Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Anxiety as a Risk Factor for Acute Mountain Sickness Among Young Chinese Men After Exposure at 3800 M: A cross‒sectional Study
Authors Tang X, Li X, Xin Q, Wang Q, Li S, Yang Y
Received 21 August 2023
Accepted for publication 16 November 2023
Published 28 November 2023 Volume 2023:19 Pages 2573—2583
DOI https://doi.org/10.2147/NDT.S436438
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Xugang Tang,1,2 Xiuchuan Li,1 Qian Xin,3 Qiang Wang,1 Shuang Li,1 Yongjian Yang1
1Department of Cardiology, The General Hospital of Western Theater Command, Chengdu, Sichuan, People’s Republic of China; 2Department of Cardiology, The No. 37 Hospital of Chinese PLA, Ya’an, Sichuan, People’s Republic of China; 3Department of Cardiology, The Sixth Medical Center of Chinese PLA General Hospital, Beijing, People’s Republic of China
Correspondence: Yongjian Yang, Department of Cardiology, The General Hospital of Western Theater Command, Chengdu, Sichuan, People’s Republic of China, Tel +86 28 8657 1255, Fax +86 28 8657 1255, Email [email protected]
Purpose: We aimed to explore whether anxiety is a risk factor for acute mountain sickness [AMS] in a young Chinese male population.
Patients and Methods: A total of 143 young Chinese men with a median age of 23 years (IQR, 21– 25) were employed in the present study, and they were divided into the AMS+ and AMS- groups according to the Lake Louise AMS score [AMS-S] after exposure at 3800 m for two days. Participants’ pulse oximeter saturation [SpO2] and heart rate [HR] were measured. AMS was evaluated using the AMS-S. The anxiety and sleep quality of the subjects were assessed using the Zung Self-Rating Anxiety Scale [SAS] and the Athens Insomnia Scale [AIS], respectively. Outcomes were analysed using Spearman’s partial correlation and logistic regression analysis.
Results: After two days of exposure at 3800 m, the overall prevalence of AMS was 54% in the whole group. The HR was significantly higher in the AMS+ group than in the AMS- group, as well as the SAS score and AIS score. A converse pattern was observed for SpO2. A significant difference was observed for the change in SAS and AIS score between the AMS+ and AMS- groups. Correlation analysis showed that AMS-S was positively correlated with SAS score, AIS score, HR, ΔSAS score, ΔAIS score, and ΔHR but negatively correlated with SpO2. AIS score was positively correlated with SAS score. After logistic regression analysis was adjusted for HR, SpO2, ΔAIS and ΔHR, SAS score (OR=1.446, 95% CI 1.200– 1.744, p< 0.001), AIS score (OR=1.216, 95% CI 1.033– 1.432) and ΔSAS score (OR=1.158, 95% CI 1.012– 1.327) were identified as independent risk factors for AMS.
Conclusion: The present study suggests that anxiety is a risk factor for AMS among young Chinese men, and poor sleep quality may partially mediate the association.
Keywords: anxiety, acute mountain sickness, high altitude, sleep
Introduction
Rapid ascent to altitudes above 2500 m may lead to acute mountain sickness [AMS],1 which is a common syndrome including headache, dizziness or lightheadedness, gastrointestinal symptoms (anorexia, nausea, or vomiting), weakness or fatigue, and insomnia.2 Although AMS is generally self-limited, it can develop into life-threatening high-altitude pulmonary or/and cerebral oedema.3 In addition, the precise pathogenesis of AMS remains incompletely understood.4 Therefore, early recognition of risk factors and prevention of AMS are especially important. Identified risk factors for AMS are the altitude reached, fast ascent, lack of preacclimatization, and a history of AMS.5 Recent studies have demonstrated that AMS is associated with lower pulse pressure, lower effective arterial elastance,6 right ventricular outflow tract notching,7 lower lateral mitral valve tissue motion annular displacement at sea level,8 and larger pulse oximeter saturation reduction after exercise at low altitude among young adults.9 A nested case-referent study among miners has shown that past AMS and current smoking are strong risk factors for severe AMS.10 A review article has highlighted that long-term exposure to high altitude results in adverse skeletal effects (eg, decrease bone turnover, reduce bone mineral density).11 Recent studies further suggested that the anxiety of subjects may be involved in the development of AMS.12,13 Studies have suggested a reciprocal relationship between anxiety and high-altitude-related diseases, whereby aggravating high-altitude-related symptoms and AMS can lead to greater anxiety and vice versa.14,15 Studies have also revealed that there is a potential link between anxiety at low altitudes and the development of AMS at high altitudes.16 High altitude-related symptoms such as insomnia and fatigue are more common with increasing altitude and potential triggers for increasing anxiety, which may account for the association between anxiety and AMS.17 Therefore, early recognition and intervention of anxiety may be helpful in decreasing AMS prevalence.
With the development of West China, many people, especially the Chinese young men, ascend to high altitudes for various activities (eg, work, travel), which is becoming increasingly frequent.18,19 However, no previous studies have investigated the relationship between AMS and anxiety among Chinese young men.
Given the association between anxiety and AMS, as well as the high prevalence of anxiety in young Chinese men,20,21 there is a need for investigation regarding the prevalence of AMS among young Chinese men after ascent to high altitude and whether anxiety increases AMS prevalence. Thus, the purpose of the present study was to 1) investigate the prevalence of AMS in a sample of young Chinese men upon ascent to 3800 m altitude and 2) explore whether anxiety is a risk factor for AMS among this population.
Materials and Methods
Study Design
The present study was performed in July 2018 in Aba of Sichuan Province, China. Baseline tests were performed within seven days before the participants set out on the journey. The participants ascended by bus from 500 m to 3800 m within 58 hours. The ascent profile is shown in Figure 1A. After the participants were exposed to 3800 m for two and seven days, high-altitude measurements were performed. All participants undertook the same daily regimen and refrained from vigorous exercise during the journey. All participants consumed the same meals and lived in tents.
Participants
We recruited participants who satisfied the following criteria: (1) male lowlanders from areas <500 m, (2) no high-altitude exposure over the previous six months, (3) no cardiovascular or respiratory disease, and (4) no drug administration (prescription and/or nonprescription medicines). Participants with the following conditions were excluded: cardio- or cerebrovascular diseases, respiratory diseases, diseases of the liver or kidneys, and malignant tumors. All the participants signed informed written consent and could freely withdraw from the study. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of No. 37 Hospital of People’s Liberation Army (Approval number: 2018ky002).
A total of 159 young Chinese men aged 17 to 39 years were recruited. All subjects were in good physical health (validated by electrocardiogram, echocardiogram, and chest X-ray examinations).
Screening of the Study Participants
Within seven days before the journey, clinical examinations (heart rate [HR], blood pressure [BP], pulse oximeter saturation [SpO2], electrocardiogram, echocardiogram, and chest X-ray) were performed to exclude participants with cardiovascular and pulmonary diseases from the study. Demographic characteristics of the participants (age, weight body height [BMI], drinking and smoking behaviours) were recorded in the questionnaire.
AMS Assessment
In the present study, the participants completed the Lake Louise AMS self-questionnaire before the journey and after two and seven days of 3800 m high altitude exposure. The Lake Louise AMS self-questionnaire contains four items: headache, dizziness, gastrointestinal symptoms, and fatigue/weakness.22 Every item is rated on a 4-point ordinal scale, from 0 (no symptoms present) to 3 (present with severe symptoms). AMS was diagnosed if the participant had a complaint of headache and a total Lake Louise AMS score (AMS-S) ≥3. The AMS-S is wildly used for high-altitude researches and is currently considered the reference standard for diagnosing AMS.23 According to the current literature, the sensitivity and specificity of the AMS-S >4 is 78% and 93%, respectively.24 Data on the sensitivity and specificity of the AMS-S ≥3 is currently lacking from the scientific literatures. The subjects were divided into the AMS+ and AMS- groups according to the AMS-S after exposure at 3800 m for two days.
State Anxiety and Sleep Quality Assessment
The state anxiety of the participants was evaluated by the Zung Self-Rating Anxiety Scale [SAS],25 which is suitable for the Chinese population and widely used in anxiety screening in the Chinese population,26 as well as in our previous high-altitude study.27 The SAS contains twenty items. Every item of SAS is rated on a 4-point ordinal scale, from 1 (none or a little of time) to 4 (most or all of the time). The crude total SAS score is multiplied by 1.25, which is then applied to the analysis. State anxiety was defined as a total SAS score ≥50.28 Among a Chinese population, study has shown that the sensitivity and specificity of the SAS ≥50 is 87% and 99%, respectively.29 Study has also shown that the SAS has good reliability and validity (Cronbach’s α=0.75 and Kaiser-Meyer-Olkin=0.86, respectively).30
According to the literature,27,31 the present study employed the Athens Insomnia Scale [AIS] to further evaluate the sleep quality of the subjects before and after high-altitude exposure.32 The AIS contains eight items. Every item of the AIS is rated on a 4-point ordinal scale, from 0 (no problem) to 3 (very serious problem). The AIS is a questionnaire intended to measure the subjective sleep quality of the participants over the preceding month. A higher AIS score indicates poorer quality of sleep. Insomnia is defined as a total AIS score ≥6.32 Study has shown that the sensitivity and specificity of the AIS ≥6 is 92% and 66%, respectively.33 The reliability of the AIS was found to be very satisfactory at sea level (Cronbach’s α=0.89).32
Physiological Parameter Measurements
In response to acute hypobaric hypoxia, the subjects’ HR is increased, but SpO2 is decreased, which may partly reflect the ability of the subjects to adapt to high altitude. Therefore, the subjects’ HR and SpO2 were measured before and after high-altitude exposure. After completion of the abovementioned questionnaires, the resting HR (OMRON HEM-6200; Omron Health Care, Inc., Bannockburn, IL, USA) and SpO2 (Onyx 9500; Nonin Medical, Inc., Plymouth, MN, USA) of the participants were measured three times consecutively at approximately half-minute intervals, and the mean values of the three measurements were applied in the analysis.
Statistical Analysis
The data are shown as the mean±SD, median (interquartile range), or n (%), as appropriate, and were analysed with the Statistical Package for the Social Sciences (Version 22.0; IBM Corporation, Armonk, NY, USA). The significance level was set at p<0.05 using two-sided tests. The normality of distribution of quantitative variables was assessed using the Kolmogorov–Smirnov test. Mean values were compared using a t test. The Pearson chi-squared test was used to compare the categorical variables. The Mann‒Whitney U-test was used to compare non-normally distributed variables. Correlations between variables were assessed with the Pearson correlation coefficient (r) for normally distributed variables or Spearman’s Rho (ρ) for non-normally distributed variables. After univariate analysis, factors associated with AMS (p<0.10) were entered into multivariable logistic regression analysis. AMS was the dependent variable, and smoking, drinking, age, BMI, HR, SpO2, SAS, AIS, ΔSAS and ΔAIS scores were independent variables in logistic regression analysis. Predictors entered into the regression model were defined as binary variables (yes/no), such as smoking and drinking, or as continuous variables (age, BMI, HR, SpO2, the score of SAS, and AIS). In the adjusted logistic regression analysis, the outcome was that independent risk factors for AMS were determined (Figure 1B).
Results
Participants
During the second day of ascent, four participants withdrew from the present study because of headache. During the third day of ascent, four participants withdrew from the present study because of cold. After ascending to 3800 m, three participants withdrew from the present study because of severe headache and lower SpO2 (SpO2 <80%) requiring hospital admission, and another five participants did not complete the follow-up questionnaire for reasons unknown. Thus, we analysed data obtained from the remaining 143 participants. The demographic characteristics of the participants are shown in Table 1. There were no differences between the AMS+ and AMS- groups in age, BMI, smoking, drinking, and baseline physiological parameters (HR and SpO2) (Table 1 and Figure 2).
 |
Table 1 Comparisons of Demographic Characteristics Between the AMS+ and AMS- Groups |
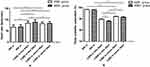 |
Figure 2 Comparisons of heart rate (A) and pulse oximeter saturation (B) between the AMS+ and AMS- groups after acute high-altitude exposure. *p<0.05, **p< 0.01. |
Physiological Parameters
As shown in Figure 2A, HR in both the AMS+ and AMS- groups was significantly increased after exposure to high altitude (all p<0.01). The HR was significantly higher in the AMS+ group than in the AMS- group after two days of exposure to 3800 m (p<0.05).
After exposure to 3800 m, a significant decrease was observed in SpO2 in both the AMS+ and AMS- groups (all p<0.01). SpO2 was significantly lower in the AMS+ group than in the AMS- group after two days of exposure to 3800 m (p<0.05) (Figure 2B).
AMS
At 500 m, no difference in AMS-S was observed between the AMS+ and AMS- groups (1 (2) vs 0 (1), p=0.176). After two days of exposure to 3800 m, AMS-S in the AMS+ and AMS- groups were 3 (1) and 1.5 (1.25), respectively.
After two days of exposure to 3800 m, the overall prevalence of AMS in the whole population was 54% (77/143). The most common symptoms reported by the subjects were headache (77%, 110/143), followed by dizziness (76%, 109/143), fatigue/ weakness (68%, 97/143), and gastrointestinal symptoms (21%, 30/143).
Anxiety and Insomnia
At 500 m, one subject with a total SAS score of 75 was diagnosed with anxiety among the 143 young Chinese men and another five subjects had a total SAS score greater than or equal to 48 but less than 50; no difference in SAS score was observed between the AMS+ and AMS- groups (31.5 (6) vs 30 (3), p=0.124). After exposure to 3800 m for two days, an increase in SAS score was observed in both the AMS+ and AMS- groups and it reached a significant level in the AMS+ group (42 (8.25) vs 31.5 (6), p<0.001). At 3800 m altitude, the SAS score in the AMS+ group was significantly higher than that in the AMS- group (p<0.01). Remarkably, the changes (500 m–3800 m 2 days and 3800 m 2 days–3800 m 7 days) in SAS score in the AMS+ group were significantly greater than those in the AMS- group (p<0.01) (Table 2).
 |
Table 2 Comparisons of SAS and AIS Score Between the AMS+ and AMS- Groups |
At 500 m, no difference in AIS score was observed between the AMS+ and AMS- groups (3 (4) vs 2 (4), p=0.073). After exposure to 3800 m for two days, a significant increase in AIS score was observed in both the AMS+ and AMS- groups (both p<0.01) and the AIS score in the AMS+ group was significantly higher than that in the AMS- group (p<0.01). Furthermore, the changes (500 m–3800 m 2 days and 3800 m 2 days–3800 m 7 days) in AIS score in the AMS+ group were significantly greater than those in the AMS- group (both p<0.01) (Table 2).
Correlational Analysis
Correlation analysis between total AMS-S and other factors was performed to assess the potential risk factors related to AMS. Our data showed that total AMS-S was positively correlated with HR, SAS score, AIS score, ΔHR (500 m–3800 m 2 days), ΔSAS score (500 m–3800 m 2 days), and ΔAIS score (500 m–3800 m 2 days) but negatively correlated with SpO2 (Figure 3A-G). AMS-S was not correlated with age, BMI, smoking and drinking status (data not shown).
As presented in Figure 3H-J, our results showed that the SAS score was positively correlated with HR and AIS score but negatively correlated with SpO2.
Logistic Regression Analysis
The univariable logistic regression analysis among all participants showed that SpO2 had a significant negative association with AMS, while HR, SAS, AIS, ΔSAS and ΔAIS had a significant positive association with AMS (Table 3). However, age, BMI, smoking, and drinking status were not associated with AMS (Table 3).
 |
Table 3 Risk Factors for AMS Among All Participants After Exposure to 3800 m for Two Days |
After adjusted logistic regression analysis, SAS, AIS, and ΔSAS were independent risk factors for AMS (Table 3).
Discussion
The main finding of the present study is that there was a 54% overall prevalence of AMS in a young Chinese male population upon ascent to 3800 m, and anxiety among this population was associated with AMS, as confirmed by multivariable logistic regression analysis (Table 3). The present study may provide insights into the prevention of AMS among young Chinese male population using anxiety interventions, which remains to be verified by further studies.
In the present study, we observed a 54% overall prevalence of AMS among 143 subjects after two days of exposure to 3800 m upon a 58-hour ascent by bus, which was higher than Karinen et al’s study34 but lower than Murdoch’s report.35 The main reason for this should be subjects with different ascent rates between our study and the others. It has been suggested that fast ascent is an identified risk factor for AMS.5 Subjects in the present study spent almost 36 hours from 1300 m to 3800 m altitude; however, subjects in Karinen et al’s study spent almost three days from 1800 m to 3760 m altitude. The ascent rate in the present study was higher than that in Karinen et al’s study. In Murdoch’s study, 84% of the subjects who flew directly to 3740 m and slept at 3860 m suffered from AMS. We believe that the ascent rate partly explains the difference in AMS prevalence between our study and the others at approximately the same altitude. We also compared the prevalence of AMS in the present study with Yuan et al’s report, which was performed at 4100 m altitude among young Chinese men.7 Although the participants were young Chinese men in both our study and Yuan et al’s study, the prevalence of AMS in the present study was higher than that in Yuan et al’s study (54% vs 29%). The participants in Yuan et al’s study spent seven days ascending from 400 m to 4100 m altitude and were diagnosed with AMS with the latest version of the Lake Louise AMS scoring system (2018 version) with insomnia removed. The difference in ascent rate may explain the discrepancy in the prevalence of AMS between the two studies. In another study with Chinese soldier participants,36 Bian et al reported a prevalence of 56% for AMS diagnosed with the original version of the Lake Louise AMS scoring system (1993 version). In Bian et al’s research, the participants ascended from 500 m to 3700 m by plane within two hours, and AMS was assessed within 18–24 hours after the participants’ arrival at 3700 m. The ascent rate in Bian et al’s research was significantly higher than that in the present study. The duration of altitude exposure is another factor that can directly affect the prevalence of AMS. The literature has suggested that AMS generally occurs within six to 12 hours when the subject ascends to high altitude and resolves gradually within one to two days.5 In our previous study, we observed a prevalence of AMS of 60% after the subjects were exposed to 3900 m for approximately 12 hours.37 The prevalence of AMS was lower in the present study than in our previous study. We think this is reasonable because the duration of altitude exposure of the subjects was notably longer in the present study than in our previous study (two days vs 12 hours).
Anxiety in the general population is a very common mental disorder in modern societies and can be caused by diverse factors (eg, workload, financial concerns, ageing, chronic illness). Studies have shown that anxiety can influence work performance,38 decrease quality of life,39 and increase the economic burden on the family and society.40 In the Chinese general population, the prevalence of anxiety ranged from 4%41 to 6%42 evaluated with the Generalized Anxiety Disorder Scale (GAD-7). In the present study, using the Zung Self-Rating Anxiety Scale [SAS] system and defining anxiety as subject with a total SAS score ≥50, one subject was diagnosed with anxiety and another five subjects’ SAS score was closed to 50 among the 143 young Chinese men at 500 m. The prevalence of anxiety reported here was lower than that reported by others.42 We suggest that the discrepancy may be partially due to the different scale used to measure the anxiety level between our and others’ work.
Studies have shown that high altitude exposure can cause or exacerbate anxiety,12,14,15,43,44 which is associated with AMS. AMS symptoms (eg, headache and insomnia) and anxiety may affect each other. The associations between AMS and the psychological states of anxiety may be caused by their relationships with AMS-related symptoms, eg, headache and insomnia, which is partly supported by previously published studies.27,45 In the present study, we found that the prevalence of anxiety in the whole group after two days of exposure to 3800 m was significantly increased compared to that obtained at 500 m (14/143 vs 1/143). In particular, all of the subjects diagnosed with anxiety came from the AMS+ group. The SAS score in the AMS+ group was significantly higher than that in the AMS- group, as was the change in SAS score (Table 2). Our data indicated that psychological changes in AMS+ subjects were more obvious than those in AMS- subjects after high-altitude exposure. Consistent with the literature,12,16 the present study showed that subjects with anxiety were predisposed to AMS compared to subjects without anxiety. Notably, we found that anxiety is a risk factor for AMS, as confirmed by multivariable logistic regression analysis (Table 3). Our result is supported by a previous study.12 In Boos and colleagues’ study,12 British military servicemen with anxiety were at higher risk of developing AMS as compared to those without anxiety. However, Niedermeier and colleagues performed a normobaric chamber study with a sample size of 29 that did not find an association between anxiety and AMS.46 The discrepancy may be partially explained by the different types of hypoxia and sample sizes between our study and Niedermeier and colleagues’ study.
Studies have shown that poor sleep quality is a very common problem among Chinese residents, especially among those with anxiety.42,47 Our results showed that the AIS score was not statistically significantly higher in the AMS+ group than in the AMS- group at 500 m (Table 2). After high-altitude exposure, however, the AIS score was significantly higher in the AMS+ group than in the AMS- group (Table 2). Moreover, the AIS score changes (500 m-3, 800 m 2 days) in the AMS+ group were significantly greater than those in the AMS- group (Table 2). Our previous studies have shown that high-altitude exposure can cause a decrease in sleep quality,27,48 which was further validated in the present study. In addition, our results showed that the SAS score was positively correlated with the AIS score (ρ=0.581, p<0.001) and AIS score was an independent risk factor for AMS (Table 3). Taken together, the present study suggests that anxiety is associated with poor sleep quality, and poor sleep quality may be an important contributor to the increasing prevalence of AMS among young Chinese male population.
With regard to the pathogenesis of high-altitude anxiety, studies have suggested that a reduction in serotonin production caused by hypoxia could promote depression.49,50 Moreover, anxiety is a part of various psychiatric disorders, such as depression.7 Therefore, we speculate that hypobaric hypoxia can cause a reduction in serotonin production, which could in turn cause or exacerbate anxiety in the subjects. Further study is needed to test this hypothesis.
To our knowledge, the present study provides the first data to prove an association between anxiety and AMS among young Chinese male population. The strengths of our study are the identical ascent profile and the large sample sizes. However, our study has some limitations that should be acknowledged. First, three subjects who withdrew from the study because of severe headache, lower SpO2 and requiring hospital admission and were not analysed might have severe AMS. Five subjects did not complete the follow-up questionnaire for reasons unknown. Therefore, our results might be affected by nonresponse bias. Second, all the subjects were male, which may produce a sex bias. Third, the mean age of the subjects was 23 years (IQR, 21–25), which makes it difficult to extend our results to subjects with older age. Fourth, with an observational cross‒sectional study, it is difficult to determine the precise mechanisms of action. Fifth, the sleep quality of the participants in the present study was only measured as subjective sleep quality as opposed to polysomnography. These limitations should be addressed in future studies.
Conclusion
The present study suggests that anxiety is associated with AMS among young Chinese men, and poor sleep quality may partially mediate their association. Recognition of anxiety is particularly relevant in a young Chinese male population. Young Chinese men with anxiety may have a recognizable and preventable vulnerability after high-altitude exposure. Early recognition and intervention of anxiety among young Chinese men may be helpful in decreasing AMS prevalence, which remains to be verified by further studies.
Abbreviations
AIS, Athens Insomnia Scale; AMS, acute mountain sickness; BMI, body mass index; CI, confidence interval; HR, heart rate; OR, odds ratio; SAS, Zung Self-Rating Anxiety Scale; SpO2, pulse oximeter saturation.
Ethics Approval Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of No. 37 Hospital of People’s Liberation Army (Approval number: 2018ky002). All the participants signed informed written consent and could freely withdraw from the study.
Acknowledgments
The authors thank all the subjects as well as all investigators, whose participation allowed the study to be conducted.
Funding
This study was supported by the National Natural Science Foundation of China (82070289).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maggiorini M, Bühler B, Walter M, et al. Prevalence of acute mountain sickness in the Swiss Alps. BMJ. 1990;301(6756):853–855. doi:10.1136/bmj.301.6756.853
2. Roach RC, Bärtsch P, Hackett PH, et al. Hypoxia and molecular medicine. sutton JR. In: Houston CS, Coates G, editors. The Lake Louise Acute Mountain Sickness Scoring System. Burlington, VT: Queen City Printers; 1993:272–274.
3. Scherrer U, Rexhaj E, Jayet PY, et al. New insights in the pathogenesis of high-altitude pulmonary edema. Prog Cardiovasc Dis. 2010;52(6):485–492. doi:10.1016/j.pcad.2010.02.004
4. Kriemler S, Burgi F, Wick C, et al. Prevalence of acute mountain sickness at 3500 m within and between families: a prospective cohort study. High Alt Med Biol. 2014;15(1):28–38. doi:10.1089/ham.2013.1073
5. Bärtsch P, Swenson ER. Acute high-altitude illnesses. N Engl J Med. 2013;368(24):2294–2302. doi:10.1056/NEJMcp1214870
6. Chen R, Sun M, Yang J, et al. Cardiovascular indicators of systemic circulation and acute mountain sickness: an observational cohort study. Front Physiol. 2021;12:708862. doi:10.3389/fphys.2021.708862
7. Yuan F, Qin Z, Liu C, et al. Echocardiographic right ventricular outflow track notch formation and the incidence of acute mountain sickness. High Alt Med Biol. 2021;22(3):263–273. doi:10.1089/ham.2020.0196
8. Ke J, Yang J, Liu C, et al. A novel echocardiographic parameter to identify individuals susceptible to acute mountain sickness. Travel Med Infect Dis. 2021;44:102166. doi:10.1016/j.tmaid.2021.102166
9. Shen Y, Yang YQ, Liu C, et al. Association between physiological responses after exercise at low altitude and acute mountain sickness upon ascent is sex-dependent. Mil Med Res. 2020;7(1):53. doi:10.1186/s40779-020-00283-3
10. Vinnikov D, Brimkulov N, Krasotski V, et al. Risk factors for occupational acute mountain sickness. Occup Med. 2014;64(7):483–489. doi:10.1093/occmed/kqu094
11. Brent MB. A review of the skeletal effects of exposure to high altitude and potential mechanisms for hypobaric hypoxia-induced bone loss. Bone. 2022;154:116258. doi:10.1016/j.bone.2021.116258
12. Boos CJ, Bass M, O’Hara JP, et al. The relationship between anxiety and acute mountain sickness. PLoS One. 2018;13
13. Hüfner K, Sperner-Unterweger B, Brugger H. Going to altitude with a preexisting psychiatric condition. High Alt Med Biol. 2019;20(3):207–214. doi:10.1089/ham.2019.0020
14. Sracic MK, Thomas D, Pate A, et al. Syndrome of acute anxiety among marines after recent arrival at high altitude. Mil Med. 2014;179(5):559–564. doi:10.7205/MILMED-D-13-00359
15. Fagenholz PJ, Murray AF, Gutman JA, et al. New-onset anxiety disorders at high altitude. Wilderness Environ Med. 2007;18(4):312–316. doi:10.1580/07-WEME-BR-102R1.1
16. Missoum G, Rosnet E, Richalet JP. Control of anxiety and acute mountain sickness in Himalayan mountaineers. Int J Sports Med. 1992;1313:S37–39.
17. Leshem E, Pandey P, Shlim DR, et al. Clinical features of patients with severe altitude illness in Nepal. J Travel Med. 2008;15(5):315–322. doi:10.1111/j.1708-8305.2008.00229.x
18. China central television, military report, military drivers on the Qinghai-Tibet road to transport materials into Tibet; 2018. Available from: http://tv.cctv.com/2018/11/13/VIDEVH258yVvpzN6qLzVDXnZ181113.shtml.
19. China central television, China news, military drivers on the Sichuan-Tibet road; 2021. Available from: http://news.cctv.com/2021/06/15/ARTImEruZMlEtbjf8wHcmpsh210615.shtml.
20. Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi:10.1016/S2215-0366(18)30511-X
21. Geng J, Cheng C, Chen S, et al. Anxiety, depression, insomnia symptoms & associated factors among young to middle-aged adults during the resurgent epidemic of COVID-19: a cross-sectional study. Psychol Health Med. 2023;28(5):1336–1346. doi:10.1080/13548506.2022.2143542
22. Roach RC, Hackett PH, Oelz O, et al. Lake Louise AMS score consensus committee. the 2018 lake louise acute mountain sickness score. High Alt Med Biol. 2018;19(1):4–6. doi:10.1089/ham.2017.0164
23. Meier D, Collet TH, Locatelli I, et al. Does this patient have acute mountain sickness?: the rational clinical examination systematic review. JAMA. 2017;318(18):1810–1819. doi:10.1001/jama.2017.16192
24. Maggiorini M, Müller A, Hofstetter D, et al. Assessment of acute mountain sickness by different score protocols in the Swiss Alps. Aviat Space Environ Med. 1998;69(12):1186–1192.
25. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi:10.1016/S0033-3182(71)71479-0
26. Hu HH, Li G, Arao T. The association of family social support, depression, anxiety and self-efficacy with specific hypertension self-care behaviours in Chinese local community. J Hum Hypertens. 2015;29(3):198–203. doi:10.1038/jhh.2014.58
27. Dong JQ, Zhang JH, Qin J, et al. Anxiety correlates with somatic symptoms and sleep status at high altitudes. Physiol Behav. 2013;112-113:23–31. doi:10.1016/j.physbeh.2013.02.001
28. Zhong GQ, Lin BH, Chen YX, et al. Analysis of factors correlated with postoperative kinesiophobia in patients with cervical spondylotic myelopathy: a cross-sectional survey. Neuropsychiatr Dis Treat. 2023;19:1755–1761. doi:10.2147/NDT.S416271
29. Zhang Y, Liu R, Li G, et al. The reliability and validity of a Chinese-version short health anxiety inventory: an investigation of university students. Neuropsychiatr Dis Treat. 2015;11:1739–1747. doi:10.2147/NDT.S83501
30. Wang Q, Zhang B, Zhang S, et al. Anxiety and depression and their interdependent influencing factors among medical students in inner mongolia: the cross-sectional survey. BMC Med Educ. 2022;22(1):787. doi:10.1186/s12909-022-03839-0
31. Bian SZ, Zhang L, Jin J, et al. The onset of sleep disturbances and their associations with anxiety after acute high-altitude exposure at 3700 m. Transl Psychiatry. 2019;9(1):175. doi:10.1038/s41398-019-0510-x
32. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens insomnia scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48(6):555–560. doi:10.1016/S0022-3999(00)00095-7
33. Lin CY, Cheng ASK, Nejati B, et al. A thorough psychometric comparison between Athens insomnia scale and insomnia severity index among patients with advanced cancer. J Sleep Res. 2020;29(1):e12891. doi:10.1111/jsr.12891
34. Karinen H, Peltonen J, Tikkanen H. Prevalence of acute mountain sickness among Finnish trekkers on mount kilimanjaro, Tanzania: an observational study. High Alt Med Biol. 2008;9(4):301–306. doi:10.1089/ham.2008.1008
35. Murdoch DR. Altitude illness among tourists flying to 3740 meters elevation in the Nepal Himalayas. J Travel Med. 1995;2(4):255–256. doi:10.1111/j.1708-8305.1995.tb00671.x
36. Bian SZ, Jin J, Zhang JH, et al. Principal component analysis and risk factors for acute mountain sickness upon acute exposure at 3700 m. PLoS One. 2015;10(11):e0142375. doi:10.1371/journal.pone.0142375
37. Zheng CR, Chen GZ, Yu J, et al. Inhaled budesonide and oral dexamethasone prevent acute mountain sickness. Am J Med. 2014;127(10):1001–1009. doi:10.1016/j.amjmed.2014.04.012
38. Erickson SR, Guthrie S, VanEtten-Lee M, et al. Severity of anxiety and work-related outcomes of patients with anxiety disorders. Depress Anxiety. 2009;26(12):1165–1171. doi:10.1002/da.20624
39. Shen LL, Lao LM, Jiang SF, et al. A survey of anxiety and depression symptoms among primary-care physicians in China. Int J Psychiatry Med. 2012;44(3):257–270. doi:10.2190/PM.44.3.f
40. Wittchen H-U, Jacobi F. Size and burden of mental disorders in Europe--A critical review and appraisal of 27 studies. Eur Neuropsychopharmacol. 2005;15(4):357–376. doi:10.1016/j.euroneuro.2005.04.012
41. Phillips MR, Zhang J, Shi Q, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001-05: an epidemiological survey. Lancet. 2009;373(9680):2041–2053. doi:10.1016/S0140-6736(09)60660-7
42. Xu WQ, Tan WY, Li XL, et al. Prevalence and correlates of depressive and anxiety symptoms among adults in Guangdong province of China: a population-based study. J Affect Disord. 2022;308:535–544. doi:10.1016/j.jad.2022.04.089
43. Roth WT, Gomolla A, Meuret AE, et al. High altitudes, anxiety, and panic attacks: is there a relationship? Depress Anxiety. 2002;16(2):51–58. doi:10.1002/da.10059
44. Ryn Z. Psychopathology in mountaineering-mental disturbances under high-altitude stress. Int J Sports Med. 1988;9(02):163–169. doi:10.1055/s-2007-1024998
45. Nicolas M, Thullier-Lestienne F, Bouquet C, et al. An anxiety, personality and altitude symptomatology study during a 31-day period of hypoxia in a hypobaric chamber (experiment ‘everest-comex 1997’). J Environ Psychol. 1999;19(4):407–414. doi:10.1006/jevp.1999.0139
46. Niedermeier M, Waanders R, Menz V, et al. Is acute mountain sickness related to trait anxiety? A normobaric chamber study. Physiol Behav. 2017;171:187–191. doi:10.1016/j.physbeh.2017.01.004
47. Wang Q, Zhang J, Yao H, et al. Prevalence and associated factors of anxiety among the population in an urban area of China: a cross-sectional study. BMJ Open. 2022;12
48. Tang X-G, Zhang J-H, Qin J, et al. Age as a risk factor for acute mountain sickness upon rapid ascent to 3700 m among young adult Chinese men. Clin Interv Aging. 2014;9:1287–1294. doi:10.2147/CIA.S67052
49. Young SN. Elevated incidence of suicide in people living at altitude, smokers and patients with chronic obstructive pulmonary disease and asthma: possible role of hypoxia causing decreased serotonin synthesis. J Psychiatry Neurosci. 2013;38(6):423–426. doi:10.1503/jpn.130002
50. Kanekar S, Sheth CS, Ombach HJ, et al. Hypobaric hypoxia exposure in rats differentially alters antidepressant efficacy of the selective serotonin reuptake inhibitors fluoxetine, paroxetine, escitalopram and sertraline. Pharmacol Biochem Behav. 2018;170:25–35. doi:10.1016/j.pbb.2018.05.002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


