Back to Journals » International Journal of General Medicine » Volume 16
Anxiety Disorders and Gut Dysbiosis in Primary Sjögren’s Syndrome-Mediated Dry Eye Patients
Authors Zhang Y , Gan M, He Y, Liu T, Xu M
Received 18 January 2023
Accepted for publication 21 April 2023
Published 9 May 2023 Volume 2023:16 Pages 1735—1746
DOI https://doi.org/10.2147/IJGM.S405225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
Yiting Zhang, Meiqi Gan, Yuqin He, Tingting Liu, Mei Xu
The First Affiliated Hospital of Chongqing Medical University, Chongqing Key Laboratory of Ophthalmology, Chongqing Eye Institute, Chongqing Branch of National Clinical Research Center for Ocular Diseases, Chongqing Municipality Division, National Clinical Research Center for Ocular Diseases, Chongqing, People’s Republic of China
Correspondence: Mei Xu, The First Affiliated Hospital of Chongqing Medical University, Youyi Road 1, Chongqing, 400016, People’s Republic of China, Email [email protected]
Purpose: Primary Sjögren’s syndrome (pSS), a disease that is associated with a high prevalence of psychological disorders, has become increasingly important. Interactions between the gut microbiota and ocular conditions have been identified in pSS. As mental intervention is frequently needed, this study aims to investigate the relationship between anxiety disorders and the gut microbiome in patients with pSS-mediated dry eye.
Methods: Demographics and self-administered questionnaires were obtained. Faecal samples were evaluated using 16S ribosomal RNA gene sequencing.
Results: The Hospital Anxiety and Depression Scale (HADS-A) cut-off point of ≥ 8 points showed a sensitivity and specificity of 76.5% and 80.0%, respectively. In all participants, we found that the prevalence of anxiety disorder was 30.4%. Dry eye discomfort could promote an anxious state, and conversely, anxiety could threaten tear film and increase the risk of pSS activity. There was a certain correlation between anxiety disorder and gut dysbiosis. Prevotella was associated with dry eye severity (p < 0.001). Bacteroidetes (p =0.046) and Odoribacter (p =0.001) were correlated with pSS activity.
Conclusion: There is a bidirectional relationship between anxiety disorder and the gut microbiota in pSS-mediated dry eye. Alterations in certain classes of gut microbiota are associated with pSS activity and dry eye severity. Main gut microbiota alterations that have a facilitating impact on anxiety are emerging in pSS-mediated dry eye. Future studies are needed to explore specific therapeutic targets for improving mental health in pSS-mediated dry eye by microbiota intervention.
Keywords: primary Sjögren’s syndrome, dry eye, anxiety, gut microbiota
Introduction
Primary Sjögren’s syndrome (pSS) is a common systemic chronic autoimmune disease with a high prevalence of approximately 0.5–4% of the population.1 More than 6 million patients have been diagnosed in China.1 It is characterized by lymphocytic infiltrates associated with exocrine hypofunction and sicca symptoms.2 Among these symptoms, oral and ocular dryness are the most frequent complaints resulting from affection of the salivary gland and lacrimal gland. Approximately 25% of pSS individuals suffer severe systemic manifestations that may affect quality of life, including both physical and psychological aspects, and even have some social effects. Compared with patients with simple environmental dry eye, it is more difficult to provide management options for patients with pSS combined with dry eye.3 Although current treatment methods have improved ocular discomfort and visual impairment in pSS-related dry eye, disease-related risk factors remain difficult to manage.
An increased incidence of psychiatric disorders has been observed in individuals with pSS compared with the healthy population.4,5 Anxiety is a very common distress symptom in pSS patients and may lead to continuous fatigue, loss of physical function, and excess health costs.5,6 The pSS patients with mental distress have the possibility of cognitive dysfunction, which may be an important reason for their work disability and decreased quality of life. Therefore, more attention should be given to psychological disorders, and anxiety may be an effective intervention target to improve the subjective health and quality of life of pSS patients.
Alterations in the composition of the gut microbiome play a significant role in human autoimmune diseases. In a homeostatic state, human commensal microbe communities increase the stability of the host in a plethora of biological processes. Recently, interactions between gut microbiota and ocular mucosal immunity have been identified in a variety of ocular diseases, including pSS.7 Advances in the microbiome have the potential to fill the management gap in pSS. In addition, studies have revealed that gut dysbiosis may have important functions in disease manifestation, severity, and therapeutic responsiveness. In both the pSS mouse model and pSS patients, alterations in the gut microbiota led to the deterioration of ocular mucosal diseases, in which the relative abundance of commensal microbes is decreased, while that of pathobionts is increased.8
Although the understanding of autoimmune diseases has been improving, the relationship between gut microbiota and mental distress in pSS patients with dry eye is still poorly understood. We hypothesized that the gut microbiota composition plays a crucial role in pSS and mental health regulation. The purpose of this study was to investigate the interactions between anxiety behaviour, gut microbiota and pSS-induced dry eye. Furthermore, by assessing the differences in gut microbiota composition, we hope to uncover possible treatments that improve mental health via microbiotic approaches in autoimmune-mediated dry eye individuals.
Materials and Methods
Eligibility
This study was conducted in the Ophthalmology Department of the First Affiliated Hospital of Chongqing Medical University. From Jan 2019 to Jan 2022, we enrolled 56 pSS individuals with dry eye. All patients were diagnosed according to the 2016 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria.9 Patients with secondary Sjögren’s syndrome were excluded. The diagnosis of dry eye symptoms in pSS participants was made by the same academic ophthalmology clinician (Mei Xu) based on a combination of dry eye symptoms, signs, and clinical examination according to the updated diagnostic guidelines.7 These patients underwent follow-up medical management in the same hospital. The pSS participants with dry eye were divided into two groups based on HADS-A scores. The first group was composed of participants with HADS-A scores of 0–7 (normal). The second group consisted of subjects with a HADS-A score of 8–21 (presence of the anxious state). None of the patients had undergone abdominal surgery with bowel resection. Any patient who had received antibiotics within 1 week prior to faecal sampling was not selected. All subjects were of Han nationality from southwestern provinces of China. The age for inclusion was over 18 years old.
Clinical Information
Medical records of pSS patients were reviewed by trained and certified clinicians to collect relevant information. The basic clinical features of all participants were recorded, including sex, age, body mass index (BMI), education duration, smoking status, periodic exercise, sleep time, family history, pSS duration, other systemic diseases, ophthalmology intervention and laboratory tests. Laboratory tests included IgG levels, anti-SSA (anti–Sjögren’s-syndrome-related antigen A) antibodies, anti SSB (anti–Sjögren’s-syndrome-related antigen B) antibodies, antinuclear antibodies (ANA), rheumatoid factor (RF), and complement levels (complement component 3 and 4, C3 and C4). All laboratory analyses were performed in the Clinical Laboratory Department in our hospital.
The questionnaires were explained to each eligible subject by a trained clinician. All participants completed self-administered questionnaires without any interaction with clinicians. Questionnaires included the European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index (ESSDAI),10 European League Against Rheumatism Sjögren’s Syndrome Patient Reported Index (ESSPRI),11 the State Anxiety subscale of the State-Trait Anxiety Inventory,12 the HADS,13 and the Ocular Surface Disease Index (OSDI).14 The State-Trait Anxiety Inventory (STAI) was used to screen anxious states across 40 items and attitudes related to trait anxiety and state anxiety. Since the State-subscale (STAI-S) registered state anxiety rather than general anxiety disorder, it was used as the gold standard for the HADS-A. The cut-off value of STAI-S diagnostic anxiety was set to be 1.5 standard deviations greater than the normative sample. Validation in Chinese patients was performed in 2012.15 The OSDI subscale scores can be categorized according to the guidelines.16
Dry Eye Analysis
Tear break-up time (TBUT) was a clinical test for tear film. In testing for TBUT, a fluorescein paper strip was used for corneal staining. The participants were asked not to blink until tiny dry spots developed under the slit lamp. The shorter it takes, the less stable the tear film. A TBUT less than 10 seconds was considered abnormal. A TBUT of 5–10 seconds was regarded as borderline dry eye. A TBUT of less than 5 seconds was thought to indicate a high possibility of dry eye.
The Schirmer test was used to measure aqueous tear production. Sterile paper strips were applied to the inferior-temporal aspect of the conjunctival sac of both eyes. The wetted millimetres were measured at 5 minutes. A Schirmer's test score less than 5 mm without any topical anaesthesia was considered abnormal.
Intestinal Microbiota Evaluation
Faecal samples were collected from all individuals. Standardized methods for collection, preservation, sequencing, and taxonomic assignment were performed in these patients. Participants were provided faecal sample kits for home collection and stored in a freezer. A team of two research assistants collected faecal samples from each participant and stored them at −80 °C. DNA isolation of faecal samples was performed using the AllPrep DNA/RNA Mini Kit (Qiagen, Venlo, the Netherlands).
Polymerase chain reaction (PCR) was performed using extracted DNA and bacterial PCR primers. The bacterial PCR primers 515F (5’-GTGCCAGCMGCCGCGGTAA-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) contained targeted v4 variable regions of the 16S rRNA gene (Illumina, San Diego, CA).1 Gel electrophoresis was used to quantify each sample (Bio-Rad, Hervules, CA, USA). Products were purified and evaluated by a 2100 Bioanalyzer (Agilent, Santa Clara, CA) and then sequenced by a MiSeq system (Illumina, San Diego, CA). Clustering of the sequences with custom primers on the MiSeq instrument was performed (Illumina, San Diego, CA). The EzBioCloud database was used for taxonomic classification after a chimaera check.17
Statistical Analyses
All statistical analyses were performed using SPSS software version 25.0 (SPSS Inc., Chicago, IL, USA). A receiver operator characteristic (ROC) curve was generated to determine the optimal threshold scores. The cut-off for anxiety diagnosis in this study was set as ≥8 points. The ability of the HADS-A was evaluated according to ROC curves. For demographics and characteristics comparison among groups, quantitative variables were expressed as the means ± SDs, while the number of each category was also calculated. A logistic regression model was used to estimate the potential risk of anxiety in pSS subjects with dry eye symptoms. For comparison analysis of the gut microbiome among groups, the Wilcoxon rank-sum test was performed. Alpha diversity and beta diversity were calculated by Quantitative Insights Into Microbial Ecology 2.0 (QIIME).18 All p values < 0.05 were considered statistically significant.
Results
Demographics and Characteristics of Anxiety and Nonanxiety Patients with pSS-Mediated Dry Eye
A total of 56 pSS-mediated dry eye patients were enrolled in the current study. The area under the curve was higher than 0.7 (0.6–0.8) for the HADS-A scale (95% CI 0.56–0.90). The HADS-A cut-off point of ≥ 8 points showed a sensitivity and a specificity of 76.5% and 80.0%, respectively.
The demographics and clinical characteristics of the subjects were summarized considering that the HADS-A cut-off was ≥ 8 points (Table 1). In these participants, the prevalence of anxiety disorder was 30.4%. The mean age of these individuals was 55.3±4.2 years old. More females were observed than males, and the ratio of females/males was 13:1 in all subjects. Compared with the nonanxiety group, the anxiety group was characterized by a significantly lower education level (p =0.014), longer pSS duration (p =0.017), higher ESSDAI score (p =0.022), and higher OSDI score category (p =0.032). No statistical significance was observed in age, gender, BMI, smoking status, periodic exercise, sleep time, family history, other systemic disease, ophthalmic medical survey, ESSPRI score, and laboratory test between the two groups.
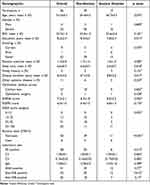 |
Table 1 Clinical Demographics and Characteristics |
Anxiety Behaviour Was Associated with pSS Activity and Ocular Surface Severity
Dry eye severity was evaluated among groups. The anxiety group showed significantly higher OSDI scores (p < 0.001, Figure 1A), lower basal and reflex tear secretion (p < 0.001, Figure 1B) and shorter TBUT (p < 0.001, Figure 1C) than the nonanxiety group. Potential risk factors for anxiety are shown in Table 2. A shorter education duration (OR, 7.781; 95% CI, 1.096–55.23; p =0.04) was significantly correlated with an anxious state. Participants with a pSS duration of <8 years (OR, 0.028; 95% CI, 0.003–0.31; p =0.004) or ESSDAI score <7 units (OR, 0.081; 95% CI, 0.011–0.614; p =0.015) had a lower incidence of anxiety. No statistically significant effect was observed for other potential risk factors.
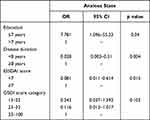 |
Table 2 Multivariate Logistic Regression of Risk Factors for pSS Dry Eye Patients with Anxiety Disorder |
Gut Microbial Landscape in pSS-Mediated Dry Eye Patients With/Without Anxiety
Profiles of the relative gut microbiome composition at the phylum and genus levels were similar between the nonanxiety and anxiety groups. The Chao1 richness index and Shannon diversity index analysis showed no significant difference (p >0.05, Figure 2). No specific anxiety disorder pattern was observed in gut samples from all participants by principal coordinate analysis (PCoA). The gut microbiota composition profiles at the phylum and genus levels showed disease-specific patterns (Figure 3A and B). Firmicutes was the major phylum in the gut samples of pSS subjects with dry eye, composing between 42.4–72.1% of all phyla (Figure 3C). Bacteroides and Faecalibacterium were the dominant genera in all subjects (Figure 3D). A lower Firmicute/Bacteroidetes (F/B) ratio was observed in anxious participants (p =0.027, Figure 3E). The anxiety group had a significant expansion of Bacteroides (p =0.011) and a depletion of Actinobacteria (p =0.001) compared with the nonanxiety group (Figure 3F and G). The relative abundances of Bifidobacterium (p =0.002) and Odoribacter (p <0.001) were elevated in the anxiety state (Figure 3H and I). However, the abundance of the genus Prevotella (p =0.001) was significantly less enriched in the anxiety group than in the nonanxiety group (Figure 3J).
 |
Figure 2 Species richness (A) and Shannon diversity analysis (B) between non-anxiety and anxiety groups. |
The Relationship Between Anxiety and Gut Microbiota in pSS-Mediated Dry Eye Patients
Further univariate linear regression analysis revealed that eye indices in pSS patients were significantly correlated with the gut microbiota. ESSDAI scores were positively correlated with the phylum Bacteroidetes (p =0.046) and negatively correlated with the genus Odoribacter (p =0.001, Figure 4A). The OSDI score category was positively correlated with the genus Prevotella (p <0.001) (Figure 4B). The phylum Actinobacteria and genus Bifidobacterium were not significantly correlated with scores on the ESSDAI or OSDI (Figure 4).
Discussion
The alterations in the composition of the gut microbiome show the possibility of a functional role in human autoimmune diseases. In this study, negative emotional states were commonly found in pSS patients with dry eye. Despite growing public concern regarding potential therapeutic options for the gut microbiome from the clinic to the laboratory, less information is available on the correlation between gut dysbiosis and mental distress in pSS-mediated dry eye individuals. We summarize the potential risk factors in pSS-mediated dry eye patients with anxiety. Then, we describe the alterations in gut microbiota components in pSS-induced dry eye participants with anxiety. Finally, we analysed the interactions between anxiety disorder and the gut microbiota.
First, the HADS-A can be used as an emotional state screening tool for identifying the presence of anxiety disorder. The sensitivity and specificity of the HADS-A subscale are similar to values published by Hall et al and Kugaya et al19,20 An optimal threshold for determining cases and noncases by the HADS-A is necessary. However, no agreement existed in previous studies. According to the clinical results, the HADS-A cut-off point of ≥ 8 points was similar to that of Abiodum et al and Razavi et al21,22 The HADS-A is a sensitive instrument with good consistency and reliability for screening anxiety in pSS patients with dry eye.
Chronic discomfort and pain caused by pSS may have negative impacts on the health of patients, including cognitive processes, psychological health, and overall quality of life.23 Patients with overlapping ocular surface discomfort or psychiatric diseases have more frequent chronic pain complaints and report more severe symptoms.24,25 Dry eye condition is the main symptom of ocular surface discomfort and an important cause of visual disturbance that affects the physical, social, and psychological functions, daily activities and work efficiency of patients.26–28
Psychological disorders are more likely to be associated with sicca symptoms but not with sicca sighs, indicating that somatization increases the perception of ocular discomfort induced by dry eye in these individuals.29,30 Galor et al reported that patients in an anxious state are more likely to be affected by central sensitization, which causes frequent complaints of ocular surface discomfort.31 The relationship between mental distress and dry eye condition is a two-way street. Dry eye discomfort can promote mental distress, and conversely, anti-anxiolytic and antidepressant medications for mental disorders can damage tear film status and increase the risk of dry eye symptoms through anti-cholinergic mechanisms.32,33 The new anti-anxiolytic, selective serotonin reuptake inhibitors (SSRIs) have no anticholinergic adverse effects. Patients who use SSRIs still have been shown to have an increased risk of dry eye syndrome, independent of the duration of SSRI dosage.34 This association may contribute to the high prevalence of anxiety in pSS patients with dry eye. Cui et al revealed that the prevalence of anxiety in Chinese pSS patients was 33.8% in 2018.35 We found a similar pattern of anxiety disorder in pSS-mediated dry eye patients. It is not practical to screen all these patients for anxiety, but focusing on individuals with these risk factors may be under an anxious state. This is of clinical importance because ophthalmology, rheumatology, and psychology clinicians need to be more aware of early mental care for pSS dry eye patients with potential risks, including lower education level, longer pSS disease duration, and higher ESSDAI and OSDI scores. Studies with longer follow-up are warranted to further understand the currently reported associations with pSS-mediated dry eye.
Many recent studies have described that the gut microbiota influences many aspects of human health, including autoimmune and psychological diseases, in all stages of life.36,37 The alteration of gut microbiota components contributes to human mood and behaviour disorders, including anxiety and stress, indicating a bidirectional relationship between gut microbiota and mental health. The mechanisms, including the gut microbiome generating short-chain fatty acids, tryptophan catabolites, and microbiota intervention, are associated with mediating the gut microbiota to mental health.38
Recent studies have reported that anxiety patients show a certain profile of gut microbiota alterations. Differences in the two major phyla, Bacteroidetes and Firmicutes and Actinobacteria, were reported.17,39,40 Anxiety in pSS-mediated dry eye can induce an imbalance in microbial diversity and richness, which decreases Bacteroidetes and increases Firmicutes and Actinobacteria. The important effect of Bacteroidetes on gut dysbiosis through T-helper 1 (Th1)-induced immune responses confirmed that stronger immune responses were present in anxious participants. This imbalance changes the immunological response. Additionally, the Actinobacteria phylum is a commensal gut bacterium enriched in healthy subjects that presents beneficial functions in intestinal health by mediating bile acid and pathogen virulence.41 However, the results are not consistent with a previous study. Zheng et al showed similar changes in Bacteroidetes phyla and Actinobacteria phyla, but no alteration was found in Firmicutes phyla.42 These conflicting findings may be explained by the lack of control of diet for these participants before collecting the faecal sample. Since diet quality and patterns have an important impact on the components of the gut microbiota, they may contribute to the lack of consistency among these studies.
Since region of residence and ethnic background can influence the results of gut microbiota analysis, the cases included in this study were selected from similar geographic areas, and all were in the Chinese Han population. A specific proportion of differences in gut bacterial composition were observed among the subjects, which may be related to a strong female predisposition. However, the gut microbiota component varies among individuals. Certain autoimmune diseases have been associated with specific patterns of gut microbiota. Additionally, biologic variations are more important than possible factors, including geography, ethnicity, sex, age, technology, diet or medication differences.43 The above biological factors showed characteristic alterations in gut bacterial composition among the pSS group and the population control group. Thus, pSS activity and ocular surface severity are considered to be the main biological factors contributing to alterations in the gut microbiota.
As approaches to treat phenotypes of autoimmune diseases, diet modification, probiotics, prebiotics and faecal microbial transplantation (FMT) are currently explored strategies to restore a healthy gut microbiome. Animal studies showed that mice on a high-fat diet presented severe dry eye, increased inflammation and decreased lacrimal gland function.44 Multivariate analysis showed that vegetable and fish consumption decreased the odds of having Sjögren’s syndrome.45 Western-style diets and decreased prebiotic consumption have been associated with an increasing incidence of anxiety and stress.46 FMT has been used as a therapy for improving dry eye symptoms in autoimmune diseases.47 With FMT being successful in a number of autoimmune diseases, gut microbiome alteration by FMT has opened up more possibilities in various diseases. Studies have found that FMT can transfer behavioural phenotypes, including anxiety. More information is needed on how diet affects the gut microbiota in autoimmune diseases.
Among the Actinobacteria phylum, the relative composition of Bifidobacterium is reduced in chronic inflammatory states.48 This trend is also observed in anxious pSS-induced dry eye patients. Fructooligosaccharides and galactooligosaccharides are commonly studied prebiotics that show increased richness in Bifidobacterium. Bifidobacterium can generate B vitamins, antioxidants, polyphenols and immunoglobulins to promote immune system function.42,49,50 Moreover, Bifidobacterium produces lactate and acetate, which can be utilized by butyrate-producing gut bacteria to produce butyrate. This phenomenon is a cross-feeding interaction between Bifidobacterium and other gut bacteria that has a butyrogenic effect. This effect contributes to maintaining intestinal barrier by regulating immunomodulation and anti-inflammation, providing health benefits to the host.51,52 Although Bifidobacterium is typically a small proportion of the human gut microbial community, it has been reported to be correlated with mental health improvement. A probiotic strain may influence mood and behaviour by regulating the central nervous system and immune system. Furthermore, prebiotics have been presented as a way to reduce anxiety and depressive behaviour by changing general hypothalamic neuronal activity and inducing brain-derived neurotrophic factor expression.46 A double-masked study revealed that probiotic supplementation significantly improved RA activity and decreased the concentrations of inflammatory markers.53 It is increasingly recognized that diet modification has an important role in health by affecting gut microbiome composition. There is probably a correlation between specific diets and the severity of autoimmune disease.
Reduced levels of the genus Odoribacter are observed in pSS-mediated dry eye patients under anxiety disorders, which is consistent with patients with SLE.54 Notably, levels of the genus Prevotella, which has an important function in the pathogenesis of RA, were typically higher in anxious cases than in controls. Elevated levels of Prevotella may be involved in pSS-mediated inflammatory pathogenesis, which has a negative effect on mental health. This finding is consistent with a previous study in the Korean population17 but differs from cases in the American population.8 A high-fibre diet has a favourable effect on human health and is strongly related to higher richness of the Prevotella genus in the gut microbiota.55 Koreans and Chinese people have similar dietary habits. More vegetable diets in Koreans and Chinese than American may explain the different composition proportions of the genus Prevotella in these different geographic populations. The most appropriate dietary intervention to assess mental distress in pSS-associated dry eye patients from a prognostic perspective is unknown. Further prospective studies on gut microbiota in pSS patients with dry eye under mental disorders should be conducted to provide effective clinical interventions.
The present study has several limitations. The small number of cases in the participants. The numbers of patients in the anxiety group and nonanxiety group were not equal. Antidepressant management was not controlled. The results of gut microbiota component analysis may not be perfectly reflected in the anxiety and nonanxiety groups. Some patients were under pressure or stress before receiving psychological diagnosis. This led to the possibility that the level of anxious state of the participants was overestimated at the time of recruitment. The ESSDAI, ESSPRI and OSDI are used to reflect current pSS activity and dry eye severity. Participants were not required to complete lacrimal gland biopsy to confirm the activity or chronicity of pSS.
Differences in gut microbiota composition have been investigated in pSS-mediated dry eye patients with anxiety behaviour compared with those without anxiety behaviour. Mental distress contributes to more severe gut dysbiosis in pSS-related dry eye patients. The alteration of certain gut microbiota components has been found to be associated with psychiatric disorders. However, discrepancies were presented in studies regarding alpha diversity and microbiota compositional differences. Dietary supplements can prevent or restore symptoms and signs of anxiety behaviour and pSS-mediated dry eye severity. Intervention in the gut microbiota may be a potential approach to have health benefits for the host. While these results are promising, whether these findings will translate into improving the behaviour of mental disorders or the phenotype of pSS-mediated dry eye is unclear. Further large studies across diverse populations on microbiota in autoimmune disease are needed. Gut microbiota modulation may be a potential therapy for pSS-mediated dry eye patients with anxiety behaviour.
Conclusions
Taken together, this study described the complex interactions between anxiety behaviour, gut microbiota, and pSS-mediated dry eye disease. Mental health is important for immune system function and ocular surface stability to promote the behaviour and phenotype of pSS-mediated dry eye. Gut dysbiosis may act as a pathogenic factor involved in the mood and behaviour of autoimmune diseases. Psychometric tests may provide relevant information for helping to predict pSS activity and ocular surface conditions in individuals. Alterations in specific profiles of the gut microbiota in pSS-mediated dry eye patients with anxiety behaviour were found. Clinicians need to have increased awareness of mental disorders when managing pSS-mediated dry eye patients. Early identification and intervention of gut dysbiosis may partially restore the adverse effects of pSS-mediated dry eye. Appropriate gut microbiota intervention may have beneficial effects on mitigating symptoms and signs of pSS-mediated dry eye under mental distress. However, the mechanism of the gut microbiota in pSS dry eye patients with anxiety disorder is less well understood. Future studies are important to identify the biological roles of the optimal microbiota composition in various populations.
Ethics Approval and Informed Consent
The study was approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University and adhered to the ethical standards of Declaration of Helsinki and its later amendments or comparable ethical standards. All the patients completed written informed consent. All the participating patients were fully informed and participated in the study voluntarily. We have obtained informed consents from all the study participants prior to study commencement.
Funding
The study was funded by the National Natural Science Foundation of China (81900887), and Basic science and frontier technology research in Chongqing (cstc2017jcyjAX0447).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fox RI. Sjogren’s syndrome. Lancet. 2005;366(9482):321–331. doi:10.1016/S0140-6736(05)66990-5
2. Mariette X, Criswell LA. Primary Sjogren’s Syndrome. N Engl J Med. 2018;378(10):931–939. doi:10.1056/NEJMcp1702514
3. Foulks GN, Forstot SL, Donshik PC, et al. Clinical guidelines for management of dry eye associated with Sjogren disease. Ocul Surf. 2015;13(2):118–132. doi:10.1016/j.jtos.2014.12.001
4. Anyfanti P, Pyrpasopoulou A, Triantafyllou A, et al. Association between mental health disorders and sexual dysfunction in patients suffering from rheumatic diseases. J Sex Med. 2014;11(11):2653–2660. doi:10.1111/jsm.12672
5. Kocer B, Tezcan ME, Batur HZ, et al. Cognition, depression, fatigue, and quality of life in primary Sjogren’s syndrome: correlations. Brain Behav. 2016;6(12):e00586. doi:10.1002/brb3.586
6. Inal V, Kitapcioglu G, Karabulut G, Keser G, Kabasakal Y. Evaluation of quality of life in relation to anxiety and depression in primary Sjogren’s syndrome. Mod Rheumatol. 2010;20(6):588–597. doi:10.3109/s10165-010-0329-z
7. Tsigalou C, Stavropoulou E, Bezirtzoglou E. Current insights in microbiome shifts in Sjogren’s Syndrome and possible therapeutic interventions. Front Immunol. 2018;9:1106. doi:10.3389/fimmu.2018.01106
8. de Paiva CS, Jones DB, Stern ME, et al. Altered mucosal microbiome diversity and disease severity in Sjogren syndrome. Sci Rep. 2016;6:23561. doi:10.1038/srep23561
9. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76(1):9–16. doi:10.1136/annrheumdis-2016-210571
10. Seror R, Ravaud P, Bowman SJ, et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69(6):1103–1109. doi:10.1136/ard.2009.110619
11. Seror R, Ravaud P, Mariette X, et al. EULAR Sjogren’s Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjogren’s syndrome. Ann Rheum Dis. 2011;70(6):968–972. doi:10.1136/ard.2010.143743
12. Spielberger CD. State-Trait Anxiety Inventory (form Y). Mind Garden; 1983.
13. Snaith RP. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes. 2003;1:29. doi:10.1186/1477-7525-1-29
14. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615
15. Zhang J, Gao Q. Validation of the trait anxiety scale for state-trait anxiety inventory in suicide victims and living controls of Chinese rural youths. Arch Suicide Res. 2012;16(1):85–94. doi:10.1080/13811118.2012.641440
16. Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94–101. doi:10.1001/archophthalmol.2009.356
17. Moon J, Choi SH, Yoon CH, Kim MK. Gut dysbiosis is prevailing in Sjogren’s syndrome and is related to dry eye severity. PLoS One. 2020;15(2):e0229029. doi:10.1371/journal.pone.0229029
18. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi:10.1038/nmeth.f.303
19. Hall A, A’Hern R, Fallowfield L. Are we using appropriate self-report questionnaires for detecting anxiety and depression in women with early breast cancer? Eur J Cancer. 1999;35(1):79–85. doi:10.1016/S0959-8049(98)00308-6
20. Kugaya A, Akechi T, Okuyama T, Okamura H, Uchitomi Y. Screening for psychological distress in Japanese cancer patients. Jpn J Clin Oncol. 1998;28(5):333–338. doi:10.1093/jjco/28.5.333
21. Abiodun OA. A validity study of the hospital anxiety and depression scale in general hospital units and a community sample in Nigeria. Br J Psychiatry. 1994;165(5):669–672. doi:10.1192/bjp.165.5.669
22. Razavi D, Delvaux N, Farvacques C, Robaye E. Screening for adjustment disorders and major depressive disorders in cancer in-patients. Br J Psychiatry. 1990;156:79–83. doi:10.1192/bjp.156.1.79
23. Fine PG. Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med. 2011;12(7):996–1004. doi:10.1111/j.1526-4637.2011.01187.x
24. Galor A, Covington D, Levitt AE, et al. Neuropathic ocular pain due to dry eye is associated with multiple comorbid chronic pain syndromes. J Pain. 2016;17(3):310–318. doi:10.1016/j.jpain.2015.10.019
25. Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, Galor A. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol. 2017;101(2):227–231. doi:10.1136/bjophthalmol-2015-308214
26. Messmer EM. Neue Therapieoptionen für das Trockene Auge. [Dry eye - functional visual acuity and quality of life are affected]. Ophthalmologe. 2014;111(5):411. German.
27. Grubbs JR
28. Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1(2):51–57. doi:10.1007/s40135-013-0009-1
29. Kim KW, Han SB, Han ER, et al. Association between depression and dry eye disease in an elderly population. Invest Ophthalmol Vis Sci. 2011;52(11):7954–7958. doi:10.1167/iovs.11-8050
30. Labbe A, Wang YX, Jie Y, Baudouin C, Jonas JB, Xu L. Dry eye disease, dry eye symptoms and depression: the Beijing Eye Study. Br J Ophthalmol. 2013;97(11):1399–1403. doi:10.1136/bjophthalmol-2013-303838
31. Galor A, Felix ER, Feuer W, et al. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol. 2015;99(8):1126–1129. doi:10.1136/bjophthalmol-2014-306481
32. Moss SE, Klein R, Klein BE. Long-term incidence of dry eye in an older population. Optom Vis Sci. 2008;85(8):668–674. doi:10.1097/OPX.0b013e318181a947
33. Wong J, Lan W, Ong LM, Tong L. Non-hormonal systemic medications and dry eye. Ocul Surf. 2011;9(4):212–226. doi:10.1016/S1542-0124(11)70034-9
34. Kocer E, Kocer A, Ozsutcu M, Dursun AE, Krpnar I. Dry eye related to commonly used new antidepressants. J Clin Psychopharmacol. 2015;35(4):411–413. doi:10.1097/JCP.0000000000000356
35. Cui Y, Xia L, Li L, Zhao Q, Chen S, Gu Z. Anxiety and depression in primary Sjogren’s syndrome: a cross-sectional study. BMC Psychiatry. 2018;18(1):131. doi:10.1186/s12888-018-1715-x
36. Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9(10):577–589. doi:10.1038/nrgastro.2012.156
37. Lyte M. Microbial endocrinology and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:3–24.
38. Peirce JM, Alvina K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97(10):1223–1241. doi:10.1002/jnr.24476
39. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi:10.1016/j.bbi.2015.03.016
40. Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26(8):1155–1162. doi:10.1111/nmo.12378
41. Jeong Y, Kim JW, You HJ, et al. Gut microbial composition and function are altered in patients with early rheumatoid arthritis. J Clin Med. 2019;8:5. doi:10.3390/jcm8050693
42. Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21(6):786–796. doi:10.1038/mp.2016.44
43. van der Meulen TA, Harmsen HJM, Vila AV, et al. Shared gut, but distinct oral microbiota composition in primary Sjogren’s syndrome and systemic lupus erythematosus. J Autoimmun. 2019;97:77–87. doi:10.1016/j.jaut.2018.10.009
44. He X, Zhao Z, Wang S, et al. High-fat diet-induced functional and pathologic changes in lacrimal gland. Am J Pathol. 2020;190(12):2387–2402. doi:10.1016/j.ajpath.2020.09.002
45. Machowicz A, Hall I, de Pablo P, et al. Mediterranean diet and risk of Sjogren’s syndrome. Clin Exp Rheumatol. 2020;38(4):216–221.
46. Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12(5):303–310. doi:10.1038/nrgastro.2015.47
47. Watane A, Cavuoto KM, Rojas M, et al. Fecal microbial transplant in individuals with immune-mediated dry eye. Am J Ophthalmol. 2022;233:90–100. doi:10.1016/j.ajo.2021.06.022
48. Zhong D, Wu C, Zeng X, Wang Q. The role of gut microbiota in the pathogenesis of rheumatic diseases. Clin Rheumatol. 2018;37(1):25–34. doi:10.1007/s10067-017-3821-4
49. Gagnon M, Savard P, Riviere A, LaPointe G, Roy D. Bioaccessible antioxidants in milk fermented by Bifidobacterium longum subsp. longum strains. Biomed Res Int. 2015;2015:169381. doi:10.1155/2015/169381
50. Barka EA, Vatsa P, Sanchez L, et al. Taxonomy, physiology, and natural products of actinobacteria. Microbiol Mol Biol Rev. 2016;80(4):iii. doi:10.1128/MMBR.00044-16
51. Riviere A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi:10.3389/fmicb.2016.00979
52. Mandl T, Marsal J, Olsson P, Ohlsson B, Andreasson K. Severe intestinal dysbiosis is prevalent in primary Sjogren’s syndrome and is associated with systemic disease activity. Arthritis Res Ther. 2017;19(1):237. doi:10.1186/s13075-017-1446-2
53. Zamani B, Golkar HR, Farshbaf S, et al. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. Int J Rheum Dis. 2016;19(9):869–879. doi:10.1111/1756-185X.12888
54. Luo XM, Edwards MR, Mu Q, et al. Gut microbiota in human systemic lupus erythematosus and a mouse model of lupus. Appl Environ Microbiol. 2018;84:4. doi:10.1128/AEM.02288-17
55. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi:10.1126/science.1208344
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



