Back to Journals » OncoTargets and Therapy » Volume 12
Apatinib Plus Temozolomide for Recurrent Glioblastoma: An Uncontrolled, Open-Label Study
Authors Wang Y , Meng X, Zhou S, Zhu Y, Xu J, Tao R
Received 11 August 2019
Accepted for publication 23 November 2019
Published 3 December 2019 Volume 2019:12 Pages 10579—10585
DOI https://doi.org/10.2147/OTT.S226804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Yong Wang, Xiangji Meng, Shizhen Zhou, Yufang Zhu, Jun Xu, Rongjie Tao
Department of Neurosurgery, Shandong Cancer Hospital Affiliated to Shandong University; Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, People’s Republic of China
Correspondence: Rongjie Tao; Jun Xu
Department of Neurosurgery, Shandong Cancer Hospital Affiliated to Shandong University, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, No.440. Jiyan Road, Jinan 250117, People’s Republic of China
Email [email protected]; [email protected]
Objective: This study aimed to determine the efficacy and tolerability of apatinib plus dose-dense temozolomide (TMZ) as first-line treatment for recurrent glioblastoma (rGBM).
Methods: Patients with rGBM were enrolled in this study. Patients were subjected to concurrent treatment of apatinib (500 mg qd) and dose-dense TMZ (100 mg/m2, 7 days on with 7 days off) until disease progression or intolerable toxicity. Efficacy was evaluated using Response Assessment in Neuro-Oncology criteria for high-grade glioma. Safety was assessed using NCI-CTCAE 4.0. Survival was estimated with Kaplan–Meier curve and log rank test.
Results: From March 2016 to January 2018, 20 eligible patients who had relapsed from the standard chemoradiotherapy regimen (TMZ and radiotherapy) were enrolled in this study. The median follow-up time was 12 months. All patients were eligible for efficacy analysis. The objective response rate (ORR) was 45%. The disease control rate (DCR) was 90%. The median progress-free survival time was 6 months (95% CI, 5.3 to 7.8 months). The 6-month progression-free survival rate was 50%. The median overall survival was 9 months (95% CI, 8.2 to 12.2 months). The most common treatment-related adverse events were hypertension (21%), hand–foot syndrome (16%), leukopenia (14%), and thrombocytopenia (12%).
Conclusion: Apatinib combined with dose-dense TMZ was effective in terms of PFS, ORR, and DCR and was well tolerated after appropriate dose reduction in the Chinese population tested. Further randomized controlled clinical studies are needed to confirm the efficacy of apatinib combined with TMZ for treatment of rGBM.
Keywords: central nervous system, recurrence, glioblastoma, apatinib, temozolomide, vascular endothelial growth factor receptor
Introduction
Glioblastoma (GBM) is the most common primary aggressive malignant brain tumor of the central nervous system and one of the most lethal forms of cancer in humans.1 Despite various treatment modalities, including surgery, radiation, and chemotherapy, the prognosis for patients with GBM remains poor. Current treatment options for recurrent GBM (rGBM) are limited.2–4 No unified and effective treatment for rGBM is presently available. Given that the growth of GBM is dependent on the formation of new blood vessels, inhibitors targeting tumor vasculation are promising therapeutic agents for these patients.5
Apatinib, a novel small molecular anti-angiogenic inhibitor, can highly, selectively bind to vascular endothelial growth factor receptor 2 (VEGFR-2). Apatinib inhibits the activation of VEGFR-2 to block vascular endothelial growth factor (VEGF), mediate signal transduction, and inhibit angiogenesis to control tumor growth.6,7 Apatinib has broad anti-tumor profiles, such as for refractory gastric cancer and non-small-cell lung cancer.8,9
Wang et al10 reported a pilot clinical study of apatinib plus irinotecan for treatment of patients with recurrent high-grade glioma. In this clinical study, the objective response rate (ORR) and the disease control rate (DCR) were 55% (5/9) and 78% (7/9), respectively. The median progress-free survival time (mPFS) was 8.3 months. Many case reports indicated that patients with rGBM can benefit from apatinib.11–13 Temozolomide (TMZ) can prolong the survival rate of patients with newly diagnosed GBM. At recurrence, alternate dosing of TMZ can further deplete methyl-guanine-methyltransferase (MGMT), conferring added activity for patients who have progressed on the standard dosing regimen.14
We hypothesized that apatinib combined with dose-dense TMZ could lead to prolonged 6-month progression-free survival rate (PFS-6) and/or overall survival (OS). We also assessed the toxicity and tolerability of the combination of these drugs. The value of analyzing the patient’s gene status (ATRX, 1p/19q, MGMT, TERT, etc.), except for IDH1, is limited due to the small sample size of this study and was therefore not included.
Materials and Methods
Patient Selection
Patients with rGBM who failed standard chemoradiotherapy regimen (TMZ and radiotherapy) were enrolled in this single-arm, open-label, Phase II trial. This study was approved by the ethics committee of Shandong Cancer Hospital Affiliated to Shandong University and was registered with ClinicalTrials.gov under identifier NCT03660761. All patients signed a consent form prior to enrollment and were willing to comply with treatment and follow-up assessments and procedures. Patients included in the study must meet the following criteria.
The inclusion criteria are as follows: (1) age of 18–70 years; Karnofsky performance scale (KPS) of ≥60; (2) histologically confirmed diagnosis of GBM, World Health Organization Grade IV; (3) measurable or evaluable disease by magnetic resonance imaging (MRI) confirmation and a minimum life expectancy of 8 weeks; (4) progressive disease (relapse) on MRI defined by Response Assessment in Neuro-Oncology (RANO) criteria after the standard Stupp protocol; the time interval for the start of treatment was at least 12 weeks from prior radiotherapy unless in the presence of histopathologic confirmation of recurrent tumor or new contrast enhancement on MRI outside of the radiotherapy treatment field; (5) adequate bone marrow function (leukocyte count ≥4000/μL, neutrophil count ≥1500/µL, platelet count ≥100,000/µL, hemoglobin ≥8.0 g/dL), renal function (serum creatinine ≤150 μmol/L, 24 hrs urine protein ≤3.4 g), and liver function (total bilirubin ≤34 μmol/L and aspartate and alanine aminotransferase ≤120 U/L).
The exclusion criteria are as follows: (1) extracranial metastatic disease, (2) Gliadel wafer treatment, (3) severe cardiopulmonary insufficiency, (4) status epilepticus, (5) pregnancy, (6) gastrointestinal bleeding, (7) uncontrolled blood pressure with medication (>140/90 mm Hg), (8) swallowing difficulties, and (9) HIV positivity and treatment of antiretroviral therapy.
Drug Administration
Apatinib was provided by Jiangsu Hengrui Medicine Co., Ltd. A starting dose of apatinib was administered 500 mg p.o. once daily. Drug doses were withheld and/or reduced for intolerable grade 2 or grade 3–4 toxicity. A maximum of two dose-level reductions were permitted (500 mg, then 250 mg). The dosage was modified to 250 mg if patients experienced ≧grade 2 hematologic adverse events, hand and foot syndrome, proteinuria, fecal ocular blood, or grade 3/4 hypertension or other grade 3/4 adverse events. Apatinib was administered until disease progression, unacceptable toxicity, or death.
TMZ was administrated to patients with the dosage of 100 mg/m2 (7 days on with 7 days off) on a 28-day cycle.
Efficacy and Adverse Events
All the lesions were measurable. The baseline evaluation included MRI, questionnaires on health-related quality of life, neurocognitive testing, KPS, complete blood count, blood chemical analyses, and urinalysis. Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were measured by RANO criteria. The primary endpoint was PFS, and secondary endpoints included OS, ORR, and DCR. PFS was defined as the time from the beginning of intervention treatment to PD or death from any cause; ORR = (CR + PR)/total number of cases ×100; DCR = (CR + PR + SD)/total number of cases × 100.
Adverse events (AEs) were estimated with National Cancer Institute-Common Terminology Criteria Adverse Events version 4.0 (NCI-CTCAE 4.0) at regular intervals during each cycle. Physical examination, hematology, biochemistry tests, and electrocardiograms were used to monitor AEs. Medical and clinical monitoring of the study as well as data management was conducted by the authors. Clinical laboratory assessments were performed by local laboratories.
Statistics
Patient characteristics and AEs were described with counts and percentages in frequency tables, respectively. PFS rates were estimated using Kaplan–Meier method and log rank test. The survival curve was plotted using GraphPrism 7.
Results
Characteristics of Patients
The characteristics of the patients are summarized in Table 1. From March 2016 to January 2018, 20 patients with rGBM received the combination therapy of apatinib and TMZ. All of them were evaluated in this analysis. Among them, 35% were females and the median age was 50.5 years. The median KPS score before treatment was 70.
 |
Table 1 Clinical Characteristics of 20 Patients with rGBM Treated with Apatinib Plus TMZ |
Efficacy
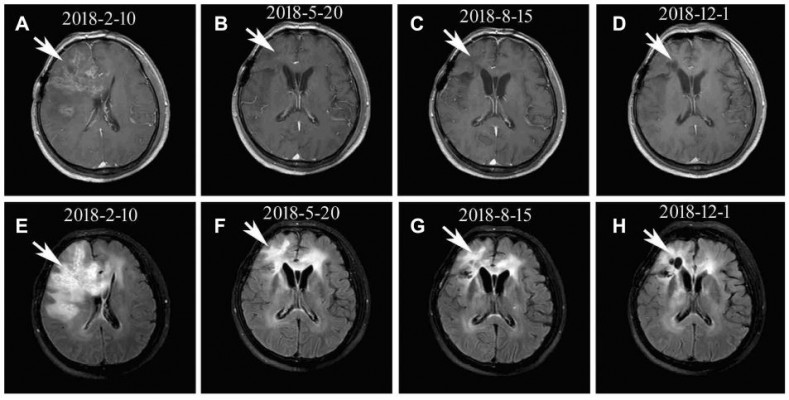
As described in Table 1, the KPS scores improved in 60% (12/20) of the patients after treatment. Radiographic benefits were assessed according to RANO criteria. The patients included one with CR, eight with PR, nine with SD, and two with PD. Three patients were still alive. The ORR was 45% (9/20), and the DCR was 90% (18/20). The median PFS for all patients were 6 months (95% confidence interval [CI]: 5.3 to 7.8 months). The median OS was 9 months (95% CI, 8.2 to 12.2 months). Survival curves for PFS and OS were estimated using Kaplan–Meier method (Figures 1 and 2). Typical imaging changes in rGBM are shown in Figure 3.
 |
Figure 1 Kaplan–Meier cures of progression-free survival in all patients. |
 |
Figure 2 Kaplan–Meier curves of overall survival in all patients. |
Adverse Events
AEs for patients are summarized in Table 2. The toxicity profiles occurred in patients included the following: hypertension (21%), hand–foot syndrome (16%), leukopenia (2%), thrombocytopenia (12%), albuminuria (9%), gastrointestinal reaction (7%), fecal ocular blood (5%), anemia (5%), fatigue (12%), grade 3/4 AE included hypertension (5%), leukopenia (2%), and thrombocytopenia (2%). These AEs were quickly reduced and recovered after a dose reduction or interruption. Therefore, detecting the toxicity of the drug and adjusting the dosage from 500 to 250 mg were critical. Half of the patients received a dose adjustment. No treatment-related death occurred.
 |
Table 2 Adverse Events in the Combination Therapy of Apatinib and Temozolomide |
Discussion
Up to now, no standard treatment regimen has been proposed for rGBM. Molecular-targeted drugs have been the focus of research on rGBM. Apatinib, as a small molecular tyrosine kinase inhibitor (TKI) to VEGFR, can highly selectively compete for the adenosine triphosphate binding site of intracellular VEGFR-2 to block downstream signal transduction and inhibit tumor angiogenesis.14 The present study is the first clinical trial to investigate the safety and efficacy of apatinib combined with dose-dense TMZ for patients with rGBM. Our data demonstrated that apatinib in combination with dose-dense TMZ was effective in terms of PFS, ORR, and DCR and was well tolerated after dose reduction in the Chinese population evaluated.
Studies revealed that new vessels provide nutrient and oxygen for tumors. Restraining angiogenesis can inhibit tumor growth, development, and metastasis.15 VEGF/VEGFR, which is overexpressed on the surface of various tumors, is an important set of ligand and receptor that affects angiogenesis. Researchers have attempted to restrict the growth of tumor by restraining the combination between VEGF and VEGFR to stop the activation of the downstream pathway.16,17 The targeted therapy of anti-VEGF/VEGFR includes reducing the concentration of activated and freed VEGF and cutting off the VEGFR signal system.
In clinics, agents that target the VEGF/VEGFR-2 pathway are used, such as anti-VEGF antibody (e.g., bevacizumab), inhibitors of angiogenic receptor tyrosine kinases (e.g., sunitinib, pazopanib, sorafenib, and regorafenib), inhibitors of VEGFR-2 tyrosine kinases (e.g., apatinib), and anti-VEGFR antibody (e.g., ramucirumab). Apatinib, which mainly targets VEGFR-2 and receptor tyrosine kinases (RTKs), such as c-kit, RET, and c-src, is the first generation of oral anti-angiogenesis drug invented in China.18 VEGF-2 promotes endothelial proliferation by activating the mitogen-activated protein kinase signaling pathway during angiogenesis.19
Given that apatinib is a novel VEGFR-2 inhibitor produced in China, the participants of most clinical trials were almost entirely Asians. Some clinical trials, such as NCT03407976, NCT03396211, and NCT03042611, are undergoing in the United States of America.20 Additional data on the efficacy of apatinib in different populations will be obtained in the next few years.
At present, bevacizumab is the most widely used anti-angiogenic drug for treatment of rGBM.21,22 Friedman et al23 showed that patients in bevacizumab alone and bevacizumab-plus-irinotecan groups had estimated 6-mPFS rates of 42.6% and 50.3%, respectively; ORR of 28.2% and 37.8%, respectively; and mOS of 9.2 and 8.7 months, respectively. Apatinib and bevacizumab are anti-angiogenic agents, but the former has some advantages over bevacizumab. Apatinib is an oral small molecular TKI that targets the intracellular domain of VEGFR-2. Apatinib reportedly could promote tumor cell apoptosis via intracellular autocrine VEGF signaling, whereas bevacizumab could not. Moreover, apatinib can reverse multidrug resistance by inhibiting the function of multiple ABC transporters.24,25
In this study, the population tested had ORR of 45% (9/20), DCR of 90% (18/20), mPFS of 6 months, and mOS of 9 months. In a previous study of dose-dense regimens of TMZ alone for rGBM, the patients had ORR of 11.1%, DCR of 30%, mPFS of 4.3 months, and mOS of 6.9 months.26 However, studies using bevacizumab alone reported ORR of 38%, mPFS of 3 months, and mOS of 8 months.27 Compared to previous studies, the present clinical study showed relatively satisfactory results and met our expectations. However, the good response data based on the results of MRI may be an effect of pseudo-response similar to what is known with bevacizumab.28 In our clinical study, patients who failed treatment with apatinib had particularly rapid tumor recurrence and more diffused cerebral edema. Therefore, administering chemotherapy drugs at the same time is important.
Wei et al29 performed a systematic review and meta-analysis to identify the efficacy and safety of various dose-dense regimens of TMZ for recurrent high-grade glioma. In this study, a dosing schedule of 7 days on/7 days off for treatment of grade IV gliomas was significantly superior to the standard 5-day regimen with respect to PFS-6 and PFS-12. Compared with the standard schedule, the proposed regimen achieved higher OS-12 in grade III gliomas. The standard TMZ regimen requires discontinuation of the drug for 2–3 weeks during a continuous treatment cycle, which may result in the survival or regeneration of some vascular endothelial cells, leading to tumor angiogenesis and tumor cell regeneration. The therapeutic advantage of the TMZ dose-density regimen may be its reduced tumor resistance to TMZ and enhanced anti-tumor angiogenesis. Alternative TMZ dosing schedules may result in sustained depletion of MGMT and overcome TMZ resistance. Rhythmic or continuous administration may produce anti-angiogenic effects by targeting vascular endothelial cells.30–32 In this regard, we finally selected 7 days on/7 days off for this clinical trial.
The side effect of apatinib combined with dose-dense TMZ was slightly higher than that of bevacizumab combined with bi-weekly TMZ for rGBM. Badruddoja et al33 reported that the most common AEs included fatigue 30% (9/30), infection 30% (9/30), nausea 13.3% (4/30), insomnia 10% (3/30), leukopenia 10% (3/30), and hypertension 10% (3/30). Moreover, grade 3/4 AE reactions accounted for 20% (10/30). By contrast, in the present work, the most common AEs were hypertension 45% (9/20) (20.9% of the total AEs), hand–foot syndrome 35% (7/20) (16.3% of the total AEs), and leukopenia 30% (6/20) (14% of the total AEs). Grades 3/4 AE reactions were 20% (4/20) and 9% of the total AEs. Half of the patients had their apatinib dose halved because of the side effects. Nevertheless, the results of the trial were still as expected. The optimal dosing schema of apatinib remains unknown, and low-dose apatinib may still be an appealing therapeutic option that could be further investigated in a large sample trial.
This study presents limitations, such as small number of patients evaluated and single-center trial. The patients’ genetic status was not examined due to the small sample size. Nevertheless, the present study is the first to report the efficacy and safety of apatinib with dose-dense TMZ for rGBM with relatively large samples.
Abbreviations
GBM, glioblastoma; VEGFR, vascular endothelial growth factor receptor; DCR, disease control rate; ORR, objective response rate; PFS, median progress-free survival; MGMT, methyl-guanine-methyltransferase; MRI, magnetic resonance imaging; CT, computed tomography; CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease; AEs, adverse events; NCI-CTCAE 4.0, National Cancer Institute-Common Terminology Criteria Adverse Events version 4.0; KPS, Karnofsky performance status; TMZ, temozolomide; RT, radiation therapy.
Ethics Approval and Consent to Participate
This research was approved by the research ethics committee of Shandong Cancer Hospital, and written informed consent for publication of the clinical details and images was obtained from the patient.
Data Sharing Statement
We would like to share all clinical trial data from these 20 patients except information that contains patient privacy. Please contact the corresponding author for data requests.
Acknowledgments
No specific funding has been used for data collection, analyses, reporting of results, or manuscript writing.
Disclosure
The authors declare that they have no competing interests.
References
1. Alexander BM, Cloughesy TF. Adult glioblastoma. J Clin Oncol. 2017;35:2402–2409. doi:10.1200/JCO.2017.73.0119
2. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi:10.1001/jama.2013.280319
3. Halani SH, Babu R, Adamson DC. Management of glioblastoma multiforme in elderly patients: a review of the literature. World Neurosurg. 2017;105:53–62. doi:10.1016/j.wneu.2017.04.153
4. Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol. 2017;28(7):1457–1472. doi:10.1093/annonc/mdx106
5. Zorzan M, Giordan E, Redaelli M, Caretta A, Mucignat-Caretta C. Molecular targets in glioblastoma. Future Oncol. 2015;11:1407–1420. doi:10.2217/fon.15.22
6. Roviello G, Ravelli A, Polom K, et al. Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett. 2016;372:187–191. doi:10.1016/j.canlet.2016.01.014
7. Peng QX, Han YW, Zhang YL, et al. Apatinib inhibits VEGFR-2 and angiogenesis in an in vivo murine model of nasopharyngeal carcinoma. Oncotarget. 2017;8:52813–52822. doi:10.18632/oncotarget.17264
8. Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–1454. doi:10.1200/JCO.2015.63.5995
9. Wu F, Zhang S, Xiong A, et al. A phase II clinical trial of apatinib in pretreated advanced non-squamous non-small-cell lung cancer. Clin Lung Cancer. 2018;19:e831–e842. doi:10.1016/j.cllc.2018.06.002
10. Wang L, Liang L, Yang T, et al. A pilot clinical study of apatinib plus irinotecan in patients with recurrent high-grade glioma: clinical trial/experimental study. Medicine (Baltimore). 2017;96:e9053. doi:10.1097/MD.0000000000009053
11. Zhang H, Chen F, Wang Z, Wu S. Successful treatment with apatinib for refractory recurrent malignant gliomas: a case series. Onco Targets Ther. 2017;10:837–845. doi:10.2147/OTT.S119129
12. Ding X, Sun J, Fan T, Li B. A case report of targeted therapy with apatinib in a patient with recurrent high grade glioma. Medicine (Baltimore). 2018;97:e10859. doi:10.1097/MD.0000000000010859
13. Yu D, Han G, Liu H, Gao L, Verma V. Treatment of adult brainstem glioma with combined antiangiogenic therapy: a case report and literature review. Onco Targets Ther. 2019;12:1333–1339. doi:10.2147/OTT.S195783
14. Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther. 2015;9:6075–6081. doi:10.2147/DDDT.S97235
15. Li T, Kang G, Wang T, Huang H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol Lett. 2018;16:687–702. doi:10.3892/ol.2018.8733
16. Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72:1909–1914. doi:10.1158/0008-5472.CAN-11-3406
17. Weathers SP, de Groot J. VEGF manipulation in glioblastoma. Oncology (Williston Park). 2015;29:720–727.
18. Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51:223–229. doi:10.1358/dot.2015.51.4.2320599
19. Liu K, Ren T, Huang Y, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. 2017;8:e3015. doi:10.1038/cddis.2017.422
20. Scott LJ. Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs. 2018;78:747–758. doi:10.1007/s40265-018-0903-9
21. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377:1954–1963. doi:10.1056/NEJMoa1707358
22. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi:10.1056/NEJMoa1308573
23. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi:10.1200/JCO.2008.19.8721
24. Peng S, Zhang Y, Peng H, et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by apatinib. Cancer Lett. 2016;373:193–202. doi:10.1016/j.canlet.2016.01.015
25. Peng H, Zhang Q, Li J, Zhang N, Hua Y, Xu L. Apatinib inhibits VEGF signaling and promotes apoptosis in intrahepatic cholangiocarcinoma. Oncotarget. 2016;7:17220–17229. doi:10.18632/oncotarget.7948
26. Santoni M, Paccapelo A, Burattini L, et al. Protracted low doses of temozolomide for the treatment of patients with recurrent glioblastoma: a phase II study. Oncol Lett. 2012;4:799–801. doi:10.3892/ol.2012.788
27. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–953. doi:10.1016/S1470-2045(14)70314-6
28. Auer TA, Breit HC, Marini F, et al. Evaluation of the apparent diffusion coefficient in patients with recurrent glioblastoma under treatment with bevacizumab with radiographic pseudoresponse. J Neuroradiol. 2019;46:36–43. doi:10.1016/j.neurad.2018.04.002
29. Wei W, Chen X, Ma X, Wang D, Guo Z. The efficacy and safety of various dose-dense regimens of temozolomide for recurrent high-grade glioma: a systematic review with meta-analysis. J Neurooncol. 2015;125:339–349. doi:10.1007/s11060-015-1920-0
30. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi:10.1056/NEJMoa043331
31. Brandes AA, Franceschi E, Paccapelo A, et al. Role of MGMT methylation status at time of diagnosis and recurrence for patients with glioblastoma: clinical implications. Oncologist. 2017;22:432–437. doi:10.1634/theoncologist.2016-0254
32. Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. Clinically relevant imaging features for MGMT promoter methylation in multiple glioblastomastudies: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2018;39:1439–1445. doi:10.3174/ajnr.A5711
33. Badruddoja MA, Pazzi M, Sanan A, et al. Phase II study of bi-weekly temozolomide plus bevacizumab for adult patients with recurrentglioblastoma. Cancer Chemother Pharmacol. 2017;80:715–721. doi:10.1007/s00280-017-3405-7
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.