Back to Journals » Infection and Drug Resistance » Volume 16
Application of Different Sampling Methods Combined with Metagenomic Next-Generation Sequencing to Detect Pathogens in Children with Severe Pneumonia on Mechanical Ventilation
Authors Nan X , Yu J , Liang Y, Lou P, Pan G
Received 23 April 2023
Accepted for publication 5 July 2023
Published 12 July 2023 Volume 2023:16 Pages 4545—4553
DOI https://doi.org/10.2147/IDR.S418339
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xiangzhen Nan, Jijie Yu, Yafeng Liang, Pengcheng Lou, Guoquan Pan
Department of Pediatric Intensive Care Unit, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China
Correspondence: Guoquan Pan, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China, Tel +86-13806882183, Email [email protected]
Objective: The aim of this study was to explore the value of applying different sampling methods combined with metagenomic next-generation sequencing (mNGS) to detect pathogens in children with severe pneumonia on mechanical ventilation.
Methods: Forty children with severe pneumonia on mechanical ventilation were selected, and routine endotracheal suctioning and bronchoalveolar lavage fluid (BALF) sampling methods were performed. The diagnostic efficacy of different sampling methods combined with mNGS versus traditional etiological pathogen detection strategies was compared.
Results: The positive rate of mNGS pathogen detection after routine endotracheal suctioning and BALF sampling was higher than that of traditional etiological detection strategies (P < 0.05). There was no significant difference in the positive rates of pathogen detection by routine endotracheal suctioning + mNGS and BALF + mNGS (P > 0.05).
Conclusion: Compared with traditional etiological detection strategies, mNGS is more efficient for diagnosing pathogens. In clinical practice, an appropriate sampling method should be selected for mNGS-based detection according to the condition of the patient. These findings could be of great importance in the diagnosis and treatment of severe pneumonia.
Keywords: sampling method, next-generation sequencing, severe pneumonia, metagenomics, mechanical ventilation
Introduction
Pulmonary infection is one of the most common diseases of the respiratory system, and severe infections in patients with underlying diseases are associated with a high mortality rate and substantial damage to human health. The rate of detecting bacteria and fungi by traditional culture is 21.7%.1 Identifying pathogenic bacteria and administering antibacterial drugs according to drug susceptibility are key to the treatment of pulmonary infection and may prevent the complication of more serious diseases such as cystic fibrosis. Chronic bacterial infections and pathogen colonization occur in the respiratory tract of patients with cystic fibrosis; infection with Haemophilus influenzae or Staphylococcus aureus usually occurs first followed by chronic infection with Pseudomonas aeruginosa or Klebsiella cepacia. Pseudomonas biofilms cause therapeutic failures in many clinical infections.2 The persistence of chronic P. aeruginosa infections, especially in immunocompromised patients, is attributed to biofilm formation, which enhances bacterial adhesion to cell walls and evasion of host immune functions.3 Traditional pathogen detection methods involve assessment of sputum, bronchoalveolar lavage fluid (BALF), blood smear and microbial culture, but the positive rates of these methods are generally low; thus, administering targeted follow-up treatment is challenging. Metagenomic next-generation sequencing (mNGS) technology, referred to simply as NGS, has gradually been recognized in clinical practice due to its lack of bias, wide applicability, high throughput, speed and accuracy. After 2004, the cost of a single test dropped significantly, and mNGS is currently widely used. Li et al4 retrospectively analyzed etiological diagnoses based on mNGS analysis of alveolar lavage fluid samples of children with severe pneumonia. Pathogens were detected in 32 of 34 children (detection rate of 94.1%). After symptomatic treatment such as anti-infection therapy, 33 children recovered and were discharged, and one child died. These findings indicate that mNGS can improve the pathogen detection rate in children with severe pneumonia and play a guiding role in clinical treatment.
This study evaluated 40 children with clinically diagnosed severe pneumonia on mechanical ventilation who were hospitalized in the PICU of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, China, from April 2020 to April 2022. Samples were collected via routine endotracheal suctioning and BALF methods and cultured, and pathogens were detected by mNGS.
Objects and Methods
Objects
This was a retrospective study involving 40 children with clinically diagnosed severe pneumonia on mechanical ventilation who were hospitalized in the PICU of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, China, from April 2020 to April 2022. All children were diagnosed with pulmonary infection according to imaging (thoracic spiral CT) and blood biochemical examinations and received routine tracheal aspiration+mNGS testing and BALF+mNGS testing. This study was approved by the Medical Ethics Committee of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University.
Methods
Specimen Collection
After admission, all children underwent complete blood count (CBC), C-reactive protein (CRP), procalcitonin (PCT), blood biochemical, immune and other examinations; the same medical and nursing team collected samples from all enrolled children first using routine endotracheal suctioning and then BALF sampling methods. After sampling, routine bacterial, fungal, and acid-fast smears, culture, and inspection were performed for 13 respiratory pathogens (mycoplasma, chlamydia, adenovirus, respiratory syncytial virus, influenza A virus (InfA), influenza A virus (H1N1), influenza A virus (H3N2), influenza B virus, parainfluenza virus, Boca virus, rhinovirus, metapneumovirus, coronavirus), and blood samples were collected for mycoplasma antibody, tuberculosis infection T-cell, and fungal (Aspergillus galactomannan, fungal D glucan) detection as well as infectious disease (human immunodeficiency virus (HIV) antibody, hepatitis C virus (HCV) antibody, Treponema pallidum antibody) detection. Additionally, after communicating with the family of the child, they voluntarily submitted samples for mNGS examination. Samples collected by routine endotracheal suctioning and BALF sampling methods were sent to BGI Genomics for mNGS. The collection, preservation and transportation of mNGS specimens were strictly in accordance with the company’s standardized specifications.
Sample Processing and DNA Extraction, Library Construction and Sequencing, and Data Analysis (BGI)
A total of 0.5–3 mL of sputum was collected, mixed and shaken, and DNA was extracted according to the kit instructions using a microsample genomic DNA extraction kit (DP316, Beijing Tiangen Biochemical Technology Co., Ltd., China). Extracted DNA was used for DNA library construction.5 Selecting the Agilent 2100 Bioanalyzer quality control library insert size, a Qubit dsDNA HS detection kit (Thermo Fisher Scientific) was used to control the DNA library concentration, and a single-chain ring structure was formed by cyclization. The cyclized library was subjected to rolling circle amplification (RCA) to generate DNA nanospheres. The prepared DNA nanospheres were loaded into the sequencing chip and sequenced using BGISEQ-50/MGISEQ-200/MGISEQ-2000.6 After the sequencing data were downloaded, low-quality data and data measuring less than 35 bp were removed to obtain high-quality data; then, data aligned with the human reference genome sequence were removed by Burrows‒Wheeler alignment (BWA: http://biobwa.sourceforge.net/).7 After removing the low-complexity sequence numbers (reads), the remaining data were compared with 4 large microbial databases of bacteria, fungi, viruses, and parasites to identify pathogen matches. Possible pathogens were determined by high or low sequence numbers and other clinical tests.
Statistical Analysis
SPSS 26.0 software was used for data analysis and processing. Measurement data are expressed as the mean plus standard deviation, count data are expressed as percentages (%), and the χ2 test was used for intergroup comparisons. P<0.05 was considered statistically significant.
Results
Clinical Data
Among the 40 children, 26 were male and 14 were female. The age ranged from 8 months to 14 years old, with an average age of 6.44±5.14 years. Thirty-seven of 40 children had fever; all children had cough symptoms. Three were considered to have congenital immunodeficiency disease, and 1 had trisomy 21 syndrome. More details of the clinical data are shown in Table 1.
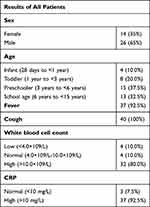 |
Table 1 Clinical Data and Routine Blood and CRP Tests |
Routine Blood Tests and CRP Detection
Among the 40 children, 32 had leukocytosis, 4 had leukopenia, and 37 had increased CRP. More details of the blood tests are shown in Table 1.
Microbial Spectrum
Routine endotracheal suctioning+ mNGS detection, BALF + mNGS detection and routine culture + smear + PCR detection were used to analyze the pathogenic microorganisms in the patients.
The rate of positive pathogen detection by BALF+mNGS was 80.0% (32/40), and 40 pathogenic microbial strains were identified. The top three were Streptococcus pneumoniae (9 strains), Staphylococcus aureus (4 strains), and adenovirus (3 strains). The rate of positive pathogen detection by routine endotracheal suctioning+ mNGS was 77.5% (31/40). A total of 53 pathogenic microorganisms were detected, the top 3 of which were S. pneumoniae (9 strains), S. aureus (4 strains) and adenovirus (3 strains). The rate of positive pathogen detection by routine culture + smear + PCR was 42.5% (17/40). The results for these 17 patients were consistent with the BALF + mNGS results. The top 3 pathogens were S. pneumoniae (5 strains), S. aureus (3 strains), and adenovirus (2 strains); of these, 13 strains were detected by routine culture, 1 strain of acid-fast bacillus was detected by smear, and the 3 strains of virus were detected by PCR. The rate of positive pathogen detection by routine endotracheal suctioning+ mNGS and BALF+ mNGS was significantly higher than that of traditional etiological detection (P < 0.05). The rate of positive pathogen detection by routine endotracheal suctioning+mNGS was similar to that by BALF+mNGS, and there was no significant difference (P > 0.05).
Among the 32 positive samples detected by BALF+mNGS, 24 were single pathogen infections, and 8 were mixed infections. The most common single infective agent was S. pneumoniae (6 patients). Among the 31 positive samples detected by routine endotracheal suctioning+ mNGS, 18 were single pathogen infections, and 13 were mixed infections. The most common single infective agent was S. pneumoniae (6 patients). The 17 positive specimens detected by routine culture, smear and PCR all contained a single pathogen. More details are shown in Table 2 and 3.
 |
Table 2 Data for the 20 Patients with Single-Pathogen Infections |
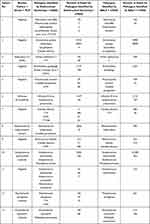 |
Table 3 Data for the 13 Patients with Multiple Pathogen Infections |
The sensitivity of mNGS was significantly higher than that of culture, while the specificity of culture was significantly higher than that of mNGS. The sensitivity and specificity of mNGS and culture detection of pathogens are shown in Table 4 and 5.
 |
Table 4 Comparison of Sensitivity and Specificity Between Endotracheal Suctioning + mNGS and Culture |
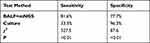 |
Table 5 Comparison of Sensitivity and Specificity Between BALF + mNGS and Culture |
Treatment and Outcomes
The children were empirically treated with antibiotics according to their medical history, clinical manifestations and laboratory test results when they were admitted to the hospital. After the mNGS results were reported, the treatment plan was adjusted. Overall, 2 patients died, 1 patient abandoned treatment, and 1 patient was transferred to another hospital. After active comprehensive treatment, the remaining children were all cured and discharged.
Discussion
Severe pneumonia due to infection seriously threatens the safety of children, so early and accurate identification of the pathogen is crucial. When the etiology of LRTI is not clear, it is often impossible to administer targeted drug treatment, which delays treatment and patient recovery. If the treatment is not delivered in a timely manner, the patient may die.8 However, the existing methods for detecting the etiology of pulmonary infection are not satisfactory. A nationwide study of hospitalized patients with community-acquired pneumonia conducted by Cao et al in 2014 showed that sputum culture examinations were completed in 68.9% of patients after admission, of which only 18.5% were qualified sputum specimens, and only 12.7% had possible or confirmed pathogens.9 In a prospective investigation of the etiology of lower respiratory tract infections in adults in 11 European countries, Leven et al showed that even with combined microbial culture, molecular biology methods such as polymerase chain reaction (PCR) and serological screening of respiratory pathogens, the positive rate for detecting a pathogen was approximately 59%, and 40% of lower respiratory tract infections were of unknown etiology.10
In recent years, with the rapid development of genomics technology, mNGS technology has been increasingly applied for pathogen detection, as it is capable of covering a wider range of pathogens, including viruses, bacteria, fungi, mycoplasma, chlamydia, and parasites, than other methods. A short time is needed to determine the pathogen, and the drug resistance of bacteria can also be predicted, which plays an important guiding role in the diagnosis and treatment of pneumonia. The application of this technology opens a new chapter in the accurate clinical diagnosis and treatment of severe pneumonia.11
The results of this study showed that the rates of positive pathogen detection by routine endotracheal suctioning + mNGS, BALF+mNGS, and routine culture + smear + PCR in the 40 enrolled children were 77.5% (31/40), 80.0% (32/40), and 42.5% (17/40), respectively. The rate of positive pathogen detection by routine endotracheal suctioning + mNGS and BALF+ mNGS was significantly higher than that of traditional etiological detection (P < 0.05), suggesting that mNGS has more advantages in pathogen detection than traditional detection methods. The reason is that mNGS screening for pathogens directly yields the nucleic acid sequence information of the pathogen from the specimen and then compares and analyzes it through bioinformatics methods to identify the pathogen. The detection rate of this method is improved because it detects nucleic acids and is not easily affected by antimicrobial drugs.12 In contrast, culture and smear methods may be affected by culture conditions and antimicrobial use, resulting in lower positive detection rates.13
The rate of positive pathogen detection by routine endotracheal suctioning+ mNGS was similar to that detected by BALF+mNGS (P > 0.05). The routine transtracheal intubation suctioning method does not pass through the oral cavity, which reduces the possibility of contamination with viruses, bacteria, and fungi in the oropharynx. For critically ill infants, the diameter of the tracheal intubation tube is small, and there is difficulty in inserting the bronchoscope. Routine BALF sampling cannot be performed, so routine transtracheal intubation sampling can be used instead for inspection.
In this study, routine endotracheal suctioning +mNGS and BALF+mNGS detected 9 cases of S. pneumoniae, while routine culture and smear detected 5 strains, all of which were the most common pathogens, consistent with the results of reports on the most common pathogens in community-acquired pneumonia in recent years13,14 and those of Tang et al.15 These findings also confirmed that S. pneumoniae is a common pathogen in severe childhood pneumonia.
As a more ideal detection method for infectious pathogens, mNGS is used worldwide for clinical diagnosis. A systematic review published in Nature Reviews Genetics in 2016 detailed the application of mNGS in respiratory system infections, blood infections, and nervous system infections.16 Chinese scholars have also reported cases of the successful application of mNGS technology in diagnosing pathogens in different fields.17–20 In the newly released “Clinical practice expert consensus for the application of metagenomic next generation sequencing”,21 the guidelines recommend that if no pathogen is found in traditional etiological testing in 3 days and the effect of anti-infective empirical treatment is poor, respiratory samples should be submitted for mNGS detection. For immunosuppressed patients with respiratory tract infections or critically ill patients, while performing traditional etiological testing, respiratory samples should be sent for mNGS testing as soon as possible for the early identification of rare pathogens or possible mixed infections. Therefore, mNGS can be used as an effective supplement to traditional etiological diagnosis, and the combined application of the two can improve the overall pathogen detection rate.
As a detection method with high sensitivity and specificity, mNGS can provide clinicians with valuable insights into clinical treatment.22 BALF and mNGS are broad-spectrum tests that can be performed rapidly and have high diagnostic efficiency, which are ideal for critically ill children.23
Conclusion
In summary, compared with traditional etiological detection, mNGS has better diagnostic performance for detecting pathogens. In clinical practice, an appropriate sampling method should be selected for mNGS detection according to the actual patient condition. The application of mNGS is of great importance in the diagnosis and treatment of patients with severe pneumonia.
Due to the high cost of mNGS examination, the sample size of this study was relatively small. In future research, we will design a multicenter study and enroll more children to obtain results more consistent with evidence-based medicine.
Ethics Approval and Informed Consent
2021-K-114-01. The medical ethics committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University approved this study.Data were collected in strict confidentiality.The hospital has an ethical exemption for such retrospective research articles, witch were also discussed by the Medical Ethics Committee of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University in the mid-term and found to be compliance with the Declaration of Helsinki.
Funding
Wenzhou Science and Technology Plan Project: Y2020070.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yang W, Tang Y, Zhu H, et al. Etiological study of bronchopneumonia in critically ill children using metagenomic Next-Generation Sequencing technology. J Clin Pulmonary Med. 2021;6:863–868.
2. Al-Qaysi AK, Al-Ouqaili MT, Al-Meani SA. Ciprofloxacin- and gentamicin-mediated inhibition of Pseudomonas aeruginosa biofilms is enhanced when combined the volatile oil from Eucalyptus camaldulensis. SRP. 2020;11(7):98–105.
3. Al-Qaysi AK, Al-Ouqaili MTS, Al-Meani SA. Effect of pyocyanin as secondary metabolite on pseudomonal biofilm and in increasing the resistance degree to antipseudomonal agents. Drug Invention Today. 2020;14(3):125.
4. Li X, Wang L, Zhang L, et al. Observation on the application of Next-Generation Sequencing of alveolar lavage fluid in the pathogenic diagnosis of severe pneumonia in children. Chin J Pract Pediatr. 2019;34(6):513–516.
5. Long Y, Zhang Y, Gong Y, et al. Diagnosis of sepsis with cell-free DNA by Next-Generation Sequencing technology in ICU patients. Arch Med Res. 2016;47(5):365–371. doi:10.1016/j.arcmed.2016.08.004
6. Jeon YJ, Zhou Y, Li Y, et al. The feasibility study of non-invasive fetal trisomy 18 and 21 detection with semiconductor sequencing platform. PLoS One. 2014;9(10):e110240. doi:10.1371/journal.pone.0110240
7. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi:10.1093/bioinformatics/btp324
8. Huang C, Hu R, Zhang Y, Li S. The next-generation sequencing technology for diagnosis of pulmonary tuberculosis and pulmonary nontuberculous mycobacterial diseases: two cases report and literature review. Chin J Respirat Crit Care Med. 2019;18(1):21–25.
9. Chen L, Zhou F, Li H, et al. Disease characteristics and management of hospitalised adolescents and adults with community-acquired pneumonia in China: a retrospective multicentre survey. BMJ Open. 2018;8(2):e018709. doi:10.1136/bmjopen-2017-018709
10. Ieven M, Coenen S, Loens K, et al. Aetiology of lower respiratory tract infection in adults in primary care: a prospective study in 11 European countries. Clin Microbiol Infect. 2018;2018:S1198743X18301526.
11. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14:319–338. doi:10.1146/annurev-pathmechdis-012418-012751
12. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic Next-generation Sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–s240. doi:10.1093/cid/ciy693
13. National Health Commission of the People’s Republic of China, National Administration of Traditional Chinese Medicine. Guideline for diagnosis and treatment of community-acquired pneumonia in Children (2019 version). Chin J Clin Infect Dis. 2019;12(1):6–13. doi:10.3760/cma.j.issn.1674-2397.2019.01.002
14. Ning G, Wang X, Wu D, et al. The etiology of community-acquired pneumonia among children under 5 years of age in mainland China, 2001–2015: a systematic review. Hum Vaccin Immunother. 2017;13(11):2742–2750. doi:10.1080/21645515.2017.1371381
15. Tang Q, Xin L, Kang Y. Value of metagenomics next-generation sequencing in bronchoalveolar lavage fluid in the etiological diagnosis of children with severe pneumonia. Clin Res Pract. 2023;8(1):92–96. doi:10.19347/j.cnki.2096-1413.202301025
16. Salzberg SL, Breitwieser FP, Kumar A, et al. Next-generation sequencing in neuropathologic diagnosis of infections of the nervous system. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e251. doi:10.1212/nxi.0000000000000251
17. Cheng J, Hu H, Zhang S, et al. Application of next-generation sequencing in detecting pathogens in heart valve tissues of patients with infective endocarditis. Chin J Infect Control. 2019;18(04):277–282.
18. Jin W, Li N, Zhou X, et al. Etiological diagnostic value of metagenomic next-generation sequencing in spinal infection. Chin J Clin Med. 2020;27(04):567–571.
19. Xu Y, Li S, Xu W. Application of next-generation sequencing technology in diagnosis of periprosthetic joint infection. Chin J Joint Surg. 2019;13(05):584–588.
20. Zhao Z, Zhao E, Liu T, et al. Clinical analysis of 6 cases of Streptococcus suis meningitis. China Trop Med. 2020;20(05):469–472. doi:10.13604/j.cnki.46-1064/r.2020.05.16
21. Editorial Board of Chinese Journal of Infectious Diseases. Clinical practice expert consensus for the application of metagenomic next generation sequencing. Chin J Infect Dis. 2020;38(11):681–689. doi:10.3760/cma.j.cn311365-20200731-00732
22. Li Q, Han L, Ma Y, Zhang L, Zhang G. Clinical significance of macrogenomic second- generation sequencing technology for the detection of pathogenic bacteria in pulmonary infections. Shenzhen J Integrated Trad Chin West Med. 2020;30(9):66–67. doi:10.16458/j.cnki.1007-0893.2020.09.031
23. Yang W, Tang Y, Zhu H, et al. Study of metagenomic next generation sequencing for pulmonary infectious pathogens detection to intensive care children with pneumonia. J Clin Pulmonary Med. 2021;26(6):863–868. doi:10.3969/j.issn.1009-6663.2021.06.011
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
