Back to Journals » Drug Design, Development and Therapy » Volume 17
Application of Butorphanol versus Sufentanil in Multimode Analgesia via Patient Controlled Intravenous Analgesia After Hepatobiliary Surgery: A Retrospective Cohort Study
Authors Xu X, Tao Y, Yang Y , Zhang J, Sun M
Received 31 August 2023
Accepted for publication 13 December 2023
Published 20 December 2023 Volume 2023:17 Pages 3757—3766
DOI https://doi.org/10.2147/DDDT.S433136
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Xiaodong Xu,* Yuan Tao,* Yitian Yang, Jiaqiang Zhang, Mingyang Sun
Department of Anesthesiology and Perioperative Medicine, Zhengzhou University People’s Hospital, Henan Provincial People’s Hospital, Zhengzhou, 450003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiaqiang Zhang; Mingyang Sun, Department of Anesthesiology and Perioperative Medicine, Zhengzhou University People’s Hospital, Henan Provincial People’s Hospital, Zhengzhou, 450003, People’s Republic of China, Tel +0 371 6558 0728, Email [email protected]; [email protected]
Purpose: We investigate the efficacy and safety of butorphanol in multimodal analgesia combined with dexmedetomidine and ketorolac via patient-controlled intravenous analgesia (PCIA) after hepatobiliary surgery, as compared with sufentanil.
Patients and Methods: Postoperative follow-up data of hepatobiliary surgery patients in Henan Provincial People’s Hospital from March 2018 to June 2021 were collected retrospectively and divided into butorphanol group (group B) or sufentanil group (group S) according to the postoperative intravenous controlled analgesia scheme. The baseline characteristics and surgical information of the two groups were matched through propensity score matching (PSM).
Results: A total of 3437 patients were screened, and PSM yielded 1816 patients after matching, including 908 in the butorphanol group and 908 in the sufentanil group. Compared with group S, the incidence of moderate-to-severe pain on the first postoperative day and the second postoperative day was lower in group B during rest (3.2% vs 10.9%, P< 0.001; 1.2% vs 4.6%, P< 0.001), and during movement (7.0% vs 18.9%, P< 0.001; 2.6% vs 8.7%, P< 0.001). Patients receiving butorphanol had a lower morphine consumption (50mg vs 120mg, P< 0.001). The bolus attempts of an analgesic pump in group B were significantly lower than in group S (1 vs 2, P< 0.001). Postoperative hospital length of stay was shortened in group B (11d vs 12d, P=0.017). The occurrence of postoperative vomiting was lower in group B (1.4% vs 3.0%, P=0.025) than in group S. However, more patients in group B experienced dizziness (0.9% vs 0.1%, P=0.019).
Conclusion: Compared with sufentanil, the application of butorphanol in multimodal analgesia combined with dexmedetomidine and ketorolac via PCIA ameliorated postoperative pain after hepatobiliary surgery, with reduced opioid consumption and shorter postoperative hospital length of stay.
Keywords: patient controlled intravenous analgesia, butorphanol, sufentanil, propensity score matching
Introduction
Acute postoperative pain remains one of the most common challenges in patients undergoing hepatobiliary surgery.1,2 Insufficient analgesia has been associated with increased sympathetic activation, immune suppression, higher risk of cardiovascular and respiratory systems, delayed recovery of gastrointestinal peristalsis function, and adverse psychological emotions.3,4 If not well controlled in the initial stage, acute pain may result in peripheral and central sensitization, and evolve into chronic pain after surgery.5–7 Therefore, adequate postoperative analgesia is fundamental and acts as a core principle in the context of the Enhanced Recovery After Surgery (ERAS) program.8,9 Considering that pain patterns in patients receiving hepatobiliary surgery are multifactorial and differ from other procedures, effective multimodal analgesia with the combination of differing targeting pain pathways has been suggested to be the optimal pain management in abdominal surgery.10,11
Current evidence emphasizes the growing interest in novel techniques, including intrathecal morphine, truncal nerve blocks, as well as emerging analgesics including esketamine, 5-HT3-antagonists, and so on.2,12–16 However, opioids are still utilized as the first-line choice for perioperative pain management due to their powerful rapid-onset systematic analgesic effect.17 Meanwhile, they could also induce a high incidence of opioid-related side effects, including delayed respiratory depression, nausea and vomiting, urinary retention, itching, dizziness, and so on.4 Strategies to minimize opioid consumption, whilst optimizing patient comfort and postoperative recovery are of priority.
Butorphanol, an opioid receptor agonist-antagonist, has been widely used in postoperative pain management recently. Butorphanol mainly acts on the κ receptors in the presynaptic and postsynaptic membranes of neurons in the central nervous system. By inhibiting adenylyl cyclase, it reduces the intracellular cyclic adenosine monophosphate content and intracellular protein kinase phosphorylation level, and reduces the release of glutamate, thereby reducing the excitability of postsynaptic neurons and playing an analgesic effect. Butorphanol acts as a partial agonist of the κ-opioid receptor in the G protein activation pathway and is a full agonist on the β-arrestin recruitment pathway.18 Compared with pure μ-receptor agonists, butorphanol could better inhibit visceral pain and result in a lower incidence of opioid adverse reactions and opioid addiction.18–21 In addition, butorphanol has also been proven to mitigate emergence agitation and reduce postoperative shivering.22,23 Clinical practice concerning pair-wise combinations of butorphanol with ketorolac or dexmedetomidine via patient controlled intravenous analgesia (PCIA) has been well-established recently, while the beneficial characteristic and related side effects of butorphanol in combination with ketorolac and dexmedetomidine together in postoperative multimodal analgesic strategies remain to be verified in large clinical practice.24 This retrospective study aims to investigate the efficacy and safety of butorphanol combined with dexmedetomidine and ketorolac in multimodal analgesia after hepatobiliary surgery as compared with sufentanil.
Materials and Methods
This study was approved by the Ethics Committee of Henan Provincial People’s Hospital [Ethics approval number: (2019) lun shen (102)], a tertiary academic hospital in the central region of China. Considering the nature of the retrospective design and the use of deidentified data, our institutional ethics committee waived the need for informed consent. Our study complied with the Declaration of Helsinki, and reporting of the cohort study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.25 All of the data were analyzed anonymously.
Electronic records of patients undergoing hepatobiliary surgery with general anesthesia and receiving PCIA from March 2018 to June 2021 were collected. Exclusion criteria included incomplete clinical data; preoperative long-term use of opioids (no less than 3 months); liver or kidney dysfunction; and incomplete follow-up data within 72 hr after surgery. Patients were categorized into two groups according to the opioid prescription of the analgesic pump, either the sufentanil group (group S) or the butorphanol group (group B).
Anesthesia and Analgesia Techniques
The anesthetic protocol in both groups was administered according to routine clinical practice. On admission to the operation room, peripheral venous access was established and vital signs were routinely monitored (including NIBP, ECG, SPO2, and ETCO2). After a 3-min preoxygenation with 100% oxygen, general anesthesia was induced with an intravenous injection of propofol and sufentanil.26,27 Tracheal intubation or laryngeal mask was facilitated with rocuronium or cis-atracurium. During the operation, combined intravenous-inhalation anesthesia with propofol, remifentanil, and sevoflurane was administered to maintain a BIS of 40 to 60, and adequate muscle relaxation was maintained with intermittent intravenous infusion of cis-atracurium.28,29 At the end of the operation, neuromuscular blockade was reversed with neostigmine and atropine. The tracheal tube/laryngeal mask was removed, while the patient fully awakened from anesthesia. All patients received PCIA for postoperative pain management. The solution of the electronic analgesia pump was a mixture of the following agents: one opioid (sufentanil 1.5~2.0ug/kg or butorphanol 8~12mg) + dexmedetomidine 1~1.5ug/kg + ketorolac 240~360mg + tropisetron 10~15mg, with a total capacity of 100mL. The background continuous rate was 2 mL/hr, and the bolus dose was 2 mL with a 15-min lockout interval. The type of the opioid agent was chosen according to the anesthesiologists’ preference and experience. PCIA was discontinued if hypoventilation (respiratory rate of <10 breaths per minute) or hypoxia (SPO2 < 88% while receiving nasal oxygen inhalation at 5 L/min) happened. A professionally trained postoperative APS (acute pain service) team came to the ward for postoperative evaluation every morning (from 8:00 a.m. to 10:00 a.m.) on the first, second, and third postoperative day (respectively, POD1, POD2, and POD3). Visual analogue scale (VAS; 0 mm: no pain, 100 mm: worst imaginable pain) was used to evaluate the patient’s pain score at rest and during movement (by deep breathing or forced coughing three times), and we separately recorded moderate-to-severe pain (VAS ≥ 4).30 Adverse opioid-related reactions including nausea, vomiting, dizziness, and urinary retention were recorded.
The following variables were collected (1) preoperative data: age, sex, weight, ASA grade, history of smoking, history of alcohol consumption, comorbidities (hypertension, diabetes, coronary heart disease); (2) intraoperative data: type of surgery (open or laparoscopic surgery), type of anesthesia (combined general anesthesia with nerve block or general anesthesia alone), duration of operation, total intraoperative fluid volume, estimated blood loss, urine volume, blood transfusion; (3) postoperative data: postoperative VAS score during movement and at rest on POD1, POD2, and POD3, bolus attempts of analgesic pump, opioid-related adverse reactions (nausea, vomiting, dizziness, and urinary retention), postoperative hospital stay. Sufentanil and butorphanol consumption in PCIA was converted to morphine consumption.31,32
Statistical Analysis
Statistical analysis was performed using IBM SPSS statistical software (version 26.0, Chicago, USA). Categorical variables are presented as frequency and percentage, and continuous variables are presented as mean ± SD, or median and interquartile range (IQR). Variables were tested for normal distribution using the Shapiro–Wilk test. Continuous variables were analyzed with unpaired Student’s t-test for normally distributed data or Mann–Whitney U-test. Categorical variables were analyzed with χ2 or Fisher’s exact test, as appropriate. Since this was a retrospective database study, the number of eligible patients was fixed. Therefore, we estimated the statistical power rather than calculating the sample size. To minimize the effects of potential confounders, propensity score matching (PSM) using a multivariable logistic regression model with a 1:1 ratio was carried out according to age, sex, ASA, comorbidities, type of surgery, type of anesthesia, duration of operation, total intraoperative fluid volume, estimated blood loss, urine volume, and blood transfusion, with a caliper of 0.02. We performed subgroup analysis based on the type of surgery and the type of anesthesia for the primary outcome (moderate-to-severe pain within 72 hr after surgery) to further investigate subgroup-specific treatment effects. A two-sided alpha of 0.05 was used for all statistical tests.
Results
A total of 5987 patients undergoing hepatobiliary surgery were identified in our database (Figure 1). Patients with incomplete baseline information (n=2108), preoperative opioid administration (n=350), liver and kidney dysfunction (n=14), and incomplete follow-up data within 72 hr after surgery (n=78) were excluded. The remaining 3437 patients were divided into sufentanil (n=913) and butorphanol group (n=2524). Comparison of baseline characteristics and intraoperative information is shown in Table 1. Results showed that diabetes, coronary arterial disease, type of surgery, surgical duration, intraoperative total infusion volume, intraoperative blood transfusion, urine output, and blood loss differed statistically (P < 0.05) between groups. After propensity score matching, baseline characteristics and intraoperative values were well-balanced between the two groups (Table 2). Results of the multivariable logistic regression analysis are presented in Supplementary Table S1.
 |
Table 1 Baseline Characteristics and Intraoperative Information Before PSM |
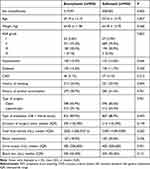 |
Table 2 Baseline Characteristics and Intraoperative Information After PSM |
 |
Figure 1 Flow diagram of the study. |
Primary Outcome
Compared with sufentanil group, the incidence of moderate-to-severe pain on POD1 and POD2 was lower in butorphanol group during rest (3.2% vs 10.9%, P<0.001; 1.2% vs 4.6%, P<0.001), and during movement (7.0% vs 18.9%, P<0.001; 2.6% vs 8.7%, P<0.001). On POD3, there was no statistical difference in the incidence of moderate-to-severe pain at rest (7.2% vs 6.3%, P=0.453), and during movement (11.9% vs 10.0%, P = 0.202), as shown in Figure 2. Compared with the sufentanil group, butorphanol group had a significantly lower VAS score during movement and at rest on POD1 and POD2 (P < 0.05), while this difference no longer existed on POD3 (P > 0.05), as shown in Table 3.
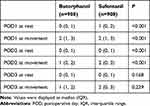 |
Table 3 VAS Pain Scores Within 72hr After Surgery |
 |
Figure 2 Incidence of moderate to severe pain during movement and at rest within 72hr after surgery. |
Secondary Outcome
As shown in Table 4, patients receiving butorphanol had a lower morphine consumption (50mg vs 120mg, P<0.001). In terms of adverse reactions, there was no significant difference in the incidence of nausea, and urine retention within 72 hr after operation between the two groups (P>0.05). The rate of postoperative vomiting was lower in butorphanol group (1.4% vs 3.0%, P=0.025) than in sufentanil group. However, more patients in butorphanol group experienced dizziness (0.9% vs 0.1%, P = 0.019). Compared with sufentanil group, the number of bolus attempts in butorphanol group was significantly lower, as well as the incidence of multiple bolus attempts (bolus attempts >5) (P<0.05). In addition, postoperative hospital stay in butorphanol group was significantly shorter than that in sufentanil group (11d vs 12d, P=0.017).
 |
Table 4 Secondary Outcomes |
Subgroup Analysis
Results of the subgroup analysis showed that our findings remained unchanged for the primary outcome (VAS ≥ 4) whether in patients undergoing laparoscopic or open surgery within 72 hr after surgery (P>0.05), as displayed in Supplementary Table S2. However, the beneficial effect of butorphanol no longer existed in those receiving general anesthesia alone on POD2, regardless of their state (at rest, 1.1% vs 4.0%, P=0.423; at movement, 3.3% vs 7.9%, P=0.163), as compared with those receiving combined general and nerve block anesthesia.
Discussion
This study demonstrated that postoperative administration of butorphanol combined with dexmedetomidine and ketorolac in PCIA was associated with reduced VAS pain score and lower incidence of moderate-to-severe pain within 48 hr after surgery, as compared with sufentanil. Furthermore, patients receiving butorphanol had a shorter hospital length of stay and a lower rate of vomiting. However, more patients in butorphanol group experienced dizziness.
Understanding the physiology and pathophysiology of pain is vital to reach optimal pain management. The traumatic stimuli during hepatobiliary surgery are mainly generated from the release of inflammatory mediators caused by local tissue damage and the activation of nociceptive receptors, direct injury of peripheral nerve, visceral pain caused by intraoperative viscera pull, laparoscopic peritoneal stretching, diaphragmatic stretching, and visceral ischemia.33,34 During the initial postoperative stage, somatic pain may dominate significantly over visceral pain because of the removed viscus (for example, gall bladder). However, with the subsiding of initial somatic pain, visceral pain increases because of the parietal peritoneal irritation and inflammation.35,36 Therefore, to achieve optimal postoperative pain relief, all these elements should be taken into account to block the transmission of afferent nociceptive impulses.
With the development of precision surgery and minimally invasive techniques, laparoscopic surgery is gaining increasing use in hepatobiliary surgery, which could reduce the incidence of incision pain to some extent.37 Peripheral nerve block anesthesia, relieving incision pain by blocking somatic nerve conduction of noxious stimuli, is also getting more utilized in recent years because of its safety profile and effectiveness. As for visceral pain, opioids and other centrally active analgesic agents may provide superior effects. Traditional opioids represented by sufentanil have powerful analgesic effects and become the first choice for postoperative pain management. As a highly selective μ receptor agonist, sufentanil can stimulate opioid receptors distributed in the respiratory, digestive, urinary system, and other parts while exerting an analgesic effect, causing respiratory depression, nausea and vomiting, urinary retention, itching, and other adverse reactions. Butorphanol is a synthetic opioid agonist-antagonist, which mainly produces analgesic effects by agonizing κ receptors, has antagonistic or partial agonistic effects on μ receptors, and has no obvious effect on δ receptors.18 Consistent with traditional opioids such as sufentanil, butorphanol inhibits the uploading of noxious stimuli in the dorsal horn of the spinal cord and activates the pain control circuit that descends from the midbrain through the rostral ventromedial region (RVM) to the dorsal horn of the spinal cord, therefore exerts the analgesic effect. In addition, compared with pure μ-receptor agonists, butorphanol has a better effect on inhibiting visceral pain and has a lower incidence of adverse reactions such as nausea, vomiting, dizziness, and respiratory depression.19,20
Alleviated postoperative pain contributes to improving medical experience and accelerating postoperative rehabilitation. Meanwhile, shortened length of hospital stay enables to speed up the turnover of beds, improve the utilization efficiency of medical resources, and reduce social and economic costs. In our study, most of the findings remained unchanged in subgroup analysis regardless of surgical technique (open or laparoscopic surgery), suggesting that the superior pain relief profile of butorphanol was consistent across different surgical populations. However, for those receiving general anesthesia without nerve block, we did not detect a difference between the groups on POD2. A possible explanation was that butorphanol as an adjuvant combined with local anesthetics might help to play a synergistic analgesic role through κ receptors in the spinal cord.38 When given butorphanol intravenously solely, the improved analgesic effect no longer exists.
Our study suggested that the application of butorphanol was associated with improved pain control and shortened hospital length of stay, which was generally consistent with published trials. The study by DU et al39 showed that for patients undergoing laparoscopic hysterectomy, dexmedetomidine combined with butorphanol for PCIA can effectively reduce the postoperative pain score and the incidence of postoperative nausea and vomiting and increase patient satisfaction. Considering opioids are becoming increasingly and excessively used worldwide, strategies to achieve optimal analgesia while minimizing opioid consumption are priorities for investigation.40,41 Our study showed that butorphanol combined with nonsteroidal anti-inflammatory drugs (NSAIDs) and dexmedetomidine reduced the morphine equivalent. A previous study showed that in patients after abdominal hysterectomy, butorphanol combined with morphine for PCIA could provide a better analgesic effect and patient satisfaction than morphine alone.42 It also reduced morphine consumption and lowered the incidence of side effects, including nausea, vomiting, dizziness, drowsiness, and so on. In addition, butorphanol could reduce postoperative hyperalgesia induced by remifentanil. Kong et al43 showed that in patients undergoing laparoscopic cholecystectomy, continuous intraoperative pumping of low-dose butorphanol can reduce the postoperative VAS score and reduce the administration of fentanyl in PACU and ward, compared with the application of remifentanil alone.
In addition to the widely performed minimally invasive surgery and truncal nerve blocks as part of the multimodal analgesia in our center, we also exercised other opioid-sparing analgesics, namely ketorolac, and dexmedetomidine. As the basic drugs for three-step analgesia, NSAIDs inhibit the metabolism of arachidonic acid by inhibiting cyclooxygenase (COX) and reducing the synthesis of prostaglandin synthesis products (PGs), thus inhibiting fever, pain, and inflammation. When given together with opioids, there is a synergistic effect, helping to save opioids, improve postoperative inflammatory pain, and reduce adverse reactions to opioids.44 Dexmedetomidine is a highly selective α2-adrenergic receptor agonist. The analgesic action mechanism of dexmedetomidine is complex, mainly acting on α2 adrenergic receptors in the dorsal horn of spinal cord, inhibiting norepinephrine release through a negative feedback mechanism, and blocking pain signal transduction.45 In recent years, it has been widely used in combination with other sedative and analgesic drugs for PCIA.46,47 In addition, dexmedetomidine also has the effect of reducing stress response, improving delirium and postoperative chills.48–50 Overall, all these analgesic elements act on different targets in the pain conduction pathway, helping to increase the pain perception threshold, reduce the activation of pain receptors, reduce, or even prevent the central and peripheral sensitization, thus exerting additive or synergistic analgesic effects.51–53
This study had certain limitations. Although a 1:1 propensity score matching was used to balance the baseline information of the two groups, the loss of a large number of patients in the butorphanol group after matching may have biased the study results, and this result should be interpreted with caution. Secondly, the electronic database was only available for early postoperative period data, and long-term follow-up including chronic postoperative pain was not performed. In addition, we administered not only opioids but also non-steroidal analgesics and dexmedetomidine in the postoperative analgesic pump, so the intrinsic interaction of these drugs, as well as other covariates and potential confounders may influence the result. Therefore, a large-sample prospective randomized controlled study should be carried out to further investigate the ideal butorphanol dosage and combinations with other analgesic agents in multimodal analgesia.
Conclusion
Compared with sufentanil, the application of butorphanol in multimodal analgesia combined with dexmedetomidine and ketorolac via PCIA could provide superior analgesia after hepatobiliary surgery, with reduced opioid consumption and shorter postoperative hospital length of stay. Further prospective large-sample studies are warranted to demonstrate the efficacy and safety of butorphanol in postoperative multimodal analgesia.
Acknowledgments
This work was supported by The Key Project of Provincial and Ministerial Cooperation of Medical Science and Technology Project of Henan Province (SBGJ202102008).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kim HC, Song Y, Lee JS, et al. Comparison of pharmacologic therapies alone versus operative techniques in combination with pharmacologic therapies for postoperative analgesia in patients undergoing laparoscopic cholecystectomy: a randomized controlled trial. Int J Surg. 2022;104:106763. doi:10.1016/j.ijsu.2022.106763
2. Bhushan S, Huang X, Su X, Luo L, Xiao Z. Ultrasound-guided erector spinae plane block for postoperative analgesia in patients after liver surgery: a systematic review and meta-analysis on randomized comparative studies. Int J Surg. 2022;103:106689. doi:10.1016/j.ijsu.2022.106689
3. Kehlet H. Postoperative pain, analgesia, and recovery-bedfellows that cannot be ignored. Pain. 2018;159(Suppl 1):S11–S16. doi:10.1097/j.pain.0000000000001243
4. Pirie K, Traer E, Finniss D, Myles PS, Riedel B. Current approaches to acute postoperative pain management after major abdominal surgery: a narrative review and future directions. Br J Anaesth. 2022;129(3):378–393. doi:10.1016/j.bja.2022.05.029
5. Pak DJ, Yong RJ, Kaye AD, Urman RD. Chronification of pain: mechanisms, current understanding, and clinical implications. Curr Pain Headache Rep. 2018;22(2):9. doi:10.1007/s11916-018-0666-8
6. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi:10.1016/S0140-6736(06)68700-X
7. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537–1546. doi:10.1016/S0140-6736(19)30352-6
8. Memtsoudis SG, Fiasconaro M, Soffin EM, et al. Enhanced recovery after surgery components and perioperative outcomes: a nationwide observational study. Br J Anaesth. 2020;124(5):638–647. doi:10.1016/j.bja.2020.01.017
9. Nimmo SM, Foo ITH, Paterson HM. Enhanced recovery after surgery: pain management. J Surg Oncol. 2017;116(5):583–591. doi:10.1002/jso.24814
10. Sjövall S, Kokki M, Kokki H. Laparoscopic surgery: a narrative review of pharmacotherapy in pain management. Drugs. 2015;75(16):1867–1889. doi:10.1007/s40265-015-0482-y
11. Liu S, Peng P, Hu Y, et al. The effectiveness and safety of intravenous dexmedetomidine of different concentrations combined with butorphanol for post-caesarean section analgesia: a randomized controlled trial. Drug Des Devel Ther. 2021;15:689–698. doi:10.2147/DDDT.S287512
12. Abdelaziz DH, Boraii S, Cheema E, et al. The intraperitoneal ondansetron for postoperative pain management following laparoscopic cholecystectomy: a proof-of-concept, double-blind, placebo-controlled trial. Biomed Pharmacother. 2021;140:111725. doi:10.1016/j.biopha.2021.111725
13. Pirie K, Doane MA, Riedel B, Myles PS. Analgesia for major laparoscopic abdominal surgery: a randomised feasibility trial using intrathecal morphine. Anaesthesia. 2022;77(4):428–437. doi:10.1111/anae.15651
14. Desai N, El-Boghdadly K, Albrecht E. Epidural vs. transversus abdominis plane block for abdominal surgery - A systematic review, meta-analysis and trial sequential analysis. Anaesthesia. 2021;76(1):101–117. doi:10.1111/anae.15068
15. Yu JM, Tao QY, He Y, Liu D, Niu JY, Zhang Y. Opioid-free anesthesia for pain relief after laparoscopic cholecystectomy: a prospective randomized controlled trial. J Pain Res. 2023;16:3625–3632. doi:10.2147/JPR.S432601
16. Xie M, Liang Y, Deng Y, Li T. Effect of S-ketamine on postoperative pain in adults post-abdominal surgery: a systematic review and meta-analysis. Pain Physician. 2023;26(4):327–335.
17. Adams TJ, Aljohani DM, Forget P. Perioperative opioids: a narrative review contextualising new avenues to improve prescribing. Br J Anaesth. 2023;130(6):709–718. doi:10.1016/j.bja.2023.02.037
18. Ji J, Lin W, Vrudhula A, et al. Molecular interaction between butorphanol and κ-opioid receptor. Anesth Analg. 2020;131(3):935–942. doi:10.1213/ANE.0000000000005017
19. Rivière PJ. Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol. 2004;141(8):1331–1334. doi:10.1038/sj.bjp.0705763
20. Hoskin PJ, Hanks GW. Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs. 1991;41(3):326–344. doi:10.2165/00003495-199141030-00002
21. Lv S, Sun D, Li J, Yang L, Sun Z, Feng Y. Anesthetic effect of different doses of butorphanol in patients undergoing gastroscopy and colonoscopy. BMC Surg. 2021;21(1):266. doi:10.1186/s12893-021-01262-8
22. Zhang X, Qi S, Lin Z, et al. Pre-operative administration of butorphanol mitigates emergence agitation in patients undergoing functional endoscopic sinus surgery: a randomized controlled clinical trial. Front Psychiatry. 2022;13:1090149. doi:10.3389/fpsyt.2022.1090149
23. Wang Y, Zhao K, Wu N, et al. Effect of different doses of butorphanol on postoperative shivering in elderly patients: a randomized, double-blind, placebo-controlled trial. Drug Des Devel Ther. 2023;17:839–849. doi:10.2147/DDDT.S396309
24. Zhu Z, Zhang W. Efficacy and safety of butorphanol use in patient-controlled analgesia: a meta-analysis. Evid Based Complement Alternat Med. 2021;2021:5530441. doi:10.1155/2021/9854850
25. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi:10.1016/j.ijsu.2014.07.014
26. Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: physiologic basis, benefits, and potential risks. Anesth Analg. 2017;124(2):507–517. doi:10.1213/ANE.0000000000001589
27. Jaber S, De Jong A, Schaefer MS, et al. Preoxygenation with standard facemask combining apnoeic oxygenation using high flow nasal cannula versuss standard facemask alone in patients with and without obesity: the OPTIMASK international study. Ann Intensive Care. 2023;13(1):26. doi:10.1186/s13613-023-01124-x
28. Lu Z, Wang Q, Sun X, et al. Transcutaneous electrical acupoint stimulation before surgery reduces chronic pain after mastectomy: a randomized clinical trial. J Clin Anesth. 2021;74:110453. doi:10.1016/j.jclinane.2021.110453
29. Lu Z, Zheng H, Chen Z, et al. Effect of etomidate vs propofol for total intravenous anesthesia on major postoperative complications in older patients: a randomized clinical trial. JAMA Surg. 2022;157(10):888–895. doi:10.1001/jamasurg.2022.3338
30. Xu Y, Ye M, Liu F, et al. Efficacy of prolonged intravenous lidocaine infusion for postoperative movement-evoked pain following hepatectomy: a double-blinded, randomised, placebo-controlled trial. Br J Anaesth. 2023;131(1):113–121. doi:10.1016/j.bja.2023.03.026
31. Anderson R, Saiers JH, Abram S, Schlicht C. Accuracy in equianalgesic dosing: conversion dilemmas. J Pain Symptom Manage. 2001;21(5):397–406. doi:10.1016/S0885-3924(01)00271-8
32. De Iaco F, Mannaioni G, Serra S, et al. Equianalgesia, opioid switch and opioid association in different clinical settings: a narrative review. Eur Rev Med Pharmacol Sci. 2022;26(6):2000–2017. doi:10.26355/eurrev_202203_28349
33. Li Y, Dou Z, Yang L, Wang Q, Ni J, Ma J. Oxycodone versus other opioid analgesics after laparoscopic surgery: a meta-analysis. Eur J Med Res. 2021;26(1):4. doi:10.1186/s40001-020-00463-w
34. Wills VL, Hunt DR. Pain after laparoscopic cholecystectomy. Br J Surg. 2000;87(3):273–284. doi:10.1046/j.1365-2168.2000.01374.x
35. Boezaart AP, Smith CR, Chembrovich S, et al. Visceral versus somatic pain: an educational review of anatomy and clinical implications. Reg Anesth Pain Med. 2021;46(7):629–636. doi:10.1136/rapm-2020-102084
36. Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90(3):261–269. doi:10.1016/S0304-3959(00)00406-1
37. Mansour NO, Boraii S, Elnaem MH, et al. Evaluation of preoperative duloxetine use for postoperative analgesia following laparoscopic cholecystectomy: a randomized controlled trial. Front Pharmacol. 2022;13:944392. doi:10.3389/fphar.2022.944392
38. Fu H, Fu Y, Xu X, Gao Y. Ultrasound-guided rectus sheath block combined with butorphanol for single-incision laparoscopic cholecystectomy: what is the optimal dose of ropivacaine? J Pain Res. 2020;13:2609–2615. doi:10.2147/JPR.S265418
39. Du J, Li JW, Jin J, Shi CX, Ma JH. Intraoperative and postoperative infusion of dexmedetomidine combined with intravenous butorphanol patient-controlled analgesia following total hysterectomy under laparoscopy. Exp Ther Med. 2018;16(5):4063–4069. doi:10.3892/etm.2018.6736
40. Allen ML, Kim CC, Braat S, et al. Post-discharge opioid use and handling in surgical patients: a multicentre prospective cohort study. Anaesth Int Care. 2020;48(1):36–42. doi:10.1177/0310057X19895019
41. Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251.
42. Wang F, Shen X, Liu Y, Xu S, Guo X. Continuous infusion of butorphanol combined with intravenous morphine patient-controlled analgesia after total abdominal hysterectomy: a randomized, double-blind controlled trial. Eur J Anaesthesiol. 2009;26(1):28–34. doi:10.1097/EJA.0b013e32831a6aa2
43. Kong M, Yang L, Li J, et al. Low-dose butorphanol alleviates remifetanil-induced hyperalgesia in patients undergoing laparoscopic cholecystectomy. J Clin Anesth. 2016;34:41–45. doi:10.1016/j.jclinane.2016.03.042
44. Zhang Z, Xu H, Zhang Y, et al. Nonsteroidal anti-inflammatory drugs for postoperative pain control after lumbar spine surgery: a meta-analysis of randomized controlled trials. J Clin Anesth. 2017;43:84–89. doi:10.1016/j.jclinane.2017.08.030
45. Kaye AD, Chernobylsky DJ, Thakur P, et al. Dexmedetomidine in Enhanced Recovery After Surgery (ERAS) protocols for postoperative pain. Curr Pain Headache Rep. 2020;24(5):21. doi:10.1007/s11916-020-00853-z
46. Lin TF, Yeh YC, Lin FS, et al. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br J Anaesth. 2009;102(1):117–122. doi:10.1093/bja/aen320
47. Gao Y, Deng X, Yuan H, et al. Patient-controlled intravenous analgesia with combination of dexmedetomidine and sufentanil on patients after abdominal operation: a prospective, randomized, controlled, blinded, multicenter clinical study. Clin J Pain. 2018;34(2):155–161. doi:10.1097/AJP.0000000000000527
48. Duan X, Coburn M, Rossaint R, Sanders RD, Waesberghe JV, Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analy sis with trial sequential analysis of randomised controlled trials. Br J Anaesth. 2018;121(2):384–397. doi:10.1016/j.bja.2018.04.046
49. Liu ZX, Xu FY, Liang X, et al. Efficacy of dexmedetomidine on postoperative shivering: a meta-analysis of clinical trials. Can J Anaesth. 2015;62(7):816–829. doi:10.1007/s12630-015-0368-1
50. Miao M, Xu Y, Li B, Chang E, Zhang L, Zhang J. Intravenous administration of dexmedetomidine and quality of recovery after elective surgery in adult patients: a meta-analysis of randomized controlled trials. J Clin Anesth. 2020;65:109849. doi:10.1016/j.jclinane.2020.109849
51. White PF. Expanding role of multimodal analgesia in facilitating recovery after surgery: from fast-tracking to enhanced recovery. J Clin Anesth. 2019;55:105–107. doi:10.1016/j.jclinane.2018.12.050
52. Dunkman WJ, Manning MW. Enhanced recovery after surgery and multimodal strategies for analgesia. Surg Clin North Am. 2018;98(6):1171–1184. doi:10.1016/j.suc.2018.07.005
53. Zhang H, Yang YT, Jiang LL, Xu XD, Zhang JQ, Zhang LZ. Risk factors for moderate-to-severe acute pain after hepatobiliary and pancreatic surgery: a single-center retrospective study. BMC Anesthesiol. 2023. In Press.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
