Back to Journals » Cancer Management and Research » Volume 12
Apatinib for Treatment of Inoperable Metastatic or Locally Advanced Chondrosarcoma: What We Can Learn About the Biological Behavior of Chondrosarcoma from a Two-Center Study
Authors Xie L , Xu J, Sun X , Liu K, Li X , He F, Liu X, Gu J, Lv Z, Yang R, Tang X, Yan T , Li D , Yang Y, Dong S, Sun K, Shen D, Guo W
Received 10 March 2020
Accepted for publication 5 May 2020
Published 15 May 2020 Volume 2020:12 Pages 3513—3525
DOI https://doi.org/10.2147/CMAR.S253201
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Lu Xie,1 Jie Xu,1 Xin Sun,1 Kuisheng Liu,1 Xiaowei Li,1 Fangzhou He,1 Xinyu Liu,1 Jin Gu,2 Zhe Lv,3 Rongli Yang,1 Xiaodong Tang,1 Taiqiang Yan,1 Dasen Li,1 Yi Yang,1 Sen Dong,1 Kunkun Sun,4 Danhua Shen,4 Wei Guo1
1Musculoskeletal Tumor Center, Peking University People’s Hospital, Beijing, People’s Republic of China; 2Surgical Oncology, Peking University Shougang Hospital, Beijing, People’s Republic of China; 3Radiology Department, Peking University Shougang Hospital, Beijing, People’s Republic of China; 4Pathology Department, Peking University People’s Hospital, Beijing, People’s Republic of China
Correspondence: Wei Guo
Musculoskeletal Tumor Center, Peking University People’s Hospital, No. 11 Xizhimen South Street, Xicheng District, Beijing 100044, People’s Republic of China
Tel +86 13701195504
Fax +86 10 68318386
Email [email protected]
Purpose: For patients who have chondrosarcoma in the unresectable setting, antiangiogenic agents are reportedly effective. This multicenter, retrospective study investigated the antitumor activity of apatinib in patients with unresectable chondrosarcoma to gain insight into the biological behavior of this disease.
Methods: All of the patients with unresectable chondrosarcoma who were diagnosed between October 1, 2009, and November 1, 2019, in two sarcoma centers affiliated with Peking University were evaluated. Relevant information was collected from the medical records at both centers, from which patients receiving apatinib for systemic therapy were selected for analysis.
Results: In total, efficacy analysis was conducted in 33 patients with a median follow-up time of 22.1 (Q1, Q3, 14.6, 23.0) months. There were 20/33 (60.0%) conventional chondrosarcomas (grades 2– 3), 5/33 (15.2%) dedifferentiated chondrosarcomas, 4/33 (12.1%) mesenchymal chondrosarcomas, 3/33 (9.1%) extraskeletal myxoid chondrosarcoma, and 1/33 (3.1%) clear-cell chondrosarcomas with 87.9% in metastatic and 12.1% in locally advanced states. The objective response rate was 6/33 (18.2%). The median progression-free survival (PFS) was 12.4 months (Q1, Q3, 7.0, 21.2), while the median overall survival has not yet been reached. Rare variants of chondrosarcoma tended to have a longer PFS than conventional chondrosarcoma (P= 0.06). Based on clinicopathological factors Cox and univariate analysis, only extraskeletal myxoid chondrosarcoma and baseline target lesions < 60 mm benefited from the drug apatinib (P=0.14 and P=0.00), respectively. Grade 3 or higher adverse events were frequent in 11/33 (39.3%) of patients who discontinued apatinib due to deterioration of their general condition.
Conclusion: Apatinib had clinically meaningful activity in patients with inoperable high-grade chondrosarcoma. However, special caution should be made in managing toxicity due to the indolent behavior and slow growth pattern after using this drug. Patients with a smaller tumor size and extraskeletal myxoid chondrosarcoma subtype might benefit from this therapy more.
Clinical Trial Registration: Registered February 7, 2020, with clinicaltrials.gov: NCT04260113.
Keywords: chondrosarcoma, apatinib, inoperable, metastasis, locally advanced
Introduction
Chondrosarcomas (CS) are heterogeneous, diverse groups of tumors characterized by the production of cartilage matrix.1 They are the second most frequent primary malignant tumors of bone following osteosarcomas, accounting for 20–27% of primary malignant bone neoplasms. The majority (≥85%) are conventional CS, which can be categorized according to their location in bone into primary central and secondary peripheral CS, while the remaining 10% are rare variants of CS, which include periosteal, dedifferentiated, clear-cell, and mesenchymal CS.2 According to a study by van Maldegem et al,3 the overall survival (OS) rate for advanced, unresectable conventional central CS was 48% at 1 year, 24% at 2 years, 12% at 3 years, 6% at 4 years, and 2% at 5 years.
In the last several decades, there has been no significant improvement in the survival of patients with CS.1–3 Surgery is the mainstay of treatment for this disease, with the aim of wide surgical margins.4 In selected cases, radiotherapy may achieve encouraging results when adequate doses are delivered by conformational techniques or proton beam/carbon ion radiotherapy.5,6 For advanced cases, there is no standard systemic treatment.1–4,7 A large retrospective study showed that for unresectable CS, the objective response rate (ORR) for chemotherapy was significantly different according to histological subtype: 31% for mesenchymal, 20.5% for dedifferentiated, 11.5% for conventional, and 0% for clear-cell sarcoma (P=0.04).4 However, approximately 70% of patients with grade 3 tumors will develop metastasis, which makes this orphan disease difficult to manage.1 There is growing evidence from preclinical data, implicating angiogenesis in the pathogenesis of CS.8–10 In addition, vascularization increases with increasing histological grade.10 Imatinib,11 dasatinib,12 pazopanib,13–15 ramucirumab,15 and regorafenib16 have all been explored in CS of the advanced stage with diverse results. Apatinib is an active anti-angiogenesis tyrosine kinase inhibitor (TKI), which more selectively inhibits vascular endothelial growth factor receptor 2 with an IC50 of 0.001 μM in vitro while synchronously inhibiting c-kit (0.429 μM), PDGFR-α (>1 μM), Ret (0.013 μM), c-src (0.53 μM), EGFR (>10 μM), HER2 (>10 μM), and FGFR1 (>10 μM).17 In our off-label experience, this agent had high and durable responses in three CS patients.18
We conducted this retrospective, two-center study to summarize our prior experience on CS patients using apatinib, to gain insights into the biological behaviors and outcomes of patients with different subtypes in an advanced, unresectable setting.
Methods
Study Design and Participants
Prior to commencing the study, institutional review boards from the Medical Ethics Committee of Peking University People’s Hospital and Peking University Shougang Hospital (Beijing, China) provided approval (identifier number: 2019PHD003-03 and IRBK-2020-060-02, respectively), which included authorization to perform a retrospective search of the electronic medical records and review all radiographic images for each patient. The search was performed at these two sarcoma centers to identify high-grade CS patients treated with apatinib between March 2016 and December 2019, of whom the histological diagnosis was centrally reviewed and confirmed again by two senior pathologists (SDH and SKK) at Peking University People’s Hospital.
Criteria for the unresectable disease were judged by the multidisciplinary teams at these two hospitals and defined as complete resection of primary tumor and/or metastases was not deemed feasible due to technical inability because of the size or location of the primary tumor or extensive metastatic sites. Baseline information consisted of gender, age at first presentation of disease, date at disease diagnosis, disease location, subtypes, grades, date for initial apatinib therapy, Eastern Cooperative Oncology Group (ECOG) performance status19 at initial therapy, target or non-target lesions before apatinib therapy, previous systemic therapy, date of progression, progression patterns, date of death if available, and so on. Data regarding toxicity were also obtained from the electronic patient record. The study was conducted in accordance with Good Clinical Practice principles, the Declaration of Helsinki, and all local regulations.
Procedures
Apatinib was prescribed at 500 mg orally once daily, 30 min after the meal. Dose reductions were performed if patients’ adverse events (AEs) could not be relieved by prophylactic management or symptomatic treatment and continued to exceed grade 2 according to the Common Terminology Criteria for Adverse Events version 5.0.20 Whenever feasible, dosing was resumed at a higher ideal dose level. All of the patients had progressive disease after previous multimodal therapy and underwent a baseline scan before using apatinib. Response to treatment was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Re-staging scans were routinely performed every 2 months. The follow-up was generally done at an outpatient clinic; however, if the patient’s condition was too weak, telephones or electronic mail might be used until death.
Outcomes
Progression-free survival (PFS) was defined as the time from the start of apatinib until disease progression, death, or last follow-up. Overall survival (OS) was defined as the time from the start of apatinib until death or last follow-up. Clinical benefit rate (CBR) was defined as the proportion of patients who did not have disease progression at 6 months. The disease control rate (DCR) was the proportion of patients who did not have disease progression on record. Duration of response (DoR) was defined as the time from the first documentation of objective response to the time of the first documentation of disease progression or death, whichever came first.
Statistical Analysis
The cutoff date for statistical analysis of clinical outcome was February 2, 2020. Clinical and pathological descriptive statistics were employed to show the distribution of variables in the population. The survival curves were calculated according to the Kaplan–Meier method. Prognostic factors were identified by univariate and multivariate analyses using a Cox regression model. Variables associated with PFS and OS with a P-value <0.05 in the univariate analysis were included in the multivariate analysis. Statistical analysis was performed using SAS Statistics (version 9.4) and GraphPad Prism (version 5).
Results
Clinicopathological Characteristics
A total of 40 patients with advanced CS treated with apatinib were identified, of whom 7 were lost to follow-up. All of them had taken apatinib home without coming back to clinics. These patients were mostly from remote countryside in different provinces of China and did not accept telephone reviews. Thus, 33 patients were included in this study, whose characteristics are described in Table 1 (detailed information in Appendix Table 1). Their initial surgeries were done between October 1, 2009, and November 1, 2019, with pathological diagnosis confirmed as high-grade CS. With an average time of 19.9 months (95% confidence interval [CI]: 12.9, 27.0), these patients started apatinib because of uncontrollable disease progression between 2016 and 2019. The median age of this cohort was 41.0 years (range 17.0–72.0), with a male to female ratio of 2.7. In total, 57.6% of patients had an ECOG status of 1 before apatinib therapy, while the median number of prior surgeries was 2 (range, 1–5). We included 20/33 (60.0%) conventional CS (grades 2–3), 5/33 (15.2%) dedifferentiated CS, 4/33 (12.1%) mesenchymal CS, 3/33 (9.1%) extraskeletal myxoid CS, and 1/33 (3.1%) clear-cell CS. At the beginning of treatment, tumors were located in the lungs only in 9/33 (27.3%) patients, at musculoskeletal sites only in 4/33 (12.1%), and both at pulmonary and musculoskeletal sites as well as visceral infiltration in 20/33 (60.6%). Combination chemotherapy (doxorubicin and ifosfamide-based regimen) had been administered in 13 cases (39.4%), previous TKIs (anlotinib) had been used in 2 (6.1%) patients, while 18 patients (54.6%) had not received any systemic therapy before treatment, most of whom had conventional chondrosarcoma (grades 2–3), which was generally thought to be refractory to chemotherapy. With a median follow up of 22.1 (Q1, Q3, 14.6, 23.0) months, 23/33 (69.7%) were alive with disease, 8 (24.2%) died of disease, and 2 (6.1%) died because of deterioration of their general condition without disease progression. Interestingly, until the last follow-up, 28 patients ceased apatinib: 17/28 (60.7%) because of disease progression and 11 (39.3%) because of severe toxicity or deterioration of the general condition.
 |
Table 1 Patients’ Demographics (N=33) |
Treatment Activity
Thirty-three patients were assessable for response (Table 2). Using RECIST, no patient had complete response (CR), 6 (18.2%) had partial response (PR), and 23 (69.7%) had stable disease (SD), with median time to best response of 2.0 (Q1, Q3, 1.1, 2.2) months. Progressive disease (PD) was observed in four (12.1%) patients. The DCR at 3 months reached 78.8% (26/33), while the CBR at 6 months was 54.6% (18/33). The median DoR was 5.4 (Q1, Q3, 1.8, 11.4) months with 2/6 (33.3%) still ongoing. Moreover, the ORR was significantly different according to histological subtype: 15.0% for conventional CS (3/20), 25.0% for mesenchymal CS (1/4), 20.0% for dedifferentiated CS (1/5), 0.0% for clear-cell CS (0/1), and 33.4% for myxoid CS (1/3) (Figure 1). Median PFS was 12.4 months (95% CI 7.0–21.2) (Figure 2). The 6-, 12-, and 24-month PFS rates were 72.9% (95% CI 55.9%, 87.8%), 54.8% (33.9%, 71.6%), and 12.5% (2.2%, 32.2%), respectively. We calculated these dedifferentiated, mesenchymal, extraskeletal myxoid, and clear-cell CS into a group as rare variants of CS and found that this group of patients tended to have a longer PFS than conventional chondrosarcoma (P=0.06). For conventional central CS (n=20), the median PFS was 7.0 months (95% CI 4.5, 9.6) with a 6-month PFS rate of 50.5% (95% CI 24.5%, 75.5%), while the median OS was 24.3 months (95% CI 5.5, 43.0). However, in all, the median OS has not yet reached the 12-month OS of 75.8% (95% CI 87–100) and 24-month OS of 61.9% (95% CI 77–100) (Figure 3). On univariate analysis, only extraskeletal myxoid CS subtype and baseline target lesion size were significantly associated with PFS (P=0.08 and P=0.04, respectively). We categorized the tumor size be RECIST 1.1 using baseline target lesions by median tumor size into those ≥60mm and those < 60mm and found out the progression-free survival obviously prolonged in those smaller in tumor size (P=0.00). For extraskeletal myxoid CS, although there were only 3 cases, the median DoR was 21.2 months (range, 10.9, 28.1) with one PR and two SD. From the forest plots summarized in Figure 4, it was evident that patients with extraskeletal myxoid CS and baseline target lesion size < 60 mm benefited more from apatinib (P=0.14 and P=0.00, respectively). For multivariate analysis, only baseline target lesion size had a tendency to influence PFS (P=0.06).
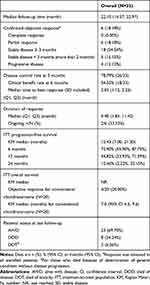 |
Table 2 Efficacy of Apatinib in Patients with Advanced Chondrosarcoma |
 |
Figure 1 Waterfall plots for target lesions for the 33 patients (according to RECIST v1.1). |
 |
Figure 2 Kaplan-Meier plots for PFS in 33 patients with advanced CS. Crosses indicate censoring. |
 |
Figure 3 Kaplan-Meier plots for overall survival in 33 patients with advanced CS. Crosses indicate censoring. |
 |
Figure 4 Forest plots of hazard ratios for disease progression in different clinical or pathological subgroups. |
Toxicity
All 33 patients (100%) experienced one or more AEs, and 11 (39.3%) had AEs leading to the withdrawal of apatinib. Withdrawals due to AEs occurred after a median of 4 months of treatment (range, 1–8). Two patients died from infections, which were deemed possibly related to treatment. Upon review of their medical records, it was unclear if the infections occurred at a local health institution with the deterioration of their general condition. Grades 3 or 4 treatment-related AEs were observed including anorexia (12 patients [36.4%]), wound dehiscence and infections (9 [27.3%]), platelet decrease (3 [9.1%]), and hypertension (2 patients [6.1%]).
Discussion
This retrospective study first described the efficacy and safety of apatinib in treating CS with adequate follow-up in the advanced setting. At the same time, it reflected the basic biological behavior of CS, obtaining prolonged disease control with antiangiogenic therapy.
The context of CS formation makes it deficient in response to chemotherapy,1 the mechanisms of which have been widely investigated but not completely understood.21 Conventional central CS is the most common subtype with the majority being low histologic grade. However, approximately 10% are histologically high grade (grade 2 or 3) with a high risk for distant metastases and/or local recurrence.22,23 In previous decades, it has been believed that no available systemic therapy improved outcome.1,2 Dedifferentiated CS represents 9–10% of all CS and is characterized by conventional low-grade chondrosarcoma with abrupt transition to foci that has dedifferentiated into a higher grade, which occurs in older age with a 5-year survival rate of less than 25%.1,23 Chemotherapy might have a role in this subgroup, especially for the high-grade part.4 Mesenchymal CS and clear-cell CS represent two rare variants occurring in younger patients, the former of which is composed of small round cells and thought to be chemosensitive.4 However, Xu et al (2015) performed a systematic review of 107 patients in 2015 and concluded that chemotherapy regime based on anthracyclines did not benefit OS of mesenchymal CS.24 Extraskeletal myxoid chondrosarcoma is a rare soft tissue sarcoma marked by low sensitivity to the chemotherapeutic drugs but with indolent behavior and slow growth. It carries chromosome 9 reciprocal translocation involved NR4A3 gene locus.13 In this study, we noticed that this subtype benefited more than other CS subtypes with more indolent behavior and took a longer time to develop secondary resistance. At the same time, tumor size influenced the outcome. Those with large tumor burden do not experience much tumor shrinkage with apatinib treatment and can quickly develop resistance.
Currently, there is an urgent requirement to recognize factors causing resistance and discover new strategies for optimal treatment for this group of patients. Signaling pathways suggested to have a role in CS include Hedgehog (Hh),25 Src,26 PI3k–Akt–mTOR,27 mutations in isocitrate dehydrogenase 1/2,28 and angiogenesis.10,29 Nevertheless, it seems that all clinical trials targeted at gene-driven molecular pathways have failed except for anti-angiogenesis,2 and the result of the latter is still controversial regarding whether it is actually beneficial for prognosis due to small sample sizes resulting in diverse prognoses.2–4,13,24 We summarized all of the anti-angiogenesis drugs, which have been tested in CS (Table 3). Pericartilage vessels are involved in supporting tumor growth, whereas intracartilage vessels might be involved in the acquisition of metastatic potential by cartilage tumors. VEGF expression is strongly associated with the presence of intracartilage vessels.10 van Maldegem et al3 reviewed a large series of 171 patients with advanced, unresectable conventional CS from the Rizzoli Institute and Leiden University Medical Center and provided a standard for OS rates. Grignani et al11 prospectively investigated imatinib on nonresectable or metastatic PDGFR expression conventional and myxoid CS and found no ORR, but a median PFS of 3 months. In a heterogeneous group with sarcoma treated with dasatinib in a Phase 2 trial, the median 6-month PFS for CS was 47% with durable disease stable in three cases.12 Pazopanib has been normatively investigated in both CS of any grade and advanced extraskeletal myxoid CS. Because of the diversity of these two subtypes’ features, the DCR at 16 weeks was 43% in conventional central CS, and 12-month PFS in extraskeletal myxoid CS reached 74%.13,14
 |
Table 3 Antiangiogenic Therapy for Advanced Chondrosarcoma |
In our study, the majority of patients had conventional CS (n=20) with an ORR of 15% and median PFS of 7.0 months, which represented a meaningful outcome and supported apatinib treatment in this group of traditionally chemotherapy-resistant disease. Regarding other CS subtypes, although response rates were diverse,11–14,16,24,29 longer PFS was observed comparing to other studies.3,11-14,16 Mi et al had reported that apatinib significantly enhanced the cytotoxicity of drugs targeted on P-glycoprotein (ABCB1), multidrug resistance protein 1 (MRP1, ABCC1), and breast cancer resistance protein (BCRP, ABCG2) and reversed ABCB1- and ABCG2-mediated multidrug resistance by inhibiting their transport function.32 We think this might partly explained the longer PFS observed in this study. Notably, the AE profile seen in this study of patients reflected what has been described in previous studies of apatinib in patients with osteosarcoma,30,31 but the patients’ tolerability was markedly lower, with nearly 40% of patients dropping out for palliative treatment. These results make us question that for this potentially lifelong-used drug for advanced cases, what kind of therapeutic options would be better? A 17-year conventional CS patient’s nearly 2 years’ treatment experience presented typical therapeutic courses of these patients, which is shown in Figures 5–8. Current basic research has revealed that dedifferentiated CS could be eligible for anti-PDL1 therapy, and more importantly immunomodulation through CSF1R+ macrophages could be a promising therapeutic approach for conventional CS.33 Maybe lowering the dose of apatinib in combination with immunotherapy might be a better option for this group of patients to better benefit disease control and quality of life.
 |
Figure 5 Chest Computed Tomography (CT) of a 17-year-old conventional CS patient before apatinib therapy on March 15, 2018. |
 |
Figure 6 Chest CT of a conventional CS patient after using apatinib for more than 7 months. |
 |
Figure 7 Chest CT of the patient after using apatinib for more than 14 months with pneumothorax. |
 |
Figure 8 Chest CT of the patient after using apatinib for almost 22 months with pleurodesis. |
In addition, our study has some limitations. Our retrospective study, despite using data from two experienced sarcoma centers in China, may have encountered some differences in the definition of unresectability between the centers that may have had some impact on the outcome of this study. Also, because of the retrospective nature, not all possible prognostic data could be reliably retrieved from the records, such as AEs, which might not be so accurate as a prospective study. Third, because of the rarity of the disease, we had an insufficient sample size to permit subset analyses, especially for CS rare subtypes, which could have reduced the statistical power.
Conclusion
In summary, patients with inoperable advanced CS had an ORR of 18.2% and median PFS of 12.4 months after apatinib therapy, which represented a meaningful outcome in our experience. However, special caution should be made in managing this drug’s toxicity due to long-term disease control. Patients with a smaller tumor size and extraskeletal myxoid CS might benefit from this therapy more.
Data Sharing Statement
We promised to cover patients’ data confidentially. But patients’ data will be made disguisedly available, including data dictionaries, for approved data sharing requests. Individual data will be shared that underlie the results reported in this Article, after de-identification and normalization of information (text, tables, figures, and appendices). The statistical analysis plan will also be available upon request. Anonymised data will be available beginning 3 months after and ending 2 years after publication of this Article to researchers after methodological review of the proposed analysis plan by the directors of Musculoskeletal Tumor center of Peking University People’s Hospital. Proposals should be directed to [email protected]. To gain access, data requestors will need to sign a data access agreement.
Acknowledgments
We thank Medlink (the third party for data analysis) for interpretation of all the clinical data and statistical analysis of this trial. We also thank LetPub for its linguistic assistance during the preparation of this manuscript.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Prof. Dr. Xiaodong Tang reports grants from The National Natural Science Foundation of China (No. 81972509) and the Beijing Science and Technology Planning Project (Fund No. Z171100001017097) during the conduct of the study. The authors report no other possible conflicts of interest in this work.
References
1. Nazeri E, Gouran Savadkoohi M, Majidzadeh AK, Esmaeili R. Chondrosarcoma: an overview of clinical behavior, molecular mechanisms mediated drug resistance and potential therapeutic targets. Crit Rev Oncol Hematol. 2018;131:102–109. doi:10.1016/j.critrevonc.2018.09.001
2. Polychronidou G, Karavasilis V, Pollack SM, Huang PH, Lee A, Jones RL. Novel therapeutic approaches in chondrosarcoma. Future Oncol. 2017;13(7):637–648. doi:10.2217/fon-2016-0226
3. van Maldegem AM, Gelderblom H, Palmerini E, et al. Outcome of advanced, unresectable conventional central chondrosarcoma. Cancer. 2014;120(20):3159–3164. doi:10.1002/cncr.28845
4. Italiano A, Mir O, Cioffi A, et al. Advanced chondrosarcomas: role of chemotherapy and survival. Ann Oncol. 2013;24(11):2916–2922. doi:10.1093/annonc/mdt374
5. DeLaney TF, Liebsch NJ, Pedlow FX, et al. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110(2):115–122. doi:10.1002/jso.23617
6. DeLaney TF, Liebsch NJ, Pedlow FX, et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74(3):732–739. doi:10.1016/j.ijrobp.2008.08.058
7. Nakashima Y, Unni KK, Shives TC, Swee RG, Dahlin DC. Mesenchymal chondrosarcoma of bone and soft tissue. A review of 111 cases. Cancer. 1986;57(12):2444–2453. doi:10.1002/1097-0142(19860615)57:12<2444::aid-cncr2820571233>3.0.co;2-k
8. Liu GT, Chen HT, Tsou HK, et al. CCL5 promotes VEGF-dependent angiogenesis by down-regulating miR-200b through PI3K/Akt signaling pathway in human chondrosarcoma cells. Oncotarget. 2014;5(21):10718–10731. doi:10.18632/oncotarget.2532
9. Sun X, Charbonneau C, Wei L, Yang W, Chen Q, Terek RM. CXCR4-targeted therapy inhibits VEGF expression and chondrosarcoma angiogenesis and metastasis. Mol Cancer Ther. 2013;12(7):1163–1170. doi:10.1158/1535-7163.MCT-12-1092
10. Ayala G, Liu C, Nicosia R, Horowitz S, Lackman R. Microvasculature and VEGF expression in cartilaginous tumors. Hum Pathol. 2000;31(3):341–346. doi:10.1016/s0046-8177(00)80248-8
11. Grignani G, Palmerini E, Stacchiotti S, et al. A phase 2 trial of imatinib mesylate in patients with recurrent nonresectable chondrosarcomas expressing platelet-derived growth factor receptor-alpha or -beta: an Italian Sarcoma Group study. Cancer. 2011;117(4):826–831. doi:10.1002/cncr.25632
12. Schuetze SM, Bolejack V, Choy E, et al. Phase 2 study of dasatinib in patients with alveolar soft part sarcoma, chondrosarcoma, chordoma, epithelioid sarcoma, or solitary fibrous tumor. Cancer. 2017;123(1):90–97. doi:10.1002/cncr.30379
13. Stacchiotti S, Ferrari S, Redondo A, et al. Pazopanib for treatment of advanced extraskeletal myxoid chondrosarcoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019;20(9):1252–1262. doi:10.1016/S1470-2045(19)30319-5
14. Chow W, Frankel P, Ruel C, et al. Results of a prospective phase 2 study of pazopanib in patients with surgically unresectable or metastatic chondrosarcoma. Cancer. 2020;126(1):105–111. doi:10.1002/cncr.32515
15. Jones RL, Katz D, Loggers ET, Davidson D, Rodler ET, Pollack SM. Clinical benefit of antiangiogenic therapy in advanced and metastatic chondrosarcoma. Med Oncol. 2017;34(10):167. doi:10.1007/s12032-017-1030-2
16. Duffaud F, Blay JY, Italiano A, et al. LBA88 - results of the randomized, placebo (PL)-controlled phase II study evaluating the efficacy and safety of regorafenib (REG) in patients (pts) with locally advanced (LA) or metastatic relapsed chondrosarcoma (CS), on behalf of the French Sarcoma Group (FSG) and UNICANCER. Ann Oncol. 2019;30:v926–v927. doi:10.1093/annonc/mdz394.088
17. Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. doi:10.1111/j.1349-7006.2011.01939.x
18. Xie L, Guo W, Wang Y, Yan T, Ji T, Xu J. Apatinib for advanced sarcoma: results from multiple institutions’ off-label use in China. BMC Cancer. 2018;18(1):396. doi:10.1186/s12885-018-4303-z
19. Verger E, Salamero M, Conill C. Can Karnofsky performance status be transformed to the Eastern Cooperative Oncology Group scoring scale and vice versa? Eur J Cancer. 1992;28A(8–9):1328–1330. doi:10.1016/0959-8049(92)90510-9
20. Kohlmann J, Kirsten H, Simon JC, Ziemer M. Refined common terminology criteria for adverse events criteria - respective systemic melanoma therapy. Melanoma Res. 2019;29(4):444–445. doi:10.1097/CMR.0000000000000554
21. Bovee JV, Cleton-Jansen AM, Taminiau AH, Hogendoorn PC. Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. Lancet Oncol. 2005;6(8):599–607. doi:10.1016/S1470-2045(05)70282-5
22. Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14(9):2519–2526. doi:10.1158/1078-0432.CCR-07-2223
23. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi:10.3322/caac.20073
24. Xu J, Li D, Xie L, Tang S, Guo W, Loeb DM. Mesenchymal chondrosarcoma of bone and soft tissue: a systematic review of 107 patients in the past 20 years. PLoS One. 2015;10(4):e0122216. doi:10.1371/journal.pone.0122216
25. Pansuriya TC, van Eijk R, d’Adamo P, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet. 2011;43(12):1256–1261. doi:10.1038/ng.1004
26. Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23(48):7906–7909. doi:10.1038/sj.onc.1208160
27. Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4(4):257–262. doi:10.1016/s1535-6108(03)00248-4
28. Amary MF, Damato S, Halai D, et al. Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nat Genet. 2011;43(12):1262–1265. doi:10.1038/ng.994
29. Bernstein-Molho R, Kollender Y, Issakov J, et al. Clinical activity of mTOR inhibition in combination with cyclophosphamide in the treatment of recurrent unresectable chondrosarcomas. Cancer Chemother Pharmacol. 2012;70(6):855–860. doi:10.1007/s00280-012-1968-x
30. Xie L, Xu J, Sun X, et al. Apatinib for advanced osteosarcoma after failure of standard multimodal therapy: an open label phase II clinical trial. Oncologist. 2019;24(7):e542–e550. doi:10.1634/theoncologist.2018-0542
31. Xie L, Xu J, Sun X, et al. Anorexia, hypertension, pneumothorax, and hypothyroidism: potential signs of improved clinical outcome following apatinib in advanced osteosarcoma. Cancer Manag Res. 2020;12:91–102. doi:10.2147/CMAR.S232823
32. Mi Y-J, Liang Y-J, Huang H-B, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70(20):7981–7991. doi:10.1158/0008-5472.CAN-10-0111
33. Iseulys R, Anne GB, Corinne B, et al. The immune landscape of chondrosarcoma reveals an immunosuppressive environment in the dedifferentiated subtypes and exposes CSFR1+ macrophages as a promising therapeutic target. J Bone Oncol. 2020;20:100271. doi:10.1016/j.jbo.2019.100271
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
