Back to Journals » Journal of Inflammation Research » Volume 16
Antrodia cinnamomea May Interfere with the Interaction Between ACE2 and SARS-CoV-2 Spike Protein in vitro and Reduces Lung Inflammation in a Hamster Model of COVID-19
Authors Li LH, Chiu HW, Wong WT, Huang KC, Lin TW, Chen ST, Hua KF
Received 10 August 2023
Accepted for publication 24 October 2023
Published 26 October 2023 Volume 2023:16 Pages 4867—4884
DOI https://doi.org/10.2147/JIR.S431222
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tara Strutt
Lan-Hui Li,1 Hsiao-Wen Chiu,2 Wei-Ting Wong,2 Ko-Chieh Huang,3 Tzu-Wen Lin,3 Shui-Tein Chen,3 Kuo-Feng Hua2,4
1Department of Laboratory Medicine, Linsen, Chinese Medicine and Kunming Branch, Taipei City Hospital, Taipei, Taiwan; 2Department of Biotechnology and Animal Science, National Ilan University, Yilan, Taiwan; 3ALPS Biotech Co. Ltd, Taipei, Taiwan; 4Department of Medical Research, China Medical University Hospital, China Medical University, Taichung, Taiwan
Correspondence: Kuo-Feng Hua, Department of Biotechnology and Animal Science, National Ilan University, No. 1, Sec. 1, Shennong Road, Yilan, 260, Taiwan, Tel +886 3 931 7626, Fax +886 3 935 4794, Email [email protected]
Purpose: Coronavirus disease 2019 (COVID-19) poses a global health challenge with widespread transmission. Growing concerns about vaccine side effects, diminishing efficacy, and religious-based hesitancy highlight the need for alternative pharmacological approaches. Our study investigates the impact of the ethanol extract of Antrodia cinnamomea (AC), a native medicinal fungus from Taiwan, on COVID-19 in both in vitro and in vivo contexts.
Methods: We measured the mRNA and protein levels of angiotensin-converting enzyme-2 (ACE2) in human lung cells using real-time reverse transcriptase-polymerase chain reaction and Western blotting, respectively. Additionally, we determined the enzymatic activity of ACE2 using the fluorogenic peptide substrate Mca-YVADAPK(Dnp)-OH. To assess the impact of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, we used SARS-CoV-2 pseudovirus infections in human embryonic kidney 293T cells expressing ACE2 to measure infection rates. Furthermore, we evaluated the in vivo efficacy of AC in mitigating COVID-19 by conducting experiments on hamsters infected with the Delta variant of SARS-CoV-2.
Results: AC effectively decreased ACE2 mRNA and protein levels, a critical host receptor for the SARS-CoV-2 spike protein, in human lung cells. It also prevented the spike protein from binding to human lung cells. Dehydrosulphurenic acid, an isolate from AC, directly inhibited ACE2 protease activity with an inhibitory constant of 1.53 μM. In vitro experiments showed that both AC and dehydrosulphurenic acid significantly reduced the infection rate of SARS-CoV-2 pseudovirus. In hamsters infected with the Delta variant of SARS-CoV-2, oral administration of AC reduced body weight loss and improved lung injury. Notably, AC also inhibited IL-1β expression in both macrophages and the lung tissues of SARS-CoV-2-infected hamsters.
Conclusion: AC shows potential as a nutraceutical for reducing the risk of SARS-CoV-2 infection by disrupting the interaction between ACE2 and the SARS-CoV-2 spike protein, and for preventing COVID-19-associated lung inflammation.
Keywords: coronavirus disease 2019, angiotensin-converting enzyme-2, severe acute respiratory syndrome coronavirus 2, Antrodia cinnamomea, the intracellular sensor NACHT, LRR, PYD domain-containing protein 3 inflammasome, cytokine
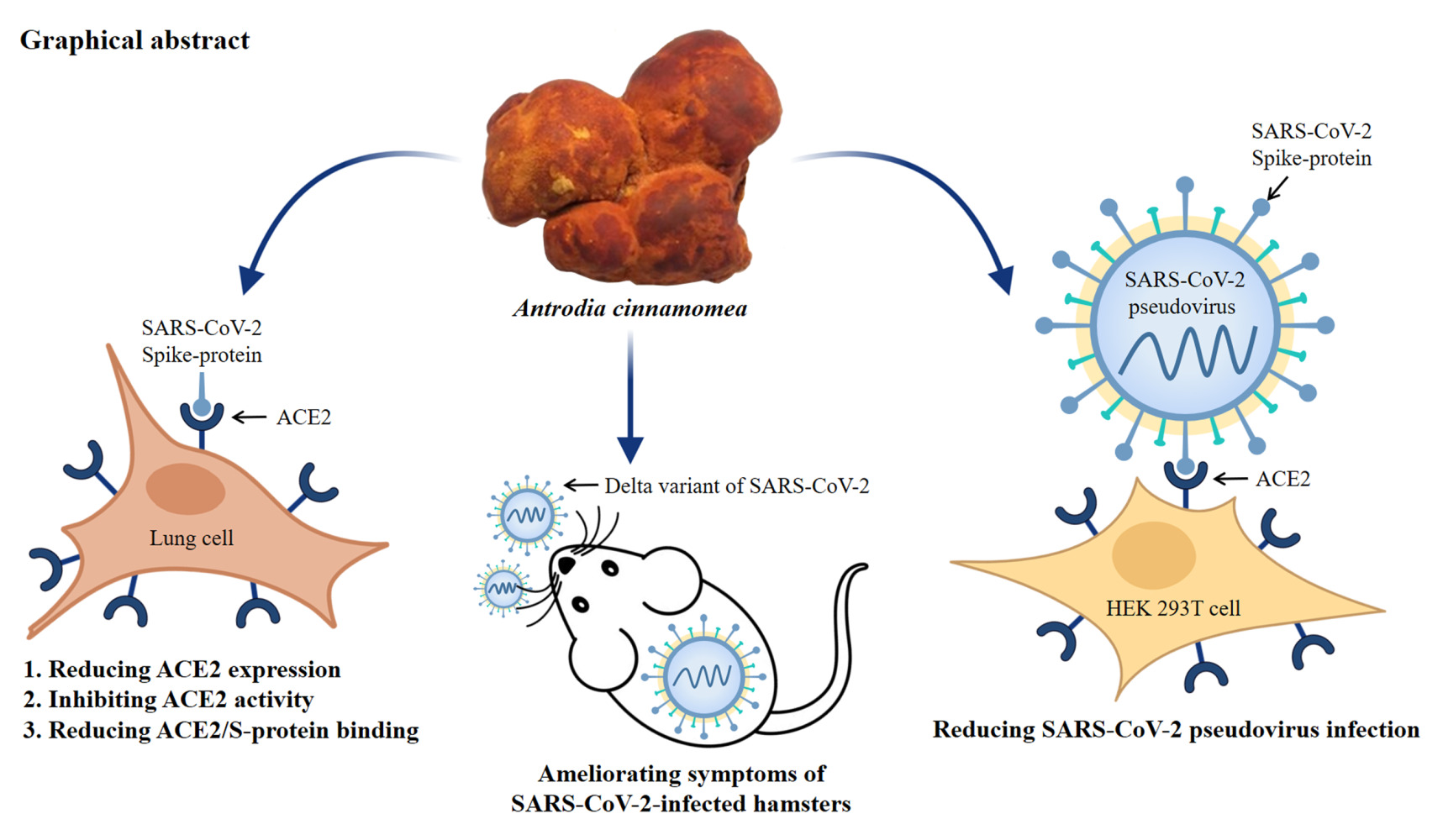
Graphical Abstract:
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the COVID-19 pandemic since 2019, shares a common cell entry receptor known as angiotensin converting enzyme II (ACE2) with the SARS-CoV virus from 2002.1 Research has shown that the initial step in viral infection involves the binding of the viral spike protein to ACE2.2 Studies have revealed that individuals with hypertension, diabetes, or chronic obstructive lung disease exhibit significantly elevated levels of ACE2 expression in their lung tissues compared to those without these conditions. These findings offer a plausible explanation for the heightened severity of Coronavirus disease 2019 (COVID-19) in patients with these underlying health issues.3 Additionally, in COVID-19 patients with heart failure, there was a notable increase in ACE2 expression within myocardial tissues, indicating that these individuals were more susceptible to SARS-CoV-2 infection in the heart, which could lead to severe complications.4 These results suggest that reducing ACE2 expression might decrease an individual’s susceptibility to SARS-CoV-2 infection and improve disease outcomes. However, it is essential to be mindful of potential side effects associated with downregulating ACE2.5
Vaccination stands out as the primary strategy for managing the COVID-19 pandemic, garnering significant attention from researchers and the pharmaceutical industry in the quest to develop effective SARS-CoV-2 vaccines.6 Nevertheless, issues such as vaccine side effects, diminishing vaccine efficacy over time, and vaccine hesitancy driven by religious convictions have hindered comprehensive vaccination coverage.7–9 Consequently, an urgent imperative exists to devise additional therapeutic or preventive measures. The concept of repurposing approved drugs against SARS-CoV-2 has captured the scientific community’s interest, mainly due to its potential to expedite the availability of medications and reduce costs. Notably, Teriflunomide, a disease-modifying agent initially sanctioned for the treatment of multiple sclerosis;10 Forodesine, a nucleoside analogue proven effective against T-cell malignancies;11,12 and Didanosine, a purine nucleoside analogue and reverse transcriptase inhibitor employed in the management of HIV/AIDS,13 have all exhibited promise in combating SARS-CoV-2 by targeting the RNA-dependent RNA polymerase. Furthermore, synthetic compounds like Taroxaz-104, belonging to the 1,3,4-oxadiazoles class,14 and Ensitrelvir, an orally active noncovalent nonpeptidic agent,15 alongside antioxidant compounds of the 1,3,4-oxadiazole or 1,3,4-thiadiazole type,16,17 and SLL-0197800, an isoquinoline derivative,18 have all demonstrated potential against SARS-CoV-2 by targeting the RNA-dependent RNA polymerase. Moreover, natural products such as Cordycepin, a recognized natural adenosine analogue of fungal origin,19 as well as compounds like ananas 26 from pineapple and zingiberenol and zingiberol from ginger,20 have shown promise in countering SARS-CoV-2 by targeting the RNA-dependent RNA polymerase. In addition to antiviral compounds targeting SARS-CoV-2 enzymes,21 there is considerable interest in disrupting the protein-protein interaction between ACE2 and the SARS-CoV-2 spike protein or targeting ACE2 using small molecules as novel approaches to reduce the incidence of SARS-CoV-2 infections.22,23
While reducing the incidence of SARS-CoV-2 infections remains the primary strategy in combating COVID-19, it is equally crucial to minimize damage in patients post-infection. A growing body of evidence underscores the pivotal role played by cytokine storms in the deterioration and fatality of COVID-19 patients.24,25 The intracellular sensor NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome, a protein complex containing caspase-1, serves as a regulator for the maturation and release of proinflammatory cytokines, namely interleukin (IL)-1β and IL-18.26 This inflammasome holds significant relevance in the development of diseases associated with inflammation.27 Both the N protein and single-stranded RNA of SARS-CoV-2 have been identified as critical pathogenic factors that trigger the activation of the NLRP3 inflammasome, thereby inducing an excessive inflammatory response.28,29 Notably, the degree of NLRP3 inflammasome activation has been linked to the severity of COVID-19 in patients.30 IL-1 inhibitors, in particular the recombinant IL-1α/β receptor antagonist anakinra, have been shown in observational studies to reduce mortality in both severe and critical COVID-19.31,32 These findings strongly suggest that targeting the NLRP3 inflammasome offers innovative therapeutic strategies for addressing COVID-19.33 Notably, NLRP3 inflammasome-derived IL-1β promotes the expression of IL-6, which has been recognized as an important mediator in COVID-19.34 It has been demonstrated that IL-6 levels in the bronchoalveolar lavage fluid of patients with COVID-19 progressively increase with higher viral load, disease severity and poor prognosis.35,36 Therefore, Therapeutic blockade of IL-1β and IL-6 may represent an effective strategy to reduce severity and prevent mortality in COVID-19 patients.37,38
Antrodia cinnamomea, also known as Taiwanofungus camphoratus, is an indigenous medicinal mushroom characterized by its orange/red fruiting bodies. This unique fungus thrives exclusively within the inner recesses of the native tree species Cinnamomum kanehirae Hayata, a member of the Lauraceae family.39 A. cinnamomea has a rich history in ethnomedicine, often utilized for its wide-ranging health benefits, including immune modulation, liver protection, neuroprotection, anticancer properties, anti-inflammatory effects, and assistance in managing diabetes.40 One of the key bioactive components found in A. cinnamomea is a group of well-defined ergostane-type triterpenoids known as antcins. These antcins have shown promise in reducing ACE2 expression in epithelial cells, suggesting a potential therapeutic role in the context of COVID-19.41 In this research study, we delve deeper into the therapeutic and preventive potential of the ethanol extract from A. cinnamomea (AC) and its pure compounds (Figure 1) in the context of COVID-19. Our investigation encompasses several aspects, including the impact of A. cinnamomea on ACE2 expression in human lung cells, the interaction between ACE2 and the SARS-CoV-2 spike protein, in vitro experiments involving SARS-CoV-2 pseudotyped lentivirus infection, and in vivo assessments using a SARS-CoV-2 infected hamster model.
 |
Figure 1 Chemical structure of pure compounds identified in AC. Antcin A (1), Antcin B (2), Antcin C (3), Antcin H (4), Antcin K (5), Dehydrosulphurenic acid (6), and Dehydroeburicoic acid (7). |
Materials and Methods
Reagents
Antibodies targeting human ACE2 (21115-1-AP) and the His-Tag (HRP-66005) were procured from Proteintech Group, Inc. (Rosemont, IL). The SARS-CoV-2 spike S1-His recombinant protein (40591-V08H) was obtained from Sino Biological US Inc. (Houston, TX). Recombinant proteins for human ACE-2 (933-ZN) and the Mca-YVADAPK(Dnp)-OH fluorogenic peptide substrate (ES007) were sourced from R&D Systems (Minneapolis, MN). We utilized the Bright-Glo Luciferase Assay System, which was acquired from Promega (Wisconsin, USA). The real-time polymerase chain reaction (PCR) primers were purchased from Genomics (Taipei, Taiwan). Adenosine triphosphate (ATP) (tlrl-atpl) was obtained from InvivoGen (San Diego, CA). Lipopolysaccharide (LPS) (L2630) was procured from Sigma‒Aldrich (St. Louis, MO). The CytoScan LDH Cytotoxicity Assay kit (786–210) was purchased from G-Bioscience (St. Louis, MO). Lastly, we employed an IL-1β ELISA kit purchased from Affymetrix eBioscience (San Diego, CA). Remdesivir was procured from Cayman Chemical (Ann Arbor, Michigan).
Preparation of A. cinnamomea Ethanol Extract (AC)
The fruiting body of A. camphorata was purchased from the Ruei-Sen Biotechnology Corporation in New Taipei City, Taiwan. A voucher specimen (AC-003) was deposited in National Ilan University and identified by China Medical University. Ethanol, with a concentration of 95%, was added to 50 grams of A. cinnamomea fruiting body dry powder, resulting in a total volume of 1 liter. This solution was allowed to sit at room temperature for 24 hours. The extraction process was repeated three times. Subsequently, the extracts were decanted and filtered through Whatman No. 1 paper. The solvent was then removed under reduced pressure using a rotary evaporator at 50°C. The resulting extract was redissolved in dimethyl sulfoxide (DMSO) and stored at −18°C for use in bioassay experiments and high performance liquid chromatography (HPLC) analysis. For the identification of active compounds in AC, an HPLC system was employed, consisting of a vacuum degasser, autosampler, and quaternary pump with a maximum pressure capacity of 400 bar (Agilent-Technologies 1100 series), along with an ultraviolet diode-array-detector. A 4.6×150 mm reversed-phase C18 analytical column with a particle size of 2.5 μm (ACE UltraCore 2.5 SuperC18 column) was utilized. The column was maintained at a temperature of 35°C, and the injected sample volume was 5 μL. The mobile phase B consisted of Milli-Q water containing 0.1% acetic acid, while mobile phase A was composed of acetonitrile. A linear gradient from 35:65 (A:B) to 100:0 (A:B) was employed over a 45-minute period, followed by a return to its initial condition. The flow rate utilized was 1.0 mL/min. The wavelength for detection was set to 248 nm, and UV spectra ranging from 190 to 400 nm were recorded for peak characterization. Each HPLC run was completed within 70 minutes. Antcin A, antcin B, antcin C, antcin H, antcin K, DA, and dehydroeburicoic acid were isolated from A. cinnamomea as described previously.42,43 The purity of these compounds above 98%, as confirmed by HPLC and nuclear magnetic resonance (NMR) analysis.
Cell Culture
Human lung CL1-5 cells were sourced from Prof. Huei-Wen Chen at the Graduate Institute of Toxicology, College of Medicine, National Taiwan University.44 Human lung MRC-5 cells, mouse J774A.1 macrophages and human embryonic kidney-293T (HEK-293T) cells were procured from the Bioresource Collection and Research Center (Hsinchu, Taiwan). All these cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), and they were cultured at 37°C in a 5% CO2 incubator.
ACE2 mRNA and Protein Expression
For the dose-response study, we exposed human lung cancer CL1-5 cells to concentrations of 12.5, 25, or 50 µg/mL of AC or 0.1% DMSO (used as a vehicle control) for a duration of 24 hours. Human lung MRC-5 cells were similarly incubated with concentrations of 25, 50, or 100 µg/mL of AC or the vehicle for 24 hours. In the time course study, human lung cancer CL1-5 cells were treated with either 50 µg/mL of AC or the vehicle for 3, 6, 12, or 24 hours. The protein levels of ACE2 in the cell lysates were assessed through Western blotting. Briefly, cells were lysed using a protease inhibitor cocktail in radioimmunoprecipitation assay (RIPA) lysis buffer. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was employed to separate the proteins in each sample (30 μg/sample). These separated proteins were subsequently transferred to polyvinylidene difluoride membranes. The membranes were incubated with 5% nonfat milk in phosphate buffered saline (PBS) buffer with 0.1% Tween 20 (blocking buffer) for 2 hours at room temperature. This was followed by incubation with primary and corresponding secondary antibodies in the blocking buffer for 2 hours and 1 hour, respectively. The membranes were then washed three times with PBS containing 0.1% Tween 20 and developed using enhanced chemiluminescence (ECL) Reagent. Signals were captured using the GE Healthcare Life Sciences Amersham Imager 600 image system (Chicago, IL). To determine ACE2 mRNA expression, human lung cancer CL1-5 cells were exposed to 50 µg/mL of AC or the vehicle for 1, 2, or 3 hours. RNA was extracted from treated cells using TRIzol reagent and subsequently real time reverse transcriptase (RT)-polymerase chain reaction (PCR) analysis using the StepOne real-time PCR system from Applied Biosystems (Foster City, CA). The ACE2 mRNA expression data are presented as relative expressions normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers used were as follows: ACE2, forward: 5’-CATTGGAGCAAGTGTTGGATCTT-3’; ACE2, reverse: 5’-GAGCTAATGCATGCCATTCTCA-3’; GAPDH, forward: 5’-TGAAGGGTGGAGCCAAAAGG-3’; GAPDH, reverse: 5’-GATGGCATGGACTGTGGTCA-3’.
Effect of AC on Lactate Dehydrogenase (LDH) Release
To evaluate the potential cytotoxicity of AC, we exposed either human lung cancer CL1-5 cells or mouse J774A.1 macrophages to AC, a lysis buffer (representing maximum LDH release), or 0.1% DMSO (representing spontaneous LDH release) for a duration of 24 hours. The levels of LDH in the supernatants were analyzed using the CytoScan LDH Cytotoxicity Assay kit following the manufacturer’s instructions. The LDH release percentage was calculated using the formula: LDH release % = 100 × (sample optical density (O.D.) - spontaneous O.D.) / (maximum O.D. - spontaneous O.D.).
Interaction Between the Spike Protein and Human Lung Cells
CL1-5 cells, with a seeding density of 2×106 cells per dish, were exposed to 50 µg/mL of AC, 20 µg/mL of DA, or the vehicle (0.1% DMSO) for a 24-hour incubation period. Subsequently, the cells were transferred to tubes at a concentration of 2×105 cells per tube. They were then fixed with 4% paraformaldehyde at 4°C for 1 hour and subsequently blocked with 1% BSA at 4°C for another 1 hour. Following the blocking step, the cells were incubated with His-tagged spike protein at a concentration of 1 µg/mL for 1 hour. This was followed by an incubation step with anti-His antibody at 2 µg/mL and anti-IgG HRP at 1 µg/mL. After thorough washing, the cells were treated with 50 µL of 3,3’,5,5’-Tetramethylbenzidine (TMB) for 15 minutes in the dark. The resulting signals were detected at an O.D. of 450 nm using an ELISA reader.
ACE2 Enzyme Activities
The enzymatic activities of ACE2 were assessed using a Mca-YVADAPK(Dnp)-OH fluorogenic peptide substrate. In a 96-well plate, we incubated 10 ng of recombinant human ACE2 protein with 1 µg of a pure test compound isolated from A. cinnamomea for 15 minutes at room temperature. This reaction was carried out in a total volume of 50 µL within an assay buffer containing 75 mM Tris and 1 M NaCl. Subsequently, the fluorogenic peptide substrate was introduced into the wells at a final concentration of 20 μM. The plate was then immediately read at excitation and emission wavelengths of 320 nm and 405 nm, respectively, followed by continuous monitoring in fluorescence kinetic mode at 1-minute intervals for a duration of 1 hour. To determine the inhibitory constant (Ki) of DA on ACE2, we incubated 10 ng of recombinant human ACE2 protein with varying concentrations of DA ranging from 0 to 2 mg/mL for 15 minutes at room temperature. This reaction also took place in a 96-well plate and in an assay buffer consisting of 75 mM Tris and 1 M NaCl. The fluorogenic peptide substrate was subsequently added to the wells at final concentrations ranging from 0 to 40 μM. The plate was immediately subjected to reading at excitation and emission wavelengths of 320 nm and 405 nm, respectively, and then continuously monitored in fluorescence kinetic mode at 1-second intervals for a duration of 1 minute.
SARS-CoV-2 Pseudotyped Lentivirus Neutralization Assay
This study was conducted at the RNAi Core of Academia Sinica in Taipei, Taiwan. In the virus neutralization assay, heat-inactivated sera were serially diluted to the desired concentration and then mixed with 250 TU of SARS-CoV-2 pseudotyped lentivirus in DMEM (supplemented with 1% FBS and 100 U/mL penicillin/streptomycin). This mixture was incubated for 1 hour at 37°C. Subsequently, the inoculated mixture was added to HEK-293T cells (10,000 cells per well) that stably expressed the human ACE2 gene. These cells were plated in a 96-well plate that had been preincubated with either 50 µg/mL of AC or 10–30 µg/mL of DA for 1 hour. At 16 hours post-infection, the culture medium was replaced with fresh complete DMEM (supplemented with 10% FBS and 100 U/mL penicillin/streptomycin), and the cells were continuously cultured for an additional 48 hours before the luciferase assay was performed. To conduct the luciferase assay, the expression levels of the luciferase gene were assessed using the Bright-Glo luciferase assay system. The relative light unit (RLU) values were measured using the Tecan i-control (Infinite 500). The percentage of inhibition was calculated as the ratio of RLU reduction in the presence of diluted serum to the RLU value of the no-serum control, as shown in the following formula:
Percentage of Inhibition = (RLU Control - RLU Serum) / RLU Control
Effect of AC on IL-1β Production in Macrophages
J774A.1 macrophages were initially incubated with 1 µg/mL of LPS for 4 hours. Following this pre-incubation period, the cells were treated with 3–30 µg/mL of AC or the vehicle (0.1% DMSO) for 0.5 hours. Subsequently, the cells were activated by exposure to 5 mM ATP for an additional 0.5 hours. The IL-1β concentration in the supernatants was then analyzed using an ELISA assay to determine the levels of this cytokine.
Hamster Model of SARS-CoV-2 Delta-Variant Infection
The SARS-CoV-2 delta variant used in these animal experiments was sourced from the Taiwan Centers of Disease Control (TCDC#1144, lot: GRC20210717) at a concentration of 1.0×106 plaque-forming unit (PFU)/mL. Syrian hamsters were acquired from the National Laboratory Animal Center in Taipei, Taiwan. They were housed in a controlled environment where the temperature was maintained at 23 ± 3°C, and relative humidity was kept between 40% to 60%. An automatic lighting system provided a 12-hour light and 12-hour dark cycle for the hamsters. The animals were provided with Altromin 1314 (Altromin GmbH, Lage, Germany) for their food and supplied with distilled water. The Syrian hamsters were randomly divided into four groups for the experiment: Group 1: Hamsters received oral administrations of distilled water five times daily, starting 48 hours before infection. Group 2: Hamsters received oral administrations of 100 mg/kg AC five times daily, starting 48 hours before infection. Group 3: Hamsters received oral administrations of 500 mg/kg AC five times daily, starting 48 hours before infection. Group 4: Hamsters received intraperitoneal (i.p.) injections of 15 mg/kg remdesivir twice daily, starting 24 hours after infection. The Syrian hamsters were anesthetized and challenged intranasally with 1.0×104 plaque-forming units of the SARS-CoV-2 delta variant in a 100-μL volume. All procedures involving the animals were conducted with a focus on minimizing any discomfort, distress, or pain experienced by the animals. The weights of the hamsters were monitored daily following the challenge with the SARS-CoV-2 delta variant. After the completion of the experiment, surviving hamsters were euthanized using carbon dioxide, and lung tissues were collected during the animal sacrifice. These Syrian hamster challenge experiments were rigorously reviewed and approved by the Institutional Animal Care and Use Committee of Academia Sinica, Taiwan, under approval number 20-06-1483, with approval granted on August 13, 2021.
Lung Histopathology
The left lung of each hamster was collected and fixed in 4% paraformaldehyde. After fixation for one week, the lung tissue was trimmed, processed, embedded, sectioned, and stained with Hematoxylin and Eosin (H&E), followed by microscopic examination. The lung section was evaluated with a lung histopathological scoring system. Each section was divided into 9 areas. Lung tissue of each area was scored using the scoring system as shown below. The average scores of these 9 areas are used to represent the histopathological score of the animal. Lung tissue of every area is scored using the scoring system: 0, Normal, no significant finding; 1, Minor inflammation with a slight thickening of alveolar septa and sparse monocyte infiltration; 2, Apparent inflammation, alveolus septa thickening with more interstitial mononuclear inflammatory infiltration; 3, Diffuse alveolar damage, with alveolus septa thickening, and increased infiltration of inflammatory cells; 4, Diffuse alveolar damage, with extensive exudation and septa thickening, shrinking of alveoli, the restricted fusion of the thick septa, obvious septa hemorrhage, and more cell infiltration in alveolar cavities; 5, Diffuse alveolar damage, with massive cell filtration in alveolar cavities and alveoli shrinking, sheets of septa fusion, and hyaline membranes lining the alveolar walls.
Quantification of Viral Titers in Lung Tissue by Cell Culture Infectious Assay
The right lobes of hamster lung tissue were collected and then homogenized in 4 mL of DMEM with 2% FBS and 1% penicillin/streptomycin using a homogenizer known as the Omni Bead Ruptor. After homogenization, tissue homogenates were subjected to centrifugation at 6000 rpm for 10 minutes, and the resulting supernatants were collected for the purpose of live virus titration. To perform the live virus titration, a series of 10-fold dilutions was prepared for each sample. These dilutions were then added to Vero E6 cell monolayers in sextuplicate within 96-well plates, followed by an incubation period of 4 days. Subsequently, the cells were fixed using 10% formaldehyde and stained with a 0.5% crystal violet solution for a duration of 20 minutes. Following staining, the plates were rinsed with tap water and examined for signs of cytopathic infection. The fifty percent tissue culture infectious dose (TCID50)/mL was then calculated using the Reed and Muench method.45 This method is commonly employed to determine the concentration of infectious agents, in this case, the live virus, within a given sample.
Real-Time RT‒PCR for SARS-CoV-2 RNA Quantification
TaqMan real-time RT-PCR was performed following established protocols.46 To quantify the levels of SARS-CoV-2 RNA, specific primers targeting the 26,141 to 26,253 region of the envelope (E) gene of the SARS-CoV-2 genome were employed. These primers consisted of the forward primer E-Sarbeco-F (5’-ACAGGTACGTTAATAGTTAATAGCGT-3’), the reverse primer E-Sarbeco-R (5’-ATATTGCAGCAGTACGCACACA-3’), and the probe E-Sarbeco-P (5’-FAM-ACACTAGCC-ZEN-ATCCTTACTGCGCTTCG-3IABkFQ-3’). To extract total RNA from each sample, 30 microlitres of RNA were collected using an RNeasy Mini Kit (QIAGEN, Germany, Cat. No. 74136) in accordance with the manufacturer’s instructions. Subsequently, 5 microlitres of RNA sample at a concentration of 200 ng/μL were combined with a 20-μL mixture of the SuperScriptTM III PlatinumTM One-Step qRT‒PCR system (Thermo Fisher Scientific, USA, Cat. No. 11732088). The final reaction mixture comprised 400 nM forward and reverse primers, 200 nM probe, 0.2 mM deoxyribonucleoside triphosphate, 3.8 mM magnesium sulfate, 50 nM ROX reference dye, and 1 μL of enzyme mixture. The PCR cycling conditions followed a one-step protocol: initial incubation at 55°C for 10 minutes for first strand cDNA synthesis, followed by 1 minute at 95°C, and then 45 amplification cycles at 95°C for 10 seconds and 58°C for 30 seconds. Data were collected and analyzed using the Applied Biosystems QuantStudio 5 (Thermo Fisher Scientific, USA). To quantify the copy numbers of the viral genome, a synthetic 113-bp oligonucleotide fragment was employed as a qPCR standard. This oligonucleotide was synthesized by Integrated DNA Technologies Pte. Ltd.
Statistical Analysis
In the animal study, Prism 6.01 (GraphPad) was used for statistical analysis. Body weight change, lung viral RNA titer and TCID50 are presented as bar plots with mean value. Differences between groups were analyzed by one-way and two-way ANOVA, with either Tukey’s multiple comparison test or Kruskal–Wallis with corrected Dunn’s multiple comparisons test used to calculate significance, as noted in respective figure descriptions. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. In the in vitro studies, statistical analysis was conducted using two-tailed t-tests for comparisons between two groups and ANOVA with Dunnett’s multiple comparisons test for comparisons involving three or more groups. The error bars in the graphs represent the standard deviations calculated from three independent experiments. Significance levels were indicated as follows: ***and *** represent p < 0.05, p < 0.01, and p < 0.001, respectively. These symbols were used to denote the level of statistical significance.
Results
AC Reduces ACE2 Expression in Human Lung Cells and Inhibits SARS-CoV-2 Spike Protein Binding to Cells
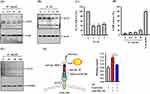
ACE2 serves as the critical entry receptor for SARS-CoV-2 infection. To investigate the potential impact of AC, human lung cancer CL1-5 cells were exposed to AC concentrations ranging from 12.5 to 50 µg/mL for a duration of 24 hours. Our findings indicate that AC led to a reduction in ACE2 protein expression (Figure 2A). In our time course study, we observed that ACE2 protein expression in CL1-5 cells continued to decrease after 24 hours of AC treatment (Figure 2B). Additionally, AC had a suppressive effect on ACE2 mRNA expression, with a noticeable reduction after only 1 hour of treatment (Figure 2C). It is important to note that a LDH release test was conducted to confirm the absence of any cytotoxic effects on CL1-5 cells when exposed to AC concentrations of up to 50 µg/mL (Figure 2D). Furthermore, AC exhibited a similar reduction in ACE2 protein expression in MRC-5 human lung fibroblast cells (Figure 2E). Lastly, when CL1-5 cells were treated with AC for 24 hours, there was a noticeable decrease in the binding between these cells and the SARS-CoV-2 spike protein (Figure 2F). These findings collectively suggest that AC may have a potential inhibitory effect on ACE2 expression and interaction with the SARS-CoV-2 spike protein.
DA from AC Inhibits ACE2 Protease Activity
To identify the active compounds present in AC, we conducted HPLC analysis to examine the triterpenoid profiles of AC. Our analysis led to the identification and isolation of seven compounds, namely antcin A (1), antcin B (2), antcin C (3), antcin H (4), antcin K (5), dehydrosulphurenic acid (DA) (6), and dehydroeburicoic acid (7), as illustrated in Figure 3A. Subsequent experiments revealed that several of these compounds exhibited inhibitory effects against ACE2 protease activity, with DA demonstrating greater potency compared to the other compounds (Figure 3B). Furthermore, the Ki of DA against ACE2 protease activity was calculated to be 1.53 µM (Figure 3C). Moreover, when CL1-5 cells were incubated with DA for a period of 24 hours, we observed a reduction in the binding between these cells and the SARS-CoV-2 spike protein (Figure 3D). These findings suggest that DA, among the compounds identified in AC, may possess notable inhibitory properties against ACE2 protease activity and the interaction between cells and the SARS-CoV-2 spike protein.
AC and DA Reduce SARS-CoV-2 Infections in a Pseudovirus Infection Cell Model
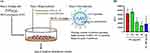
A pseudovirus is a non-infectious viral surrogate employed in experiments to replicate the behavior of the authentic virus without instigating an actual infection. In order to delve further into whether AC and its active compound, DA, diminish SARS-CoV-2 infection in cells, a pseudovirus infection cell model was employed. Human HEK-293T cells, expressing the ACE2 receptor critical for SARS-CoV-2 entry, were subjected to a 1-hour incubation with either AC or DA before exposure to the SARS-CoV-2 pseudovirus (Figure 4A). The results revealed that both AC and DA exerted a notable impact in reducing SARS-CoV-2 pseudovirus infection within the cells (Figure 4B). In summary, the experiment implies that both AC and DA possess potential inhibitory effects on SARS-CoV-2 infection in this cell model, as evidenced by their ability to mitigate the infection induced by the pseudovirus.
AC Reduces Body Weight Loss and Improves Lung Injury in SARS-CoV-2 Delta-Variant-Infected Hamsters
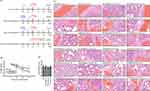
The administration of A. cinnamomea extract at a daily dosage of 100 mg/kg to mice over a period of 10 consecutive days has been shown to mitigate non-alcoholic steatohepatitis by effectively suppressing the NLRP3 inflammasome.47 Consequently, in this study, we employed A. cinnamomea at two distinct dosages: 100 mg/kg, representing the low dose, and 500 mg/kg, representing the high dose. We conducted an in vivo study to assess the prophylactic effects of AC using a hamster model infected with SARS-CoV-2, which mirrors the COVID-19 disease characterized by swift weight loss and severe lung damage (Figure 5A).48 In our investigation, we observed that hamsters infected with the SARS-CoV-2 delta variant through intranasal exposure and orally administered a vehicle solution experienced a 5.1% ± 0.9% (mean ± SD) reduction in body weight. In contrast, those hamsters that received oral doses of 500 mg/kg AC or 100 mg/kg AC twice prior to virus exposure exhibited reduced weight loss at 72 hours post-infection (hpi), with losses of 3.0% ± 1.1% (p = 0.0067 compared to the vehicle) and 4.9% ± 1.4% (p = 0.7531 compared to the vehicle), respectively (Figure 5B). Additionally, the dosage of remdesivir, an antiviral drug against SARS-CoV-2, utilized in this study was derived from a prior investigation which utilized 15 mg/kg.49 Hamsters treated with remdesivir at a dose of 15 mg/kg twice after virus challenge showed a weight loss of 3.1% ± 2.0% (p = 0.0523 compared to the vehicle) (Figure 5B). These results indicate that hamsters receiving AC at a dosage of 500 mg/kg experience a significant reduction in body weight loss when infected with the SARS-CoV-2 delta variant. However, hamsters receiving AC at 100 mg/kg or remdesivir at 15 mg/kg did not show a significant reduction in body weight loss. Furthermore, we conducted histopathological analyses of the lung tissues of infected hamsters (Figures 5C and D). The lungs of vehicle-treated hamsters sacrificed at 72 hpi exhibited typical histopathological indications of necrotizing pneumonia, including interstitial inflammatory infiltration with mononuclear cells, mild to moderate diffuse congestion, hemorrhage, thickening of alveolar septa, alveolar shrinkage, fibrin thrombus, epithelial necrosis, and inflammatory exudates in bronchioles. Interestingly, one out of six vehicle-treated hamsters displayed an unusually low lung pathology score. Conversely, hamsters administered with 500 mg/kg AC displayed reductions in COVID-19-related histopathological signs in comparison to those receiving the vehicle (*p < 0.05). However, hamsters given 100 mg/kg AC or 15 mg/kg remdesivir did not exhibit significant reductions in lung pathology scores compared to the hamsters that received the vehicle (Figures 5C and D). In summary, our findings suggest that oral administration of AC at 500 mg/kg significantly reduces body weight loss and improves lung injury in hamsters infected with the SARS-CoV-2 delta variant. However, it’s important to note that the clinical significance of these results remains uncertain at this time.
AC Reduces IL-1β Expression in vivo and in vitro
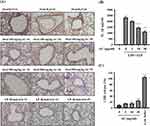
IL-1β is the final product of the NLRP3 inflammasome, a critical player in the context of COVID-19.28–30,33 In the case of SARS-CoV-2 delta variant-infected hamsters, those treated with AC or remdesivir exhibited notable reductions in IL-1β expression within their lung tissues (Figure 6A). Furthermore, when examining LPS-primed macrophages, AC demonstrated a decrease in ATP-induced IL-1β production (Figure 6B). It is important to note that AC did not exert any cytotoxic effects under the experimental conditions, as indicated in Figure 6C. These findings strongly suggest that AC effectively inhibits the NLRP3 inflammasome both in vitro and in vivo.
AC Did Not Reduce the Presence of SARS-CoV-2 in the Lungs of SARS-CoV-2 Delta-Variant-Infected Hamsters
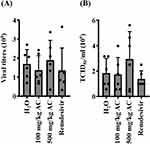
To investigate the impact of AC on the presence of SARS-CoV-2 particles in the lungs, we conducted an analysis of viral genomic RNA copy numbers in lung tissues collected 72 hours after SARS-CoV-2 delta-variant infection using real-time RT-PCR. Our findings revealed that neither AC nor remdesivir led to a reduction in the viral genomic RNA copy numbers in the lung tissues (Figure 7A). Additionally, we assessed the functional SARS-CoV-2 particle titers in the lungs through a cell culture infectious assay. We observed that the TCID50/mL (median tissue culture infectious dose per milliliter) from lung homogenates collected at 72 hours post-SARS-CoV-2 delta-variant infection remained unaltered in response to AC or remdesivir treatment (Figure 7B). These results collectively suggest that AC and remdesivir did not significantly decrease virus replication.
Discussion
In addition to vaccines, researchers and pharmaceutical companies have invested significant efforts into developing effective drugs to combat SARS-CoV-2. They have explored various inhibitors previously used to treat severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and other viral infections as potential treatments for COVID-19.50 Among these tested inhibitors, Remdesivir and Favipiravir, which target the RNA-dependent RNA polymerase, have emerged as the most promising repurposed drugs for COVID-19 treatment.51,52 Recent studies have also identified clinically used drugs such as Teriflunomide, Forodesine, and Didanosine as having the potential to combat SARS-CoV-2 by targeting the RNA-dependent RNA polymerase.10–13 In addition to targeting the RNA-dependent RNA polymerase, researchers have identified the SARS-CoV-2 main protease (Mpro) and papain-like protease (PLpro) as promising targets for inhibitory action against the virus.53 While numerous compounds have shown promise as potential inhibitors of SARS-CoV-2 in vitro, only a few have demonstrated effectiveness in vivo. Molnupiravir (EIDD-2801), a ribonucleoside prodrug that targets the viral RNA polymerase of SARS-CoV-2, and nirmatrelvir (PF-07321332), a selective inhibitor of SARS-CoV-2 Mpro, are two orally available SARS-CoV-2 inhibitors that have exhibited antiviral activity in animal models and have been validated through clinical trials.54,55 Furthermore, a recent development includes S-217622 (ensitrelvir), a small-molecule inhibitor for SARS-CoV-2 Mpro, which has demonstrated favorable bioavailability and tolerability in healthy adults during a Phase 1 clinical trial and is currently progressing through a Phase 3 clinical trial.56
In addition to inhibitors directly targeting viral particles, drugs or antibodies that focus on ACE2, thereby blocking SARS-CoV-2 from entering cells, represent another vital strategy against COVID-19. Chloroquine, an inhibitor of haem polymerase in malarial trophozoites, or its less toxic counterpart, hydroxychloroquine, has been shown to disrupt the protein-protein interaction between ACE2 and the SARS-CoV-2 spike protein. This disruption results in a reduced number of SARS-CoV-2 infections, achieved by binding to sialic acids and gangliosides on ACE2 with high affinity.57,58 In our study, we demonstrated that AC reduces ACE2 expression and interferes with the interaction between the SARS-CoV-2 spike protein and ACE2-expressing lung cells by directly binding to ACE2. This finding may partially explain why AC reduces SARS-CoV-2 infection in a pseudovirus infection cell model. However, it’s worth noting that ACE2 inhibition has been reported to have adverse effects, including increased inflammation and the promotion of lung and cardiovascular injury.5,59,60 ACE2 also plays a protective role by converting angiotensin II into angiotensin-(1-7), which activates the Mas receptor, triggering anti-inflammatory, anti-fibrotic, anti-oxidant, anti-hypertrophic, and anti-diabetic responses.61 Crucially, our research indicates that AC significantly, though not completely, reduces the expression and activity of ACE2. This suggests that AC may reduce the incidence of SARS-CoV-2 infections without causing severe adverse effects associated with complete ACE2 blockade.59,60 However, potential side effects resulting from AC-mediated ACE2 down-regulation warrant further investigation. Alternatively, neutralizing the SARS-CoV-2 spike protein with soluble ACE2 analogs can block SARS-CoV-2 infection without impacting cellular ACE2 activity.62
While drug repositioning approved by the FDA remains the predominant strategy in COVID-19 drug development, there is growing scientific interest in traditional Chinese medicine and natural products.63,64 SARS-CoV-2 infections involve several crucial proteins within both the virus and host cells. This complexity underscores the limitations of relying solely on single-structure molecular drugs that target a single protein. Natural products are distinguished by their highly diverse chemical structures, making them potential multi-target agents. This diversity serves as a valuable wellspring of inspiration for the development of SARS-CoV-2 inhibitors.65 For example, certain natural compounds like holyrine A, alotaketal C, and bafilomycin D have demonstrated effectiveness against SARS-CoV-2 Omicron subvariants BA.5 and BA.2, as well as the highly pathogenic Delta variant, in human Calu-3 lung cells.66 Moreover, natural products, particularly those derived from microbes, offer rich sources of lead compounds for drug discovery. Notably, aurasperone A, neochinulin A, as well as aspulvinone D, M, and R, isolated from Aspergillus niger, Aspergillus fumigatus, and Cladosporium sp., respectively, have demonstrated potent in vitro anti-SARS-CoV-2 activity.67–69 Furthermore, the “cytokine storm” represents one of the primary pathogenic mechanisms in COVID-19. Thus, interventions aimed at modulating the body’s cytokine response hold the potential to reduce the severity and mortality rate among COVID-19 patients. While some herbal medicines or natural products may not directly target SARS-CoV-2, their anti-inflammatory properties suggest their potential for preventing and treating COVID-19.70
A. cinnamomea is a valuable medicinal fungus with a long history of use, and one of its well-recognized biological applications is its anti-inflammatory activity.71,72 In the context of severe COVID-19, systemic hyperinflammation, often referred to as a cytokine storm, is a primary driver of acute respiratory distress syndrome. This underscores the importance of anti-inflammatory therapy in managing severe COVID-19.73–75 SARS-CoV-2 infection triggers an overproduction of IL-1β by activating the NLRP3 inflammasome, a process strongly associated with COVID-19 severity and clinical recovery in patients.30 IL-1β, induced by SARS-CoV-2 infection, promotes the secretion of tumor necrosis factor-α (TNF-α) and IL-6, along with other proinflammatory mediators, emphasizing the potential of blocking IL-1β as a valid COVID-19 treatment strategy.76 Our research found that AC significantly inhibited the NLRP3 inflammasome in macrophages, evidenced by a reduction in IL-1β production when stimulated by LPS and ATP. However, to strengthen the case, testing the effect of AC by directly stimulating macrophages with SARS-CoV-2 to activate the NLRP3 inflammasome would be more convincing. Due to safety concerns, such infection experiments should be conducted in a biosafety level 3 laboratory. Alternatively, instead of using the live SARS-CoV-2 virus, ssRNA and N-protein of SARS-CoV-2 can be employed to activate the NLRP3 inflammasome in macrophages, making it feasible in a regular laboratory setting.28,29 We plan to use ssRNA- or N-protein-stimulated macrophages in our future investigations to study AC’s anti-NLRP3 inflammasome activity. This study, along with prior work, establishes that A. cinnamomea and its bioactive components have the potential to inhibit the NLRP3 inflammasome under various pathophysiological conditions.47,77–80 We propose that AC’s ability to reduce body weight loss and mitigate lung injury in SARS-CoV-2-infected hamsters is primarily attributed to its inhibition of the NLRP3 inflammasome. Recent studies have shown that colchicine, one of the oldest anti-inflammatory drugs, can reduce the risk of hospitalization and mortality among COVID-19 patients by targeting the NLRP3 inflammasome to alleviate hyperinflammation.34,81 Interestingly, although AC was effective in reducing body weight loss and ameliorating lung injury in SARS-CoV-2-infected hamsters, it did not significantly reduce virus replication. Similarly, the widely recognized anti-COVID-19 drug, remdesivir, also did not show a significant reduction in virus replication in our study. It’s worth noting that a previous study administered remdesivir at a dosage of 15 mg/kg daily for four days, starting simultaneously with infection, and observed significant reductions in body weight loss, infectious viral progeny, and viral genomic RNA copy numbers in lung tissues.49 However, in our current study, administering 15 mg/kg remdesivir twice daily, with treatment initiated 24 hours after infection, did not result in a significant reduction in infectious viral progeny or viral genomic RNA copy numbers in lung tissues. This difference in results may be attributed to variations in the remdesivir treatment protocol employed in our study.
Several potential targets for COVID-19 drug development have been explored, including the inhibition of the viral replication cycle by targeting SARS-CoV-2 protease and RNA-dependent RNA-polymerase.82,83 However, the effectiveness of drugs targeting specific viral proteins may vary depending on SARS-CoV-2 variants and could lose efficacy due to viral mutations. Additionally, as SARS-CoV-2 continues to mutate rapidly, the number of vaccinations administered increases, but the protection conferred by vaccines weakens, and the risk of side effects rises.7,8 In comparison to vaccines and drugs designed to target SARS-CoV-2 proteins, AC, which targets ACE2, offers easy administration and is less reliant on the virus strain, providing broad-range protective activity. The advantage of AC against COVID-19 lies in its ability to improve lung injuries in the host by reducing inflammation. These findings suggest that AC offers broad protection against COVID-19, not limited to specific SARS-CoV-2 variants. Given AC’s ACE2-targeting and anti-inflammatory properties, there is a rationale for its use against SARS-CoV-2 infections to mitigate severe outcomes in COVID-19 patients. However, this study has certain limitations. Firstly, our investigation focused on evaluating AC’s effectiveness in hamsters infected with the Delta variant of SARS-CoV-2. We did not assess its efficacy against the Omicron variant, which is known for its high resistance to antibody-mediated neutralization.84 Another limitation is the absence of an assessment of AC’s impact on IL-6 expression in SARS-CoV-2-infected hamsters. IL-6 plays a crucial role in COVID-19.35,36 Previous studies have shown that AC inhibited IL-6 expression in LPS-activated myoblast cells, and its bioactive compounds Antcin A and Antcin K demonstrated the ability to suppress IL-6 expression in LPS-activated macrophages and reduce serum IL-6 levels in collagen-induced arthritis mice, respectively.85,86 However, further research is needed to investigate AC’s effect on SARS-CoV-2-induced IL-6 expression. Finally, this study did not include measurements of DA levels in plasma or tissue samples, which could have provided additional insights into the mechanisms at play. These limitations highlight potential avenues for future research and the expansion of our understanding in this field. The current observations in the functional and mechanistic experiments we conducted in cell and hamster models represent a novel finding, indicating that A. cinnamomea is an orally bioavailable natural product that is beneficial in COVID-19 by interfering with the interaction between ACE2 and the SARS-CoV-2 spike protein in vitro and reducing lung inflammation in COVID-19 in vivo.
Conclusion
The recent findings from our functional and mechanistic experiments conducted in cell and hamster models reveal a novel discovery: A. cinnamomea is beneficial in the context of COVID-19. It interferes with the interaction between ACE2 and the SARS-CoV-2 spike protein, effectively reducing lung inflammation associated with COVID-19. These results strongly suggest that A. cinnamomea can potentially serve as a functional food or nutritional supplement in the future. Such utilization could enhance the effectiveness of COVID-19 vaccines and reduce the risk of severe disease among patients.
Abbreviations
COVID-19, Coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; AC, Antrodia cinnamomea ethanol extract; DA, dehydrosulphurenic acid; ACE2, angiotensin-converting enzyme-2; IL, interleukin; NLRP3, NACHT, LRR and PYD domain-containing protein 3; RLU, relative light unit; TCID50, the fifty percent tissue culture infectious dose; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SARS, severe acute respiratory syndrome, MERS, middle East respiratory syndrome.
Acknowledgments
This research work is supported by the funding from the National Science and Technology Council, Taiwan (MOST 111-2628-B-197-001-MY3 and MOST 111-2811-B-197-001 to K.-F. H.; MOST 111-2320-B-532-002 to L.-H. L.).
Disclosure
Shui-Tein Chen is the chairman of ALPS Biotech Co. Ltd. Ko-Chieh Huang and Tzu-Wen Lin are employees in ALPS Biotech Co. Ltd. Dr Shui-Tein Chen has a patent Uses of antrodia cinnamomea extract in manufacturing products for reducing expression and treating associated diseases of angiotensin converting enzyme 2 pending to None. Dr Kuo-Feng Hua reports a patent Uses of antrodia cinnamomea extract in manufacturing products for reducing expression and treating associated diseases of angiotensin converting enzyme 2 pending to US20230181662A1. The authors report no other conflicts of interest in this work.
References
1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
2. Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi:10.1126/science.abb2762
3. Pinto BGG, Oliveira AER, Singh Y, et al. ACE2 Expression Is Increased in the Lungs of Patients With Comorbidities Associated With Severe COVID-19. J Infect Dis. 2020;222(4):556–563. doi:10.1093/infdis/jiaa332
4. Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. doi:10.1093/cvr/cvaa078
5. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi:10.1038/nm1267
6. Altmann DM, Boyton RJ. COVID-19 vaccination: the road ahead. Science. 2022;375(6585):1127–1132. doi:10.1126/science.abn1755
7. Khames Aga QA A, Alkhaffaf WH, Hatem TH, et al. Safety of COVID-19 vaccines. J Med Virol. 2021;93(12):6588–6594. doi:10.1002/jmv.27214
8. Zhuang C, Liu X, Chen Q, et al. Protection Duration of COVID-19 Vaccines: waning Effectiveness and Future Perspective. Front Microbiol. 2022;13:828806. doi:10.3389/fmicb.2022.828806
9. Kibongani Volet A, Scavone C, Catalán-Matamoros D, et al. Vaccine Hesitancy Among Religious Groups: reasons Underlying This Phenomenon and Communication Strategies to Rebuild Trust. Front Public Health. 2022;10:824560. doi:10.3389/fpubh.2022.824560
10. Rabie AM. Teriflunomide: a possible effective drug for the comprehensive treatment of COVID-19. Curr Res Pharmacol Drug Discov. 2021;2:100055. doi:10.1016/j.crphar.2021.100055
11. Rabie AM, Abdalla M. Forodesine and Riboprine Exhibit Strong Anti-SARS-CoV-2 Repurposing Potential: in Silico and In Vitro Studies. ACS Bio Med Chem Au. 2022;2(6):565–585. doi:10.1021/acsbiomedchemau.2c00039
12. Rabie AM, Abdalla M. Evaluation of a series of nucleoside analogs as effective anticoronaviral-2 drugs against the Omicron-B.1.1.529/BA.2 subvariant: a repurposing research study. Med Chem Res. 2023;32(2):326–341. doi:10.1007/s00044-022-02970-3
13. Rabie AM. Efficacious Preclinical Repurposing of the Nucleoside Analogue Didanosine against COVID-19 Polymerase and Exonuclease. ACS Omega. 2022;7(25):21385–21396. doi:10.1021/acsomega.1c07095
14. Rabie AM. Potent toxic effects of Taroxaz-104 on the replication of SARS-CoV-2 particles. Chem Biol Interact. 2021;343:109480. doi:10.1016/j.cbi.2021.109480
15. Eltayb WA, Abdalla M, Rabie AM. Novel Investigational Anti-SARS-CoV-2 Agent Ensitrelvir “S-217622”: a Very Promising Potential Universal Broad-Spectrum Antiviral at the Therapeutic Frontline of Coronavirus Species. ACS Omega. 2023;8(6):5234–5246. doi:10.1021/acsomega.2c03881
16. Rabie AM. Two antioxidant 2,5-disubstituted-1,3,4-oxadiazoles (CoViTris2020 and ChloViD2020): successful repurposing against COVID-19 as the first potent multitarget anti-SARS-CoV-2 drugs. New J Chem. 2021;45:761–771. doi:10.1039/D0NJ03708G
17. Rabie AM, Eltayb WA. Potent Dual Polymerase/Exonuclease Inhibitory Activities of Antioxidant Aminothiadiazoles Against the COVID-19 Omicron Virus: a Promising In Silico/In Vitro Repositioning Research Study. Mol Biotechnol. 2023;1–20. doi:10.1007/s12033-022-00551-8
18. Rabie AM, Abdel-Dayem MA, Abdalla M. Promising Experimental Anti-SARS-CoV‑2 Agent “SLL-0197800”: the Prospective Universal Inhibitory Properties against the Coming Versions of the Coronavirus. ACS Omega. 2023;8(39):XXXXX–XXXXX. doi:10.1021/acsomega.2c08073
19. Rabie AM. Potent Inhibitory Activities of the Adenosine Analogue Cordycepin on SARS-CoV-2 Replication. ACS Omega. 2022;7(3):2960–2969. doi:10.1021/acsomega.1c05998
20. Rabie AM. New Potential Inhibitors of Coronaviral Main Protease (CoV-Mpro): strychnine Bush, Pineapple, and Ginger could be Natural Enemies of COVID-19. Int J New Chem. 2022;9(3):225–237.
21. Seyed Hosseini E, Riahi Kashani N, Nikzad H, et al. The novel coronavirus Disease-2019 (COVID-19): mechanism of action, detection and recent therapeutic strategies. Virology. 2020;551:1–9. doi:10.1016/j.virol.2020.08.011
22. Shin YH, Jeong K, Lee J, et al. Inhibition of ACE2-Spike Interaction by an ACE2 Binder Suppresses SARS-CoV-2 Entry. Angew Chem Int Ed Engl. 2022;61(11):e202115695. doi:10.1002/anie.202115695
23. Bojadzic D, Alcazar O, Chen J, et al. Small-Molecule Inhibitors of the Coronavirus Spike: ACE2 Protein-Protein Interaction as Blockers of Viral Attachment and Entry for SARS-CoV-2. ACS Infect Dis. 2021;7(6):1519–1534. doi:10.1021/acsinfecdis.1c00070
24. Chen R, Lan Z, Ye J, et al. Cytokine Storm: the Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front Immunol. 2021;12:589095. doi:10.3389/fimmu.2021.589095
25. Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–256. doi:10.1002/jmv.26232
26. Pandey A, Shen C, Feng S, et al. Cell biology of inflammasome activation. Trends Cell Biol. 2021;31(11):924–939. doi:10.1016/j.tcb.2021.06.010
27. Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22(5):550–559. doi:10.1038/s41590-021-00886-5
28. Pan P, Shen M, Yu Z, et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun. 2021;12(1):4664. doi:10.1038/s41467-021-25015-6
29. Campbell GR, To RK, Hanna J, et al. SARS-CoV-2, SARS-CoV-1, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. iScience. 2021;24(4):102295. doi:10.1016/j.isci.2021.102295
30. Rodrigues TS, de Sá KSG, Ishimoto AY, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021;218(3):e20201707. doi:10.1084/jem.20201707
31. Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi:10.1016/S2665-9913(20)30127-2
32. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi:10.1016/S2665-9913(20)30164-8
33. Amin S, Aktar S, Rahman MM, et al. NLRP3 inflammasome activation in COVID-19: an interlink between risk factors and disease severity. Microbes Infect. 2022;24(1):104913. doi:10.1016/j.micinf.2021.104913
34. Bonaventura A, Vecchié A, Dagna L, et al. Colchicine for COVID-19: targeting NLRP3 inflammasome to blunt hyperinflammation. Inflamm Res. 2022;71(3):293–307. doi:10.1007/s00011-022-01540-y
35. Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–844. doi:10.1038/s41591-020-0901-9
36. Zizzo G, Tamburello A, Castelnovo L, et al. Immunotherapy of COVID-19: inside and Beyond IL-6 Signalling. Front Immunol. 2022;13:795315. doi:10.3389/fimmu.2022.795315
37. Potere N, Batticciotto A, Vecchié A, et al. The role of IL-6 and IL-6 blockade in COVID-19. Expert Rev Clin Immunol. 2021;17(6):601–618. doi:10.1080/1744666X.2021.1919086
38. van de Veerdonk FL, Netea MG. Blocking IL-1 to prevent respiratory failure in COVID-19. Crit Care. 2020;24(1):445. doi:10.1186/s13054-020-03166-0
39. Ganesan N, Baskaran R, Velmurugan BK, et al. Antrodia cinnamomea-An updated minireview of its bioactive components and biological activity. J Food Biochem. 2019;43(8):e12936. doi:10.1111/jfbc.12936
40. Kuang Y, Li B, Wang Z, et al. Terpenoids from the medicinal mushroom Antrodia camphorata: chemistry and medicinal potential. Nat Prod Rep. 2021;38(1):83–102. doi:10.1039/D0NP00023J
41. Senthil Kumar KJ, Gokila Vani M, Hsieh HW, et al. Antcins from Antrodia cinnamomea and Antrodia salmonea Inhibit Angiotensin-Converting Enzyme 2 (ACE2) in Epithelial Cells: can Be Potential Candidates for the Development of SARS-CoV-2 Prophylactic Agents. Plants. 2021;10(8):1736. doi:10.3390/plants10081736
42. Chen CY, Chien SC, Tsao NW, et al. Metabolite Profiling and Comparison of Bioactivity in Antrodia cinnamomea and Antrodia salmonea Fruiting Bodies. Planta Med. 2016;82(3):244–249. doi:10.1055/s-0035-1558141
43. Huang HT, Wang SL, Nguyen VB, et al. Isolation and Identification of Potent Antidiabetic Compounds from Antrodia cinnamomea-An Edible Taiwanese Mushroom. Molecules. 2018;23(11):2864. doi:10.3390/molecules23112864
44. Chu YW, Yang PC, Yang SC, et al. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am J Respir Cell Mol Biol. 1997;17(3):353–360. doi:10.1165/ajrcmb.17.3.2837
45. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27(3):493–497. doi:10.1093/oxfordjournals.aje.a118408
46. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi:10.2807/1560-7917.ES.2020.25.3.2000045
47. Yen IC, Lin JC, Chen Y, et al. Antrodia Cinnamomea Attenuates Non-Alcoholic Steatohepatitis by Suppressing NLRP3 Inflammasome Activation In Vitro and In Vivo. Am J Chin Med. 2020;48(8):1859–1874. doi:10.1142/S0192415X20500937
48. Muñoz-Fontela C, Dowling WE, Funnell SGP, et al. Animal models for COVID-19. Nature. 2020;586(7830):509–515. doi:10.1038/s41586-020-2787-6
49. Shytaj IL, Fares M, Gallucci L, et al. The FDA-Approved Drug Cobicistat Synergizes with Remdesivir To Inhibit SARS-CoV-2 Replication In Vitro and Decreases Viral Titers and Disease Progression in Syrian Hamsters. mBio. 2022;13(2):e0370521. doi:10.1128/mbio.03705-21
50. Leowattana W. COVID-19: potential Repurposing Drugs. Infect Disord Drug Targets. 2022;22(1):e110122191924. doi:10.2174/1871526521666210301143441
51. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi:10.1038/s41422-020-0282-0
52. Hassanipour S, Arab-Zozani M, Amani B, et al. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021;11(1):11022. doi:10.1038/s41598-021-90551-6
53. Tao K, Tzou PL, Nouhin J, et al. SARS-CoV-2 Antiviral Therapy. Clin Microbiol Rev. 2021;34(4):e0010921. doi:10.1128/CMR.00109-21
54. Wahl A, Gralinski LE, Johnson CE, et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591(7850):451–457. doi:10.1038/s41586-021-03312-w
55. Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586–1593. doi:10.1126/science.abl4784
56. Sasaki M, Tabata K, Kishimoto M, et al. S-217622, a SARS-CoV-2 main protease inhibitor, decreases viral load and ameliorates COVID-19 severity in hamsters. Sci Transl Med. 2023;15(679):eabq4064. doi:10.1126/scitranslmed.abq4064
57. Wang N, Han S, Liu R, et al. Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus. Phytomedicine. 2020;79:153333. doi:10.1016/j.phymed.2020.153333
58. Fantini J, Di Scala C, Chahinian H, et al. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55(5):105960. doi:10.1016/j.ijantimicag.2020.105960
59. Razeghian-Jahromi I, Zibaeenezhad MJ, Lu Z, et al. Angiotensin-converting enzyme 2: a double-edged sword in COVID-19 patients with an increased risk of heart failure. Heart Fail Rev. 2021;26(2):371–380. doi:10.1007/s10741-020-10016-2
60. Issa H, Eid AH, Berry B, et al. Combination of Angiotensin (1-7) Agonists and Convalescent Plasma as a New Strategy to Overcome Angiotensin Converting Enzyme 2 (ACE2) Inhibition for the Treatment of COVID-19. Front Med. 2021;8:620990. doi:10.3389/fmed.2021.620990
61. Cantero-Navarro E, Fernández-Fernández B, Ramos AM, et al. Renin-angiotensin system and inflammation update. Mol Cell Endocrinol. 2021;529:111254. doi:10.1016/j.mce.2021.111254
62. Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020;181(4):905–913.e7. doi:10.1016/j.cell.2020.04.004
63. Ang L, Song E, Zhang J, et al. Herbal medicine for COVID-19: an overview of systematic reviews and meta-analysis. Phytomedicine. 2022;102:154136. doi:10.1016/j.phymed.2022.154136
64. Zhao Z, Li Y, Zhou L, et al. Prevention and treatment of COVID-19 using Traditional Chinese Medicine: a review. Phytomedicine. 2021;85:153308. doi:10.1016/j.phymed.2020.153308
65. Zhao Y, Deng S, Bai Y, et al. Promising natural products against SARS-CoV-2: structure, function, and clinical trials. Phytother Res. 2022;36(10):3833–3858. doi:10.1002/ptr.7580
66. Pérez-Vargas J, Shapira T, Olmstead AD, et al. Discovery of lead natural products for developing pan-SARS-CoV-2 therapeutics. Antiviral Res. 2023;209:105484. doi:10.1016/j.antiviral.2022.105484
67. ElNaggar MH, Abdelwahab GM, Kutkat O, et al. Aurasperone A Inhibits SARS CoV-2 In Vitro: an Integrated In Vitro and In Silico Study. Mar Drugs. 2022;20(3):179. doi:10.3390/md20030179
68. Alhadrami HA, Burgio G, Thissera B, et al. Neoechinulin A as a Promising SARS-CoV-2 Mpro Inhibitor: In vitro and In Silico Study Showing the Ability of Simulations in Discerning Active from Inactive Enzyme Inhibitors. Mar Drugs. 2022;20(3):163. doi:10.3390/md20030163
69. Liang XX, Zhang XJ, Zhao YX, et al. Aspulvins A-H, Aspulvinone Analogues with SARS-CoV-2 Mpro Inhibitory and Anti-inflammatory Activities from an Endophytic Cladosporium sp. J Nat Prod. 2022;85(4):878–887. doi:10.1021/acs.jnatprod.1c01003
70. Dai YJ, Wan SY, Gong SS, et al. Recent advances of traditional Chinese medicine on the prevention and treatment of COVID-19. Chin J Nat Med. 2020;18(12):881–889. doi:10.1016/S1875-5364(20)60031-0
71. Chuang SY, Chen CY, Yang SC, et al. 2,4-Dimethoxy-6-Methylbenzene-1,3-diol, a Benzenoid From Antrodia cinnamomea, Mitigates Psoriasiform Inflammation by Suppressing MAPK/NF-κB Phosphorylation and GDAP1L1/Drp1 Translocation. Front Immunol. 2021;12:664425. doi:10.3389/fimmu.2021.664425
72. Lu MK, Lee MH, Chao CH, et al. Physiochemical changes and mechanisms of anti-inflammation effect of sulfated polysaccharides from ammonium sulfate feeding of Antrodia cinnamomea. Int J Biol Macromol. 2020;148:715–721. doi:10.1016/j.ijbiomac.2020.01.110
73. Kim JS, Lee JY, Yang JW, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–329. doi:10.7150/thno.49713
74. Amati F, Tonutti A, Huston J, et al. Glucocorticoid Therapy in COVID-19. Semin Respir Crit Care Med. 2023;44(1):100–117. doi:10.1055/s-0042-1759778
75. Wang Y, Perlman S. COVID-19: inflammatory Profile. Annu Rev Med. 2022;73(1):65–80. doi:10.1146/annurev-med-042220-012417
76. Conti P, Caraffa A, Gallenga CE, et al. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: a promising inhibitory strategy. J Biol Regul Homeost Agents. 2020;34(6):1971–1975. doi:10.23812/20-1-E
77. Yen IC, Tu QW, Chang TC, et al. 4-Acetylantroquinonol B ameliorates nonalcoholic steatohepatitis by suppression of ER stress and NLRP3 inflammasome activation. Biomed Pharmacother. 2021;138:111504. doi:10.1016/j.biopha.2021.111504
78. Han C, Shen H, Yang Y, et al. Antrodia camphorata polysaccharide resists 6-OHDA-induced dopaminergic neuronal damage by inhibiting ROS-NLRP3 activation. Brain Behav. 2020;10(11):e01824. doi:10.1002/brb3.1824
79. Liu WH, Shi LS, Chung MC, et al. Antcamphin M Inhibits TLR4-Mediated Inflammatory Responses by Upregulating the Nrf2/HO-1 Pathway and Suppressing the NLRP3 Inflammasome Pathway in Macrophages. Am J Chin Med. 2019;47(7):1611–1626. doi:10.1142/S0192415X19500824
80. Huang TT, Wu SP, Chong KY, et al. The medicinal fungus Antrodia cinnamomea suppresses inflammation by inhibiting the NLRP3 inflammasome. J Ethnopharmacol. 2014;155(1):154–164. doi:10.1016/j.jep.2014.04.053
81. Reyes AZ, Hu KA, Teperman J, et al. Anti-inflammatory therapy for COVID-19 infection: the case for colchicine. Ann Rheum Dis. 2021;80(5):550–557. doi:10.1136/annrheumdis-2020-219174
82. Banerjee R, Perera L, Tillekeratne LMV. Potential SARS-CoV-2 main protease inhibitors. Drug Discov Today. 2021;26(3):804–816. doi:10.1016/j.drudis.2020.12.005
83. Vicenti I, Zazzi M, Saladini F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert Opin Ther Pat. 2021;31(4):325–337. doi:10.1080/13543776.2021.1880568
84. Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185(3):447–456.e11. doi:10.1016/j.cell.2021.12.032
85. Yang X, Wang X, Lin J, et al. Structure and Anti-Inflammatory Activity Relationship of Ergostanes and Lanostanes in Antrodia cinnamomea. Foods. 2022;11(13):1831. doi:10.3390/foods11131831
86. Achudhan D, Liu SC, Lin YY, et al. Antcin K Inhibits TNF-α, IL-1β and IL-8 Expression in Synovial Fibroblasts and Ameliorates Cartilage Degradation: implications for the Treatment of Rheumatoid Arthritis. Front Immunol. 2021;12:790925. doi:10.3389/fimmu.2021.790925
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.