Back to Journals » Infection and Drug Resistance » Volume 16
Application of Adenosine Deaminase and γ-Interferon Release Assay in Pleural Fluid for the Diagnosis of Tuberculous Pleural Effusion in Patients Over 40 Years Old
Authors Li Z, Chen J , Zeng J, Shi P, Xiong X, Wang M, Zheng D, Zhao R, Dong L
Received 9 December 2022
Accepted for publication 9 February 2023
Published 17 February 2023 Volume 2023:16 Pages 1009—1018
DOI https://doi.org/10.2147/IDR.S400838
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zhishu Li,1 Jiayue Chen,1 Jia Zeng,2 Ping Shi,2 Xiaomin Xiong,2 Meng Wang,1 Dong Zheng,1 Rui Zhao,1 Lixia Dong1
1Department of Respiratory and Critical Care Medicine, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Guangyuan Central Hospital, Guangyuan, People’s Republic of China
Correspondence: Lixia Dong, Email [email protected]
Background: In patients with tuberculous pleural effusion (TPE) of various ages, the diagnostic accuracy of pleural biomarkers varies, and there are insufficient studies specifically in different age groups. Therefore, we investigated the adenosine deaminase cut-off value and its combination with the gamma interferon release assay for the diagnosis of TPE among patients aged ≥ 40 years.
Methods: A retrospective analysis of 198 patients who underwent medical thoracoscopy and were admitted to the hospital between 2015 and 2020 with exudative pleural effusion and either fever, night sweats, fatigue, cough, or other clinical manifestations was performed. The medical thoracoscopy, ADA, and T-SPOT results were analysed in the pleural fluid. The patients were divided into groups based on age: 18– 39, 40– 59, and 60– 87.
Results: The best cut-off values of ADA were 29.5, 31.5 and 19.5 U/L, respectively, for the aged 18– 39, aged 40– 87 and aged 60– 87 groups. The accuracy of 31.5 U/L was higher than 40 U/L for aged ≥ 40 years (86 vs 83%). The ADA diagnostic accuracy was higher than that of people under 40 years (83 vs 77%) when cut-off value of ADA was 40 U/L, but the IGRA accuracy was lower than that of people under 40 (87 vs 91%). The sensitivity of ADA or IGRA detection in patients over 40 years was 99%, and the specificity was 78%. The ADA specificity combined with IGRA for TPE was the highest (100%) in the ≥ 40 age group, and the sensitivity was 69%.
Conclusion: Our study revealed the best cut-off values of ADA for TBE in different age groups. Combining ADA and IGRA in pleural fluid improves the detection rate of TPE in patients over 40 years of age with exudative pleural effusion. ADA combined with IGRA increases specificity, and ADA or IGRA increases sensitivity substantially.
Keywords: adenosine deaminase, interferon gamma releasing assays, aged, diagnosis, pleural effusion, tuberculosis
Background
According to the latest World Health Organization (WHO) report, pulmonary tuberculosis is a serious global public health problem, particularly in developing countries. In recent years, the decline in the number of cases and incidence rates has slowed down, and mortality risks have increased.1 Tuberculous pleural effusion (TPE) is the most common extrapulmonary form of tuberculosis.2 TPE is an exudative pleural effusion caused by Mycobacterium tuberculosis infection. Early diagnosis and treatment can reduce morbidity and mortality. If untreated, 43–65% of patients can develop pulmonary tuberculosis.3 The pleural effusion aetiology is complex, particularly in middle-aged and elderly patients. Furthermore, it is difficult to distinguish from TPE, which is often a complex problem during clinical diagnosis. Combining different causative factors, TPE is still the most common cause of exudative pleural effusion in developing countries.4,5
Adenosine deaminase (ADA) is an important enzyme in purine nucleoside metabolism. When TPE occurs, the host responds to pleural lymphocytes through cellular immunity, causing monocytes and macrophages to secrete ADA2, thereby increasing ADA.6 It is widely used in TPE diagnosis because of its economic detection, convenience, and availability.7–9 However, ADA can also increase parapneumonic effusions (mainly in those complicated and empyema) along with some cases of malignant pleural effusions (mostly lymphomas, and by far less frequently other malignancies) and systemic lupus erythematosus (SLE).6 Diagnostic accuracy is affected significantly by aging. Therefore, for diagnosing suspected TPE, we chose its combination with interferon-gamma release assay (IGRA), which is not easily influenced by age.5,10–14 Lymphocytes from TPE patients exposed to Mycobacterium tuberculosis form many memory lymphocytes in the body. Moreover, when restimulated with mycobacterial antigens, lymphocytes from tuberculosis-infected patients release more interferon-gamma than lymphocytes from uninfected patients; thus, interferon-gamma can be used for the diagnosis of tuberculosis infection.15 IGRA is one of the detection methods. A study found that IGRA testing was 94.9% sensitive and 96.3% specific for tuberculous pleural effusion.16 Furthermore, IGRA may be superior to ADA testing in diagnosing TPE pleural effusion.17 However, the high cost and complexity of this detection method limit its wide application. Currently, the most accepted cut-off value of ADA for TPE diagnosis is 40 U/L, but due to the influence of tuberculosis prevalence, age, organ failure, and complications, the threshold for various ADA reports varies, as does the accompanying instruction.18 For example, in the Turkish guidelines, for patients with pleural effusion aged ≥40 and ADA >40 U/L, a pathological biopsy is still recommended to exclude other diseases, such as tumours.19 Moreover, the optimal ADA cut-offs for older and younger populations varied among studies.5,7,11,12,14 Elderly patients with pleural effusion have complex aetiologies, multiple comorbidities, and a high risk of invasive procedures. With the intensification of the aging population, the diagnostic methods of TPE in the elderly need to be continuously optimized. Based on this, we focused on TPE patients aged 40–59 and ≥60 and explored the optimal cut-off value of ADA in this population and ADA diagnostic value combined with IGRA for TPE patients of various ages.
Objectives and Methods
Subjects, Demographics, and Informed Consent
We did a retrospective analysis of demographic data (age and sex), address, smoking index, comorbidities (hypertension, diabetes, chronic obstructive pulmonary disease, neoplasm, nephrotic syndrome, liver cirrhosis, heart failure), diagnosis, diagnostic methods, and records related to biochemical index information. The above information was used for subsequent research. Inclusion criteria: Patients aged ≥18 with exudative pleural effusion were diagnosed following Light’s criteria20 and either fever, night sweats, fatigue, cough, or other clinical manifestations; and underwent pleural effusion ADA, IGRA, and medical thoracoscopy with pleural biopsy. A total of 198 patients were initially included, of which 107 were TPE and 91 were non-TPE. There were 22 patients aged 18 to 39 years, 85 patients aged 40 to 59 years, and 91 patients over 60 years (Figure 1).
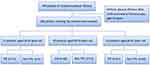 |
Figure 1 Patients selection process and grouping. |
The diagnostic criteria for TPE are as follows:21 1. Granuloma in pleural tissue; 2. Positive Lowenstein or BACTEC-MIC culture in pleural fluid, pleural biopsy or sputum; 3. Positive Ziehl Neelsen or auramine stain in pleural fluid, pleural biopsy or sputum; 4. Positive Xpert MTB/RIF in pleural fluid, pleural biopsy or sputum. This study used pathological examination, concentrated Ziehl-Neelsen staining, Lowenstein-Jensen culture, or Xpert MTB/RIF assay as diagnostic criteria for pleural tissue specimens. The information on 91 non-TPE patients is shown in Table 1. Thoracoscopic pleural biopsy consistent with the diagnosis of malignant disease was diagnosed as malignant pleural effusion. There was no macroscopic empyema in the pleura, and the biochemistry, cell type, and number met the diagnostic criteria for parapneumonic pleural effusion.22 All patients met the diagnostic criteria of the corresponding diseases based on pathology results, clinical examination, and imaging.
 |
Table 1 Diagnosis of Non-TPE (N = 91) |
This study was approved by the Guangyuan Central Hospital Ethics Committee. The need for informed consent was waived for this study. We confirm that all methods were carried out by relevant guidelines and regulations.
ADA Detection
A commercial kit (Adenosine Deaminase Detection Kit; Beijing Strong Biotechnology Co., Ltd. Beijing, China) detected ADA activity by colorimetric assay at 37 °C. One unit of ADA is defined as the amount of enzyme that produces one micromolar inosine per minute from adenosine at 37 °C. The results are expressed in international units (IU/L) per litre of pleural fluid (PF).
IGRA Detection
Forty-five milliliters of PF was collected from all subjects and tested within 6 h. PF samples were centrifuged at 500 × g for 10 min, and the sample supernatant was discarded for TB T-SPOT detection. Tuberculosis testing was performed according to the manufacturer’s instructions (Oxford Immunotec Ltd. Oxford, UK). Microspheres were resuspended in 8 mL of AIM-V medium (GIBCO, Rockville, MD, USA). Mononuclear cells were isolated using a Ficoll-Hypaque Lymphocyte Separator, washed, resuspended, and counted. Empty wells were used as negative controls, T lymphocyte mitogen lectin was used as a positive control, and ESAT-6 and CFP-10 polypeptides were used in different wells. Pleural fluid mononuclear cells were added to wells pre-coated with anti-IFN-γ monoclonal antibody (2.5 × 105 cells per well) and incubated at 37 °C for 16–20 h. Spot-forming cells (SFCs) were read using an automated enzyme-linked immunosorbent spot reader (CTL-ImmunoSpotS5 Versa analyser). The assay was valid when the positive control was >20 SFCs/106 monocytes, and the negative control was <6 SFCs/106 monocytes. Final SFCs of ESAT-6 or CFP-10 were defined as ESAT-6 or CFP-10 SFC minus negative control SFCs. The largest SFCs in the T-SPOT assay were defined as larger SFCs in the final ESAT-6 and CFP-10.15
Pleura IGRA or/and ADA
Pleura IGRA or ADA means that when one or more of the pleura IGRA, ADA is positive, it is positivity. Pleural IGRA and ADA refer to the combination of pleural IGRA and ADA, which are positivity when both are positive.
Data Analysis
The obtained data were analysed by SPSS 19.0 statistical software. Variables with normal distribution were presented as means ± standard deviation, otherwise as medians (with interquartile ranges). Enumeration data were expressed as a rate (%) using the χ2 test. The best cut-off values for IGRA and ADA with P < 0.05 were evaluated by receiver operating characteristic (ROC) curves and areas under the ROC curves (AUCs). GraphPad Prism 8.0 (GraphPad Software Inc.) was applied to create graphs.
Results
Demographics Results
Among the 198 patients, 107 patients were diagnosed with TPE and 91 patients were non-TPE. The proportion of malignant pleural effusion in non-TPE etiology was the highest (53.85%). The information on 91 non-TPE patients is shown in Table 1. The mean age of TPE and non-TPE was 52.39 ± 17.16, 59.8 ±11.03, respectively. The male percentage of TPE and non-TPE was 67.3, 61.4, respectively. Table 2 shows the general conditions, clinical characteristics, and ADA, ESR, and IGRA test results of the three groups of patients with exudative pleurisy. A summary of some previous studies on optimal cut-off values for ADA in pleural effusion in TPE patients at different ages is shown in Table 3.
 |
Table 2 Comparison of Clinical Features Between Three Groups of Patients with Exudative Pleurisy |
 |
Table 3 Previous Studies on the Best Cutoff Values for ADA from Pleural Fluid in TPE Patients |
ROC Curve Analysis and Scatterplots
For patients aged 18–39 years, and the best cut-off value was 29.5 U/L, the AUC was 0.89 (95% CI, 0.73–1.00). ROC curve analysis was performed for patients aged 40–59 years, and the best cut-off value was 27.5 U/L, the AUC was 0.94 (95% CI, 0.88–0.99). ROC curve analysis was performed for patients aged 60–87 years, and the best cut-off value was 19.5 U/L, the AUC was 0.57 (95% CI, 0.45–0.70). ROC curve analysis was performed for 40–87 years of age, and the best cut-off value was 31.5 U/L, and the AUC was 0.94 (95% CI, 0.90–0.97) (Figure 2). These scatterplots show the expression of ADA and IGRA data sensitivity and (1 – specificity) in TPE and non-TPE in different age groups (Figure 3).
 |
Figure 2 ROC analysis was performed for all patients with different ages (Y: years). |
 |
Figure 3 These scatterplots show the expression of ADA and IGRA data sensitivity and (1 - specificity) in TPE and non-TPE in different age groups. |
ADA
The diagnostic accuracy of ADA was higher than that for people under 40 years (83 vs 77%) when the cut-off value was 40 U/L. When the cut-off values were 26, 31.5 and 40 U/L, the test results of these data are shown in Table 4. For patients under 40 years old with TPE, when the cut-off value of ADA was 31.5 U/L, the sensitivity (94%) and specificity (80%) were higher than or equal to the cut-off value of ADA were 26 U/L and 40 U/L, and the diagnostic accuracy (91%) was the highest. For the patients aged 40–87 years old, the highest sensitivity (92%), the highest specificity (97%) and the highest accuracy (86%) were found when the cut-off value of ADA was 26 U/L, 40 U/L and 31.5 U/L, respectively.
 |
Table 4 Sensitivity, Specificity, LR+/−, PPV, NPV, and ACC Values According to Different ADA Cut-off Values in Patients |
IGRA and IGRA Combined with ADA
The accuracy of IGRA was lower than that for people 18–39 years (87 vs 91%). ADA or IGRA sensitivity detection in patients 40–87 years was 99%, and specificity was 78%. The ADA specificity combined with IGRA for pleural effusion was the highest (100%) in the 40–87 years group, whereas the sensitivity was 69% (Table 5).
 |
Table 5 Diagnostic Utility of Pleural IGRA, ADA and Their Integrations for the Discriminating Diagnosis of TPE and No-TPE |
Discussion
The combination of ADA and IGRA provides a high diagnostic value for TPE in patients over 40 years old. MTB detection is used for TPE diagnosis, but the MTB detection rate in pleural effusion is very low and has a high false negative rate, which is easily missed, and the culture time is long.23,24 TPE patients aged ≥40 face diagnostic challenges worldwide, but there is no consensus in the literature on diagnosis in this age group, so further studies are needed.25,26 As the population ages, the number of people over 60 is increasing. Previous studies have shown that advanced age, disease severity, and organ failure affect ADA accuracy in diagnosing TPE.13 Based on this, we included TPE patients with exudative pleural effusion and confirmed diagnosis by medical thoracoscopy biopsy and divided them into three age groups: those 18–39, 40–59, and 60–87 years. We hope this helps with the diagnosis of TPE.
The analysis found that the ADA level [cut-off value 40 U/L, sensitivity 70%] was lower in people over 40, which was close to the results reported by Arpinar Yigitbas B7 but lower than some previous studies,12,27 which may be related to the fact that the previous literature did not classify ADA by age. Moreover, we included the complexity associated with the patient’s diagnosis requiring thoracoscopic biopsy to assist in the diagnosis. Numerous previous studies have shown that ADA 40 U/L is a widely accepted cut-off value for TPE diagnosis. However, in recent years, there has been much controversy over the optimal cut-off value of ADA in a series of studies, particularly regarding the greater influence of age and local tuberculosis prevalence.4,14,28,29 We analysed the included patients with exudative pleural effusion aged 40–87 years and obtained the optimal TPE cut-off value of 31.5 U/L and AUC of 0.94 (95% CI, 0.90–0.97), which has a good clinical diagnostic value. Using ADA 40 U/L as the best cut-off value for TPE diagnosis has great differences in different research samples, populations, and regions. Moreover, ADA can also be elevated by other diseases in the body, but this severe influencing factor has not been considered in many studies.6,13,19,30 Therefore, the results of different samples differ slightly. More research is needed to accurately correct the optimal ADA threshold or to seek more accurate diagnostic indicators.
The stimulatory antigens ESAT-6 and CFP10 used in the IGRA test are unique to MTB and are unaffected by BCG and body immunity, improving diagnostic specificity. Moreover, they avoid the influence of nontuberculous bacilli and BCG on the results and have good diagnostic values for TPE.31,32 A recent study reported significant differences in IGRA between TPE and non-TPE groups due to antigen-specific responses to MTB, including malignant pleural effusion, pneumonia, and cirrhosis.33 It has been reported that IGRA has a high diagnostic accuracy of more than 90.2% for ADA-insensitive TPE patients.34
The data analysis of this group found that IGRA has a greater diagnostic advantage in the aged 60–87 than in the 40–59 years group, with high sensitivity (93 vs 88%), specificity (86 vs 81%), and diagnostic accuracy rates (89 vs 85%). The diagnostic accuracy rate of people over 40 years is lower than that of people under 40 years (87 vs 91%), which is close to the results of Mollo B’s35 study. An accuracy of 87% is lower than some other related reports, which may be related to the high comorbidity and age of this group.36 Unfortunately, we did not perform immune function tests, such as lymphocytes and their subsets, in peripheral blood and pleural effusion to judge the peripheral and local lymphocyte function in the pleural cavity to conduct a more in-depth analysis of the influencing factors of IGRA and ADA.30 Previous studies have found that ADA detection has low diagnostic sensitivity for patients aged 60–87 years. We also performed an ROC curve analysis on ADA alone for patients aged ≥60 years and found that the optimal cut-off value was 19.5 U/L. The AUC was 0.57 (95% CI, 0.45–0.70), which shows that its diagnostic value is limited, but this may be related to age (average age 69.62 ± 6.31 years), which is consistent with previous reports.11,34,37 Therefore, we analysed pleural ADA or IGRA and found that its sensitivity was 100% in the 40–59-year-old group. However, its specificity was 73%, so exudative pleural effusion in this population should be combined with other more specific biomarkers or biopsies to diagnose TPE. The sensitivity and specificity of the pleural ADA or IGRA test for people over 40 years and those under 40 years were not significantly different. These results are visually intriguing presented in scatterplots. The IGRA specificity combined with ADA detection for the aged 40–87 group was 100%, which has great clinical application for excluding TPE in patients with exudative pleural effusion who are elderly, unwilling to biopsy, and challenging to diagnose, which is consistent with the results of some Chinese related studies.38 Moreover, IGRA combined with ADA detection was more accurate for the aged 40–87 group than for the aged 18–39 group (84 vs 73%). A new meta-analysis found that combining IGRA and ADA in pleural fluid is the best method for diagnosing TPE.39 In conclusion, ADA combined with IGRA has good diagnostic and differential diagnostic value in patients with exudative pleural effusion over 40, particularly for elderly patients with suspected TBE. They are unwilling to undergo medical thoracoscopy or pleural biopsy, and those medical units do not have thoracoscopy equipment. Moreover, the application of ADA combined with IGRA detection can eliminate the pain and risk caused by invasive operations, reduce the medical burden, and promote a more accurate TBE diagnosis.
This study has some limitations. First, all data are from the retrospective analysis. Second, the sample size of patients under 40 years with TPE in our centre diagnosed by thoracoscopy pathological biopsy was small, which may influence the diagnostic value of ADA and IGRA in this population. The sample size of these patients needs to be increased later to further confirm the application value of IGRA and ADA in this age group for a definitive diagnosis requiring thoracoscopy. Third, this study was a single-centre study, and the results need to be further verified by a multicentre study. Fourth, there are geographic variations in the clinical features and test results of patients with TPE. We believe that ADA and IGRA in pleural effusion are meaningful for diagnosing TPE patients of different ages and can provide a reference for future clinical and basic research.
Conclusion
Our study revealed the best cut-off values of ADA for TBE in different age groups. Combining ADA and IGRA in pleural fluid improves the detection rate of TPE in patients over 40 years of age with exudative pleural effusion. ADA combined with IGRA increases specificity, and ADA or IGRA increases sensitivity substantially.
Abbreviations
TPE, tuberculous pleural effusion; ADA, Adenosine deaminase; IGRA, interferon gamma release test; PF, pleural fluid; SFCs, Spot-forming cells; MTB, Mycobacterium tuberculosis; SPSS, Statistical package for social sciences; CI, Confidence interval; Acc, accuracy; LR+, positive likelihood ratio; LR−, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value.
Data Sharing Statement
The data will be available on a reasonable request from the corresponding author.
Ethics Approval
All procedures performed in our study were in accordance with the ethical standards of the Medical Ethics Committee of Guangyuan Central Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As the study design was retrospective, we used the currently existing samples collected during routine medical care and did not pose any additional risks to the patients. Thus, the informed consent was waived by Medical Ethics Committee of Guangyuan Central Hospital.
Funding
This work was supported by the key R&D projects of the Science and Technology Bureau of Guangyuan City, Sichuan Province [20ZDYF0007].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Menzies NA, Quaife M, Allwood BW, et al. Lifetime burden of disease due to incident tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Global Health. 2021;9(12):e1679–e1687. doi:10.1016/s2214-109x(21
2. Porcel J. Tuberculous pleural effusion. Lung. 2009;187(5):263–270. doi:10.1007/s00408-009-9165-3
3. Gopi A, Madhavan SM, Sharma SK, Sahn SA. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007;131(3):880–889. doi:10.1378/chest.06-2063
4. Skouras VS, Kalomenidis I. Pleural fluid tests to diagnose tuberculous pleuritis. Curr Opin Pulm Med. 2016;22(4):367–377. doi:10.1097/mcp.0000000000000277
5. Jiang CG, Wang W, Zhou Q, et al. Influence of age on the diagnostic accuracy of soluble biomarkers for tuberculous pleural effusion: a post hoc analysis. BMC Pulm Med. 2020;20(1):178. doi:10.1186/s12890-020-01219-2
6. Porcel JM, Esquerda A, Bielsa S. Diagnostic performance of adenosine deaminase activity in pleural fluid: a single-center experience with over 2100 consecutive patients. Eur J Intern Med. 2010;21(5):419–423. doi:10.1016/j.ejim.2010.03.011
7. Arpinar Yigitbas B, Satici C, Kosar AF. Adenosine deaminase cutoff value when diagnosing tuberculous pleurisy in patients aged 40 years and older. Clin Respir J. 2021;15(1):109–115. doi:10.1111/crj.13277
8. Klimiuk J, Krenke R. Rola biomarkerów w diagnostyce gruźliczego wysiękowegozapalenia opłucnej [Role of biomarkers in making the diagnosis of tuberculous pleurisy]. Pneumonol Alergol Pol. 2011;79(4):288–297. Polish.
9. Zhang M, Li D, Hu ZD, Huang YL. The diagnostic utility of pleural markers for tuberculosis pleural effusion. Ann Translational Med. 2020;8(9):607. doi:10.21037/atm.2019.09.110
10. Solari L, Soto A, Van der Stuyft P. Van der Stuyft P. Performance of clinical prediction rules for diagnosis of pleural tuberculosis in a high-incidence setting. Trop Med Int Health. 2017;22(10):1283–1292. doi:10.1111/tmi.12932
11. Tay TR, Tee A. Factors affecting pleural fluid adenosine deaminase level and the implication on the diagnosis of tuberculous pleural effusion: a retrospective cohort study. BMC Infect Dis. 2013;13:546. doi:10.1186/1471-2334-13-546
12. Abrao FC, de Abreu IR, Miyake DH, Busico MA, Younes RN. Role of adenosine deaminase and the influence of age on the diagnosis of pleural tuberculosis. Int J Tuberc Lung Dis. 2014;18(11):1363–1369. doi:10.5588/ijtld.14.0257
13. Lee SJ, Kim HS, Lee SH, et al. Factors influencing pleural adenosine deaminase level in patients with tuberculous pleurisy. Am J Med Sci. 2014;348(5):362–365. doi:10.1097/maj.0000000000000260
14. Korczynski P, Klimiuk J, Safianowska A, Krenke R. Impact of age on the diagnostic yield of four different biomarkers of tuberculous pleural effusion. Tuberculosis. 2019;114:24–29. doi:10.1016/j.tube.2018.11.004
15. Losi M, Bossink A, Codecasa L, et al. Use of a T-cell interferon-gamma release assay for the diagnosis of tuberculous pleurisy. Eur Respir J. 2007;30(6):1173–1179. doi:10.1183/09031936.00067307
16. Wongtim S, Silachamroon U, Ruxrungtham K, et al. Interferon gamma for diagnosing tuberculous pleural effusions. Thorax. 1999;54(10):921–924. doi:10.1136/thx.54.10.921
17. Jiang J, Shi HZ, Liang QL, Qin SM, Qin XJ. Diagnostic value of interferon-gamma in tuberculous pleurisy: a metaanalysis. Chest. 2007;131(4):1133–1141. doi:10.1378/chest.06-2273
18. Sun J, Zhang H, Song Z, et al. The negative impact of increasing age and underlying cirrhosis on the sensitivity of adenosine deaminase in the diagnosis of tuberculous peritonitis: a cross-sectional study in eastern China. Int j Infect Dis. 2021;110:204–212. doi:10.1016/j.ijid.2021.07.061
19. Kim SB, Shin B, Lee JH, et al. Pleural fluid ADA activity in tuberculous pleurisy can be low in elderly, critically ill patients with multi-organ failure. BMC Pulm Med. 2020;20(1):13. doi:10.1186/s12890-020-1049-6
20. Porcel JM. Pleural fluid biomarkers: beyond the Light criteria. Clin Chest Med. 2013;34(1):27–37. doi:10.1016/j.ccm.2012.11.002
21. Gelalcha AG, Kebede A, Mamo H. Light-emitting diode fluorescent microscopy and Xpert MTB/RIF® assay for diagnosis of pulmonary tuberculosis among patients attending Ambo hospital, west-central Ethiopia. BMC Infect Dis. 2017;17(1):613. doi:10.1186/s12879-017-2701-5
22. Porcel JM, Light RW. Derrame pleural paraneumónico y empiema en adultos: guía práctica[Parapneumonic pleural effusions and empyema in adults: current practice]. Rev Clin Esp. 2009;209(10):485–494. doi:10.1016/s0014-2565(09)72634-7. Spanish.
23. Lei X, Wang J, Yang Z, Zhou S, Xu Z. Diagnostic Value of Pleural Effusion Mononuclear Cells Count and Adenosine Deaminase for Tuberculous Pleurisy Patients in China: a Case-Control Study. Front med. 2019;6:301. doi:10.3389/fmed.2019.00301
24. Ling DI, Flores LL, Riley LW, Pai M. Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression. PLoS One. 2008;3(2):e1536. doi:10.1371/journal.pone.0001536
25. Demirer E, Miller AC, Kunter E, Kartaloglu Z, Barnett SD, Elamin EM. Predictive models for tuberculous pleural effusions in a high tuberculosis prevalence region. Lung. 2012;190(2):239–248. doi:10.1007/s00408-011-9342-z
26. Xu HY, Li CY, Su SS, et al. Diagnosis of tuberculous pleurisy with combination of adenosine deaminase and interferon-γ immunospot assay in a tuberculosis-endemic population: a prospective cohort study. Medicine. 2017;96(47):e8412. doi:10.1097/md.0000000000008412
27. Beukes A, Shaw JA, Diacon AH, Irusen EM, Koegelenberg CFN. The Utility of Pleural Fluid Lactate Dehydrogenase to Adenosine Deaminase Ratio in Pleural Tuberculosis. Respir Int Rev Thoracic Dis. 2021;100(1):59–63. doi:10.1159/000509555
28. Chang KC, Chan MC, Leung WM, et al. Optimising the utility of pleural fluid adenosine deaminase for the diagnosis of adult tuberculous pleural effusion in Hong Kong. Hong Kong med j. 2018;24(1):38–47. doi:10.12809/hkmj176238
29. Hamada T, Sanaka M, Hata E, Hasegawa T. [Pleural adenosine deaminase levels in tuberculous pleurisy--its diagnostic performance under the different prevalences in the different age of population]. Japanese J Thoracic Cardiovascular Surg. 1998;46(1):51–57. doi:10.1007/bf03217722. Japanese.
30. Keng LT, Shu CC, Chen JY, et al. Evaluating pleural ADA, ADA2, IFN-γ and IGRA for diagnosing tuberculous pleurisy. J Infect. 2013;67(4):294–302. doi:10.1016/j.jinf.2013.05.009
31. Zhang Q, Zhou C. Comparison of laboratory testing methods for the diagnosis of tuberculous pleurisy in China. Sci Rep. 2017;7(1):4549. doi:10.1038/s41598-017-04872-6
32. Krenke R, Safianowska A, Paplinska M, et al. Pleural fluid adenosine deaminase and interferon gamma as diagnostic tools in tuberculosis pleurisy. J Physiol Pharmacol. 2008;59(Suppl 6):349–360.
33. Liao M, Yang Q, Zhang J, et al. Gamma interferon immunospot assay of pleural effusion mononuclear cells for diagnosis of tuberculous pleurisy. Clin Vaccine Immunol. 2014;21(3):347–353. doi:10.1128/cvi.00680-13
34. Yang X, Zhang J, Liang Q, et al. Use of T-SPOT.TB for the diagnosis of unconventional pleural tuberculosis is superior to ADA in high prevalence areas: a prospective analysis of 601 cases. BMC Infect Dis. 2021;21(1):4. doi:10.1186/s12879-020-05676-2
35. Mollo B, Jouveshomme S, Philippart F, Pilmis B. Biological markers in the diagnosis of tuberculous pleural effusion. Ann Biol Clin (Paris). 2017;75(1):19–27. doi:10.1684/abc.2016.1201
36. Feng M, Sun F, Wang F, Cao G. The diagnostic effect of sequential detection of ADA screening and T-SPOT assay in pleural effusion patients. Artif Cells, Nanomed Biotechnol. 2019;47(1):3272–3277. doi:10.1080/21691401.2019.1647221
37. Santos AP, Corrêa RDS, Ribeiro-Alves M, et al. Application of Venn’s diagram in the diagnosis of pleural tuberculosis using IFN-γ, IP-10 and adenosine deaminase. PLoS One. 2018;13(8):e0202481. doi:10.1371/journal.pone.0202481
38. Li Y, Xu H, Su S, et al. Clinical validation of a highly sensitive assay to detect EGFR mutations in plasma cell-free DNA from patients with advanced lung adenocarcinoma. PLoS One. 2017;12(8):e0183331. doi:10.1371/journal.pone.0183331
39. Tong X, Li Z, Zhao J, Liu S, Fan H. The value of single or combined use of pleural fluid interferon gamma release assay in the diagnosis of tuberculous pleurisy. Trop Med Int Health. 2021;26(11):1356–1366. doi:10.1111/tmi.13659
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
