with insufficient information about one of the vital variables either in the registration book or in the card were not eligible. As a result, 73 patients were ruled out of the study due to not meeting the inclusion criteria.
Statistical Methods
Multivariate survival analysis is a branch of survival analysis that deals with more than one event time per subject.18 In analysis of such multivariate survival data, the key element is an appropriate account for dependence between event times. In survival analysis, the term survival time refers to the time elapsed from an origin to the occurrence of an event.
Copulas for Bivariate Event Times
The copula model was used to join two event times (time to relapse and time to death of women cervical cancer) by specifying their dependence between failure times.19,20
The bivariate survival functions of two end points are given by:
where a function 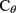 is called bivariate survival copula or simply survival copula21 and a parameter θ describes the degree of dependence between two end points. The most popular bivariate copulas are listed below:
is called bivariate survival copula or simply survival copula21 and a parameter θ describes the degree of dependence between two end points. The most popular bivariate copulas are listed below:
Kendall’s tau (τ) is used to assess the degree of dependence between time to relapse and time to death of women cervical cancer patients and given as: 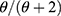 for Clayton.22
for Clayton.22
Shared Frailty Model
Shared frailty model was used to incorporate unexplained heterogeneity in the risks of experiencing an event for women cervical cancer patients. In this study, the patients are collected from different regions of Ethiopia. In shared frailty models, it is assumed that each region has its own unobserved factor (called the frailty term) influencing the risks of all patients in the region. Hence, the patients in the same region share the same frailty term.
The shared frailty model is defined on the hazard function for the jth patient in the ith cluster:
Where 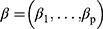 are unknown coefficients,
are unknown coefficients, 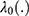 is an unknown baseline hazard function, and ui > 0, =1, 2, 3, …, C are unobserved frailty terms. In this study, we use cervical cancer dataset consisting of C = 6 independent clusters, and each cluster has, Addis Ababa = 234, Oromia = 207, Amhara = 143, Tigray = 121, SNNP = 122 and others = 80 patients (total 907 patients).
is an unknown baseline hazard function, and ui > 0, =1, 2, 3, …, C are unobserved frailty terms. In this study, we use cervical cancer dataset consisting of C = 6 independent clusters, and each cluster has, Addis Ababa = 234, Oromia = 207, Amhara = 143, Tigray = 121, SNNP = 122 and others = 80 patients (total 907 patients).
The most popular choice for the frailty distribution is the gamma density.
The mean and variance are 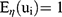 and
and 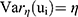 . Hence, the variance parameter
. Hence, the variance parameter 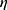 represents the amount of heterogeneity in the risk of the event.
represents the amount of heterogeneity in the risk of the event.
Joint Frailty-Copula Model
The joint frailty-copula model is defined as
Penalized Likelihood with Splines
Penalized likelihood approach used in this study because the idea of Cox partial likelihood does not carry out in a simple manner, since the integration over frailty induces a complicated form of this likelihood.23 Thus, the penalized likelihood approach has the advantage that while making no parametric assumption on the hazard or intensity functions, it yields smooth estimates of these functions. The spline method aims to obtain smooth estimate for 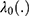 and
and 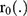 as a weighted sum of cubic polynomial functions, called basis functions.23 In this study, the goodness of fit of the models is provided by an approximate likelihood cross-validation criterion (LCV).24
as a weighted sum of cubic polynomial functions, called basis functions.23 In this study, the goodness of fit of the models is provided by an approximate likelihood cross-validation criterion (LCV).24
- R software version 4.0.5 with joint. Cox package was used for data analysis.
Result
This study includes 907 women patients who received cervical cancer treatment at TASH from September 2011 to September 2015. Of this, 275 (30.32%) experienced a relapse, 353 (38.92%) died, and 554 (61.08%) were censored (Table 1). The overall survival time ranged from 7 months to 48 months, with a median overall survival time of 34 months. The median relapse time was 29 months, ranging from 24 months to 48 months.
 |
Table 1 Descriptive Summary of Cervical Cancer Data (2011–2015) |
In this study, the explanatory variables include age, smoking status, HIV status, family plan, abortion, family history, age at marriage, age at first birth, treatment received, and stage. Analyses with a single variable and many variables are both employed. To determine the variables that might be included in the multivariate analysis, the model that contains each covariate one at a time is fitted in a univariate analysis. Covariates having p-values less than 25% in the univariate analysis were taken into account for the multivariate analysis. The factors that are irrelevant in univariate analysis are not considered in multivariate analysis.
The multivariable survival analysis makes the assumption that the Gamma frailty distribution and the Clayton Archimedean copula families exist. According to analyses using the Gamma-Clayton Archimedean copula model, age, smoking status, family planning, HIV status, family history, abortion, and stage are the most reliable predictors of both time to relapse and time to death of cervical cancer patients, whereas age at marriage and age at first birth are the predictive variables for time to death of cervical cancer patients (Table 2).
 |
Table 2 The Gamma-Clayton Archimedean Copula Multivariable Analysis |
The estimate of the copula parameter (θ = 1.476, 95% CI: 1.082, 1.870) shows moderate amount of dependence between time to relapse and time to death (τ = 0.425). This suggests that the cancer relapse may predict death in women cervical cancer patients. The estimate of the variability (heterogeneity) parameter in the population of clusters (region) is η = 0.495, 95% CI: 0.101, 0.889. This suggests that there is cluster effect on women cervical cancer patients (Table 2).
The relative risk of age of patients who are grouped in 41–60 years on time-to- relapse is 1.466 times more than as compared to that of the patients age less than or equals to 40 [RR = 1.466, 95% CI: 1.014, 2.121]. The relative risk of age of patients who are grouped in greater than 60 on time-to-death is 2.473 times more than the patients age less than or equals to 40 [RR = 2.473, 95% CI: 1.711, 3.572].
The relative risk of cervical cancer relapse for women smoker is 1.546 times more than that of nonsmoker women [RR = 1.546, 95% CI: 1.15, 2.08], which implies that women with smoker were significantly increases the risk of relapse of cervical cancer. Similarly, the relative risk of smoker patients on time-to-death is 2.252 times more than as compared to of nonsmoker patients [RR = 2.252, 95% CI: 1.756, 2.888].
The relative risk of cervical cancer relapse for women using family plan is 1.301 times more than women did not use family plan [RR = 1.301, 95% CI: 1.016, 1.667]. This indicated that women using family plan were significantly increases the risk of relapse of cervical cancer. The relative risk of women using family plan on time-to-death is 1.306 times more than women did not use family plan [RR = 1.306, 95% CI: 1.048, 1.627].
The relative risk of cervical cancer relapse for women with HIV positive is 1.557 times more than women with HIV negative (RR = 1.557, 95% CI: 1.227, 1.975). This indicated that HIV status was significantly increases the risk of relapse of cervical cancer. The relative risk of women with HIV positive on time-to-death is 1.501 times more than as compared to of the women with HIV negative (RR = 1.501, 95% CI: 1.213, 1.857) which implies that HIV status was significantly increases the risk of deaths of cervical cancer patients.
Regarding family history of women cervical cancer, the relative risk of cervical cancer relapse for having family history is 1.488 times more than women with no family history (RR = 1.488, 95% CI: 1.109, 1.995), which implies that women having family history have a significant effect on the increasing relapse of cervical cancer. The relative risk of having family history on time-to-death is 1.875 times more than as compared to women with no family history (RR = 1.875, 95% CI: 1.45, 2.424).
For women with cervical cancer, having an abortion increases the chance of relapse by 1.432 times compared to not having one (RR = 1.432, 95% CI: 1.097, 1.868), which implies that women having abortion have a significant effect on the increasing relapse of cervical cancer. The relative risk of having abortion on time-to-death is 1.647 times more than as compared to women with no abortion (RR = 1.647, 95% CI: 1.302, 2.084).
Looking for stages of women with cervical cancer, the relative risk of stage III and IV on time-to- relapse were 1.778 with [95% CI: 1.075, 2.940] and 2.955 with [95% CI: 1.694,5.157], respectively, and this indicates that women with advanced stages have a greater risk for relapse of cervical cancer. Thus, the relative risk of women with relapse of cervical cancer increased by 1.778 and 2.955 for stage III and IV, respectively, as compared to stage I. Also, the relative risk of cervical cancer for stage IV is 2.349 times more than as compared to women with cervical cancer of stage I (RR = 2.349, 95% CI: 1.523, 3.624) and this indicates that women with advanced stages have a greater risk for death of cervical cancer.
Finally, the relative risk of age at marriage who are grouped in 16–20 years on time-to-death is 0.771 times less than as compared to that of the patients age less than or equals to 15 [RR = 0.771, 95% CI: 0.613, 0.970]. Also, the relative risk of age at first birth who are grouped in 16–20 years on time-to-death is 0.677 times less than as compared to that of the patients age less than or equals to 15 [RR = 0.677, 95% CI: 0.542, 0.846].
The correlation between the time of relapse and the time to death from cervix cancer was shown using the scatter plot of the joint survival distribution. The scatter plot (Figure 1) indicates the dependency between the time to relapse and time to death of cervix cancer, with the plots appearing to act more closely or condensed.
 |
Figure 1 The scatter plots of the joint survival distribution of the Clayton Archimedean copula. |
Discussion
This study used the state-of-the-art statistical Joint Frailty Copula model on a dataset on cervical cancer obtained from Tikur Anbessa Specialized Hospital. According to this study, the patients’ time to relapse and time to death were associated. This demonstrates that the risk of death can increase dramatically after experiencing reoccurring progression, which could be explained by the fact that the same patient was engaged in both events. This solidifies the idea that the time to bi-variate events are related.25–28 The study also shows that there was a clustering (frailty) effect, which may have been influenced by the heterogeneity in the patients’ countries of origin, given that those from the same region share similar risk factors for cervical cancer.29,30
The study found that age significantly increased both the death and relapse rates for women with cervical cancer. This is because the likelihood of causing the body genetic harm (mutations) rises as we live longer. In addition, our bodies’ capacity to repair genetic damage declines with age. This aligns with past studies.31,32 Abortion had a strong positive correlation with both the time to relapse and the time to death for women with cervical cancer. This proves that cervical cancer patients who have previously had an abortion have a higher risk of dying and experiencing a relapse than those who have not. These findings are consistent with a study that was conducted.33
Another important factor that significantly affected how long it took for women with cervical cancer to die and experience a relapse of their disease was the usage of family plans. Using a family plan may increase your risk of dying from cervical cancer and its relapse, according to this information. These investigation’s findings are consistent with earlier ones.34,35 Smoking status is a significant risk factor that significantly increases the relapse and death of cervical cancer in women. The most recent study confirms earlier results.36,37 The results of this study also showed that a woman’s family history has a major impact on her chances of dying from cervical cancer and having it come back. The study that served as the basis for this one was.38
Additionally, consistent with research, the HIV status at diagnosis was significantly linked to an increased risk of cervical cancer death and relapse.39 The stages of the disease have a major impact on the time to relapse and the time to death for women with cervical cancer. This shows that women with advanced cervical cancer are more likely to die and experience relapse. Additionally, this result supports the investigations.40 The study’s concluding finding was that women’s death from cervical cancer is significantly influenced by their marital and childbearing ages. These findings are consistent with a study that was conducted.41,42
Conclusion
The most significant risk variables for relapse and death in women cervical patients were age, smoking status, family planning, HIV status, family history, abortion, and more advanced stage. While the risk of dying from cervical cancer in women is greatly influenced by age at marriage and age at first birth. The significant association between the time to relapse and the time to die for women with cervical cancer suggests that the copula model must be used to account for such a dependence. In the Tikur Anbessa Specialized Hospital, there was a significant heterogeneity effect, which reveals that one needs to employ the suitable clustered time to event frailty models to account for this clustering effect.
Abbreviations
HPV, Human papilloma virus; LCV, Likelihood Cross-validation Criterion; MPL, Maximum Penalized Log-likelihood; SNNP, South Nation Nationality and People of Ethiopia; TASH, Tikur Anbessa Specialized Hospital; WHO, World Health Organization.
Data Sharing Statement
The corresponding author will provide the datasets used and/or analyzed during the current work upon reasonable request.
Ethics Approval and Consent to Participate
The ethical review committee of the College of Natural Sciences, Assosa University approved the study protocol. Besides, a support letter was obtained from the medical director of Tikur Anbessa Specialized Hospital to access the medical records of patients. Informed consent was not taken from all respondents enrolled in the study since data were collected from the patient’s follow-up chart. Confidentiality during all phases of research activities was kept and data was held on a secured password-protected system. All the procedures are based on the principles of Helsinki declaration.
Acknowledgment
The authors gratefully acknowledge Tikur Anbessa Specialized Hospital for the provision of the data.
Author Contributions
F.S. Conceived the idea. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there were no conflicts of interest in this study.
References
1. Singh D, Vignat J, Lorenzoni V, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO global cervical cancer elimination initiative. Lancet Glob Health. 2023;11(2):e197–e206. doi:10.1016/S2214-109X(22)00501-0
2. Tugizova D, Amonova M, Farmonova D. Prognostic factors of cervical cancer in pregnant women. Sci Innov. 2022;1(D7):292–296.
3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. PMID: 33538338. doi:10.3322/caac.21660
4. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. PMID: 25220842. doi:10.1002/ijc.29210
5. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. Erratum in: CA Cancer J Clin. 2011 Mar-Apr;61(2):134. PMID: 21296855. doi:10.3322/caac.20107
6. Parkin DM, Bray F, Ferlay J, Jemal A. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev. 2014;23(6):953–966. PMID: 24700176. doi:10.1158/1055-9965.EPI-14-0281
7. Mboumba Bouassa RS, Prazuck T, Lethu T, Jenabian MA, Meye JF, Bélec L. Cervical cancer in sub-Saharan Africa: a preventable noncommunicable disease. Expert Rev Anti Infect Ther. 2017;15(6):613–627. PMID: 28440679. doi:10.1080/14787210.2017.1322902
8. Federal ministry of health Ethiopia (FMOH) and ICF. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF; 2016.
9. Abera N. Cervical cancer: Ethiopia’s outlook. J Gynecol Women's Health. 2017;5(2):555660.
10. Gurmu SE. Assessing survival time of women with cervical cancer using various parametric frailty models: a case study at Tikur anbessa specialized hospital, Addis Ababa, Ethiopia. Ann Data Sci. 2018;5(4):513–527. doi:10.1007/s40745-018-0150-7
11. Aweke YH, Ayanto SY, Ersado TL. Knowledge, attitude and practice for cervical cancer prevention and control among women of childbearing age in Hossana Town, Hadiya zone, Southern Ethiopia: community-based cross-sectional study. PLoS One. 2017;12(7):e0181415. PMID: 28742851; PMCID: PMC5526548. doi:10.1371/journal.pone.0181415
12. Yaya S, Bishwajit G. Age at first sexual intercourse and multiple sexual partnerships among women in Nigeria: a cross-sectional analysis. Front Med. 2018;5:171. doi:10.3389/fmed.2018.00171
13. Getachew S, Getachew E, Gizaw M, Ayele W, Addissie A, Kantelhardt EJ. Cervical cancer screening knowledge and barriers among women in Addis Ababa, Ethiopia. PLoS One. 2019;14(5):e0216522. doi:10.1371/journal.pone.0216522
14. Rondeau V, Pignon JP, Michiels S; MACH-NC collaborative Group. A joint model for the dependence between clustered times to tumour progression and deaths: a meta-analysis of chemotherapy in head and neck cancer. Stat Methods Med Res. 2015;24(6):711–729. Epub 2011 Oct 23. PMID: 22025414. doi:10.1177/0962280211425578
15. Emura T, Nakatochi M, Matsui S, Michimae H, Rondeau V. Personalized dynamic prediction of death according to tumour progression and high-dimensional genetic factors: meta-analysis with a joint model. Stat Methods Med Res. 2018;27(9):2842–2858. PMID: 28090814. doi:10.1177/0962280216688032
16. Emura T, Chen YH. Analysis of Survival Data with Dependent Censoring: Copula-Based Approaches. Singapore: Springer; 2018.
17. Fleming TR, Prentice RL, Pepe MS, Glidden D. Surrogate and auxiliary endpoints in clinical trials, with potential applications in cancer and AIDS research. Stat Med. 1994;13(9):955–968. PMID: 8047747. doi:10.1002/sim.4780130906
18. Gill RD. Multivariate survival analysis. Theory Probab Appl. 1993;37(2):284–301. doi:10.1137/1137061
19. Nelsen RB. An Introduction to Copulas. Springer Science & Business Media; 2007.
20. Carley H, Taylor MD. A new proof of Sklar’s theorem. In: Distributions with Given Marginals and Statistical Modelling. Dordrecht: Springer; 2002:29–34.
21. Gray R. Flexible methods for analyzing survival data using splines, with application to breast cancer prognosis. JASA. 1992;87:942–951. doi:10.1080/01621459.1992.10476248
22. Clayton DG. A model for association in bivariate life tables and its application in epidemiological studies of familial tendency in chronic disease incidence. Biometrika. 1978;65(1):141–151. doi:10.1093/biomet/65.1.141
23. Nielsen G, Gill R, Andersen P, Sorensen T. A counting process approach to maximum likelihood estimation in frailty models. Scand J Stat. 1992;19:25–43.
24. Ramsay J. Monotone regression spline in action. Statis Sci. 1988;3:425–461.
25. Gari FS, Gelcho GN. Bivariate survival copula analysis of glaucoma patients during blindness: glaucoma cases at Alert Hospital in Addis Ababa City of Ethiopia. J Res Health Sci. 2022;22(2):e00547. PMID: 36511259; PMCID: PMC9818039. doi:10.34172/jrhs.2022.82
26. Shewa F, Endale S, Nugussu G, Abdisa J, Zerihun K, Banbeta A. Time to kidneys failure modeling in the patients at adama hospital medical college: application of copula model. J Res Health Sci. 2022;22(2):e00549. PMID: 36511261; PMCID: PMC9818037. doi:10.34172/jrhs.2022.84
27. Joe H. Multivariate Models and Multivariate Dependence Concepts. CRC Press; 1997.
28. Sun T, Ding Y. CopulaCenR: copula based regression models for bivariate censored data in R. R J. 2020;12(1):266. doi:10.32614/RJ-2020-025
29. Gorfine M, Hsu L, Parmigiani G. Frailty models for familial risk with application to breast cancer. J Am Stat Assoc. 2013;108(504):1205–1215. PMID: 24678132; PMCID: PMC3963469. doi:10.1080/01621459.2013.818001
30. Mazroui Y, Mathoulin-Pélissier S, Macgrogan G, Brouste V, Rondeau V. Multivariate frailty models for two types of recurrent events with a dependent terminal event: application to breast cancer data. Biom J. 2013;55(6):866–884. PMID: 23929494. doi:10.1002/bimj.201200196
31. Ioka A, Tsukuma H, Ajiki W, Oshima A. Influence of age on cervical cancer survival in Japan. Jpn J Clin Oncol. 2005;35(8):464–469. doi:10.1093/jjco/hyi125
32. Rositch AF, Nowak RG, Gravitt PE. Increased age and race‐specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120(13):2032–2038. doi:10.1002/cncr.28548
33. Miller B, Morris M, Rutledge F, et al. Aborted exenterative procedures in recurrent cervical cancer. Gynecol Oncol. 1993;50(1):94–99. doi:10.1006/gyno.1993.1170
34. Mwaka AD, Orach CG, Were EM, Lyratzopoulos G, Wabinga H, Roland M. Awareness of cervical cancer risk factors and symptoms: cross‐sectional community survey in post‐conflict northern Uganda. Health Expect. 2016;19(4):854–867. doi:10.1111/hex.12382
35. Tadesse SK. Socio-economic and cultural vulnerabilities to cervical cancer and challenges faced by patients attending care at Tikur Anbessa Hospital: a cross sectional and qualitative study. BMC Women's Health. 2015;15(1):1–2. doi:10.1186/s12905-015-0231-0
36. Mileshkin L, Paramanathan A, Kondalsamy-Chennakesavan S, Bernshaw D, Khaw P, Narayan K. Smokers with cervix cancer have more uterine corpus invasive disease and an increased risk of recurrence after treatment with chemoradiation. Int J Gynecol Cancer. 2014;24(7):1286–1291. doi:10.1097/IGC.0000000000000170
37. Colivicchi F, Mocini D, Tubaro M, Aiello A, Clavario P, Santini M. Effect of smoking relapse on outcome after acute coronary syndromes. Am J Cardiol. 2011;108(6):804–808. doi:10.1016/j.amjcard.2011.04.033
38. Kerber RA, O’Brien E. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer. 2005;103(9):1906–1915. doi:10.1002/cncr.20989
39. Ferreira MP, Coghill AE, Chaves CB, et al. Outcomes of cervical cancer among HIV-infected and uninfected women treated at the Brazilian National Institute of Cancer (2001–2013). AIDS. 2017;31(4):523. doi:10.1097/QAD.0000000000001367
40. Machida H, Iwata T, Okugawa K, et al. Fertility-sparing trachelectomy for early-stage cervical cancer: a proposal of an ideal candidate. Gynecol Oncol. 2020;156(2):341–348. doi:10.1016/j.ygyno.2019.11.021
41. Hanske J, Meyer CP, Sammon JD, et al. The influence of marital status on the use of breast, cervical, and colorectal cancer screening. Prev Med. 2016;89:140–145. doi:10.1016/j.ypmed.2016.05.017
42. Zhai Z, Zhang F, Zheng Y, et al. Effects of marital status on breast cancer survival by age, race, and hormone receptor status: a population-based Study. Cancer Med. 2019;8(10):4906–4917. PMID: 31267686; PMCID: PMC6712463. doi:10.1002/cam4.2352
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.