Back to Journals » OncoTargets and Therapy » Volume 7
Antitumor activity of total flavonoids from Tetrastigma hemsleyanum Diels et Gilg is associated with the inhibition of regulatory T cells in mice
Authors Feng Z, Hao W, Lin X, Fan D, Zhou J
Received 3 February 2014
Accepted for publication 7 April 2014
Published 9 June 2014 Volume 2014:7 Pages 947—956
DOI https://doi.org/10.2147/OTT.S61794
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Zhengquan Feng,1,2 Wanrong Hao,3 Xiaoyang Lin,3 Daping Fan,4 Juhua Zhou5
1Department of Oncology, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang, People's Republic of China; 2Department of Oncology, Guang An Men Hospital, China Academy of Chinese Medical Sciences, Beijing, People's Republic of China; 3Department of Chinese Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People's Republic of China; 4Department of Cell Biology and Anatomy, School of Medicine, University of South Carolina, Columbia, SC, USA; 5Institute for Tumor Immunology, Ludong University College of Life Sciences, Yantai, Shandong, People's Republic of China
Objective: To determine the antitumor activity of Radix tetrastigmae flavonoids and their inhibitory effect on regulatory T cells (Tregs) in mice.
Materials and methods: Total flavonoids were isolated from Radix tetrastigmae, the root of Tetrastigma hemsleyanum Diels et Gilg, and administered to C57BL/6 mice by oral gavage after inoculation with Lewis lung carcinoma (LLC) cells. The effects of total flavonoids on tumor growth in vivo were examined. Flow cytometry was used to study the effects on Tregs, and enzyme-linked immunosorbent assay was used to analyze the changes in the serum levels of transforming growth factor ß, prostaglandin E2, and cyclooxygenase 2 after tumor inoculation and flavonoid administration.
Results: Total flavonoids from T. hemsleyanum Diels et Gilg significantly inhibited tumor growth in C57BL/6 mice inoculated with LLCs. These flavonoids dramatically suppressed regulatory T-cell development in tumor-bearing mice. Further studies revealed that total flavonoids significantly decreased the serum levels of transforming growth factor β, prostaglandin E2, and cyclooxygenase 2 in tumor-bearing mice, which may be responsible for the inhibition of Tregs.
Conclusion: The antitumor activity of total flavonoids from T. hemsleyanum Diels et Gilg is associated with the inhibition of Tregs in a mouse tumor model. Total flavonoids from T. hemsleyanum Diels et Gilg may be used as antitumor agents in cancer prevention and treatment.
Keywords: flavonoids, radix tetrastigmae, regulatory T cells, tumor growth, TGF-β1
Introduction
It has been recognized that flavonoids have antitumor activity at several stages in cancer development and progression, and thus can potentially be used as antitumor agents.1 However, the mechanisms of flavonoid-mediated antitumor functions are not fully understood. It has been reported that flavonoids from plants such as Macrothelypteris viridifrons and Vitex rotundifolia have strong antiproliferative effects on tumor cells.2,3 They may induce tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in cancer cells.4 Other studies showed that flavonoids could inhibit the activation of nuclear factor kappa-light chain enhancer of activated B cells and suppress the activities of epidermal growth factor and kinase insert domain receptor tyrosine kinases, and thus induce apoptosis in tumor cells.5 Flavonoids also inhibit the release of angiogenic factors, particularly vascular endothelial growth factor, from human breast cancer cells, and thus suppress tumor development and progression.6 Furthermore, flavonoids inhibit the expression of vascular cell adhesion molecule 1 in human umbilical vein endothelial cells and murine pulmonary endothelial cells7 and attenuate the expression of E-selectin and intercellular adhesion molecule 1 in human endothelial cells,8 suggesting flavonoids may impair the interactions between tumor cells and endothelial cells, and thus inhibit melanoma lung metastasis in mice. Therefore, flavonoids may be used as effective preventive and therapeutic agents, although the mechanisms of their actions are unclear.9–11
It has been documented that regulatory T cells (Tregs) play an important inhibitory role in the regulation of immune responses.12,13 Tregs express CD4, CD25, and Foxp3.14 They are often associated with solid tumors in both cancer patients and animal models. Increased numbers of Tregs have been observed in breast, colorectal, and ovarian cancers.15 Inhibitory activities of Tregs on other CD4 T cells and cytotoxic CD8 T cells depend on cell–cell contact and secretion of inhibitory cytokines such as interleukin 10 and transforming growth factor beta (TGF-β).16 Tregs contribute to cancer development and are responsible for the failure of current therapies mediated by antitumor responses.17 Several factors such as interleukin 2 and TGF-β are essential for Treg development and homeostasis.18,19 Chemotherapeutic agents such as cyclophosphamide and paclitaxel inhibit Tregs, which are partially responsible for their antitumor activities.20–22 However, little is known about the role of flavonoids in the regulation of Treg development and antitumor immunity.
Our previous studies indicated that total flavonoids isolated from Radix tetrastigmae (the root of Tetrastigma hemsleyanum Diels et Gilg) could inhibit the cell growth of SGC-7901 human gastric cancer cell line in vitro.23 In the current study, we found that total flavonoids from T. hemsleyanum Diels et Gilg could significantly inhibit the tumor growth of the Lewis lung carcinoma (LLC) cell line and Tregs in mice. The results suggest that the antitumor activity of total flavonoids from T. hemsleyanum Diels et Gilg is associated with the inhibition of Tregs in vivo in a mouse tumor model.
Materials and methods
Materials and total flavonoid isolation
Radix Tetrastigmae, the root of T. hemsleyanum Diels et Gilg, was purchased from the pharmaceutical store in the Zhejiang Chinese Medical University-affiliated First Hospital, Hangzhou, People’s Republic of China. Total flavonoids were extracted from Radix Tetrastigmae according to the method reported by Liu et al.24 Briefly, the powdered root (100 g) of T. hemsleyanum Diels et Gilg was extracted twice with 600 mL of 60% ethanol in a boiling water bath for 90 minutes and then filtered. The filtrate was concentrated under vacuum at 45°C, freeze-dried, and stored at 4°C. Approximately 2 g of the total flavonoid extract was obtained. The quality of the total flavonoid extract was determined by colorimetric HCl-Mg and aluminum chloride reactions, followed by high-performance liquid chromatography.24,25
Mice and tumor inoculation
Healthy wild-type C57BL/6 (B6) mice without immunosuppression and other immunological disorders were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, People’s Republic of China, and housed in the Laboratory Animal Center at Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China. The animal protocol was approved by the Institutional Animal Use Committee of Zhejiang Chinese Medical University. All mice were maintained under pathogen-free conditions.
The LLC cell line was purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences, Shanghai, People’s Republic of China, and maintained in RPMI 1640 medium with 5% fetal calf serum in a humidified 5% CO2 incubator. After washing twice, LLC cells were suspended in phosphate-buffered saline (PBS). LLC cells (2×106) in 200 μL PBS were subcutaneously injected in the right axillary fossa of each 2-month-old mouse. The same volume of PBS was subcutaneously injected in the right axillary fossa of each 2-month-old mouse as control.
Total flavonoid administration
Starting on the second day after tumor inoculation, the total flavonoids extracted from Radix Tetrastigmae were dissolved in 0.9% saline solution and administered in mice by oral gavage once daily at doses of 0, 5, 10, or 15 mg/kg mouse body weight. Each group consisted of 10 mice. Changes of mouse appearance, mouse body weight, and tumor size were examined and recorded daily. Tumor volumes were calculated on the basis of the measurement of tumor sizes according to the previously published method of Euhus et al.26 At 14 days after tumor inoculation and total flavonoid administration, mice were killed for tumor weight determination, peripheral blood collection, and splenocyte preparation.
Examination of transmission electron microscopy
Tumor cells were separated from mice at day 14 after tumor cell inoculation and flavonoid administration and were fixed in 4% glutaraldehyde for 2 hours. After washing with PBS, the cells were fixed in 1% OsO4 for 1 hour. Then, the cell samples were dehydrated by graded alcohol, embedded with Epon 812, and sectioned. After staining with uranium acetate and lead citrate, the sections were examined under Hatachy H-600 transmission electron microscope.
Flow cytometry
After mice were killed, peripheral blood samples were collected in heparin-coated tubes. Peripheral blood (50 μL) from each mouse was centrifuged to prepare serum. The remaining peripheral blood was used for peripheral blood leukocyte preparation by Ficoll centrifugation. Spleen was removed and pulverized for splenocyte separation. After red blood cell lysis, washing, and centrifugation, splenocytes were obtained.
Peripheral blood leukocytes and splenocytes were first stained with fluorescein isothiocyanate-conjugated anti-mouse CD4 and phycoerythrin-conjugated anti-mouse CD25 (eBioscience, San Diego, CA, USA). For Foxp3 staining, cells were treated with allophycocyanin-conjugated anti-mouse Foxp3 according to the manufacturer’s instructions (eBioscience). After staining, cells were analyzed with a FACSCalibur flow cytometer (BD-Bioscience, San Jose, CA, USA), using FlowJo software (Tree Star, San Carlos, CA, USA). The percentage of Tregs in total peripheral blood leukocytes and splenocytes was calculated.
TGF-β1, PGE2, and COX2 measurement
Serum samples from mice were used for the measurement of serum levels of TGF-β1, prostaglandin E2 (PGE2), and cyclooxygenase 2 (COX2) by the enzyme-linked immunosorbent assay (ELISA). The serum levels of TGF-β1 were measured using a Quantikine mouse TGF-β1 ELISA Kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions.27 The serum levels of PGE2 and COX2 were measured using a mouse PGE2 ELISA Kit and a mouse COX2 ELISA kit (MyBioSource, San Diego, CA, USA), respectively, according to the manufacturer’s instructions.
Statistical analysis
Quantitative data were expressed as the mean ± standard deviation. The statistical analysis was carried out using SPSS Statistics 17.0 software (SPSS Inc., Chicago, IL, USA). Statistical differences between control group without flavonoid administration and other groups were compared using the Wilcoxon rank sum test for P-value calculation. All P-values lower than 0.05 were considered significant.
Results
Effects of total flavonoids on mouse growth
All groups of mice had similar baseline body weights (Figure 1A). At the end point, the group of mice receiving the LLC cell inoculation but without flavonoid treatment had significantly decreased body weights compared with the control group (Figure 1B), suggesting tumor growth suppressed the increase of mouse body weights. Administration of total flavonoids from Radix Tetrastigmae at higher doses significantly rescued the body weight gain in mice inoculated with LLC cells (Figure 1B). The results indicated that total flavonoids from Radix Tetrastigmae improved the overall health of the mice by suppressing tumor growth in mice.
Effects of total flavonoids on tumor growth
After LLC cell inoculation, tumor lesions became visible at day 5. After day 7, the administration of total flavonoids significantly inhibited tumor growth in mice at a dose-dependent manner in comparison with the untreated group (Figure 2A). At day 14, the lower-dose group also showed a significant reduction of tumor volumes compared with the untreated group (Figure 2B). The results suggested that total flavonoids from Radix Tetrastigmae suppressed tumor growth in mice.
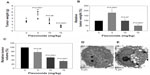
At day 14, the administration of total flavonoids from Radix Tetrastigmae at a high dose significantly suppressed the increase of tumor weights in mice (Figure 3A and B). Interestingly, the administration of total flavonoids from Radix Tetrastigmae at a low dose at day 14 also significantly inhibited the increase of tumor volumes in mice (Figure 3C). As compared with tumor cells from mice without flavonoid administration (Figure 3D), analysis by transmission electron microscopy indicated that the treatment by a low dose of total flavonoids induced the formation of irregular nucleus and bubble-like structures in the cytoplasm and loss of normal nucleoli (Figure 3E), suggesting that flavonoid administration led to apoptosis of tumor cells in mice. Thus, it appeared that flavonoids from Radix Tetrastigmae suppressed tumor growth in vivo.
Effects of total flavonoids on Treg number
Because flavonoids from Radix Tetrastigmae could inhibit tumor growth in mice (Figures 2 and 3), and Tregs play a critical role in the suppression of antitumor immunity,12,13,17 we aimed to investigate whether flavonoids could inhibit Treg development in mice. Tregs were defined as CD25+ and FoxP3+ CD4+ T cells (Figure 4) and can inhibit the functions of other CD4 T cells and cytotoxic CD8 T cells by means of cell–cell contact or secretion of the inhibitory cytokines interleukin 10 and TGF-β.16 The results showed that flavonoids from Radix Tetrastigmae significantly reduced Treg number in peripheral blood leukocytes (Figures 4E, F and 5A) and, at higher doses, decreased Treg number in the spleen as well (Figure 5B). The results suggested that flavonoids from Radix Tetrastigmae suppress Treg development, and thus inhibit tumor growth in mice.
Effects of total flavonoids on the expression of TGF-β1, PGE2, and COX2
It has been reported that several factors such as TGF-β1, PGE2, and COX2 play important roles in the induction, maintenance, and functions of Tregs.28,29 Thus, to understand the mechanisms of flavonoid-mediated downregulation of Tregs, we examined the effects of total flavonoids on these factors. ELISA analysis showed that the administration of total flavonoids from Radix Tetrastigmae significantly decreased the serum levels of TGF-β1, PGE2, and COX2 in the peripheral blood of mice (Figure 6), suggesting that flavonoids from Radix Tetrastigmae may downregulate the expression of TGF-β1, PGE2, and COX2, leading to the suppression of Tregs and inhibition of tumor growth in mice.
Discussion
It has been shown that flavonoids can induce apoptosis and inhibit proliferation of tumor cells, and thus have antitumor activities.2–4 Our previous studies revealed that flavonoids from Radix Tetrastigmae induce apoptosis and inhibit the growth of gastric cancer cells in vitro.23 Our current study revealed that administration of flavonoids from Radix Tetrastigmae significantly inhibits tumor growth in a murine cancer model (Figures 2 and 3). Therefore, flavonoids may be used as antitumor agents in the prevention and treatment of cancer. Clinical studies have shown that flavonoids may have great implications in the prevention and treatment of cancer in humans.30–32 The results suggest that flavonoids from Radix Tetrastigmae may be good candidates for clinical trials in the prevention and treatment of human cancers.
It is obvious that the administration of total flavonoids from Radix Tetrastigmae at a low dose in mice significantly suppressed the increase of tumor volumes (Figures 2 and 3C) but increased the tumor weights at day 14 after LLC tumor inoculation (Figure 3A and B). Analysis by transmission electron microscopy indicated that treatment by a low dose of total flavonoids stimulated the formation of bubble-like structures in the cytoplasm of tumor cells in mice (Figure 3E), suggesting that the administration of flavonoids may induce apoptosis of tumor cells. It is possible that the bubble-like structures in the cytoplasm of tumor cells may be full of fluids, which may be responsible for the increase of tumor weights in mice at day 14 after tumor inoculation and flavonoid administration at a low dose (Figure 3A and B). The results suggested that a higher dose of total flavonoids from Radix Tetrastigmae would be preferred for cancer treatment.
It has been well documented that antitumor immune responses play a pivotal role in cancer development and treatment.33–35 Tregs have important functions in the regulation of antitumor immune responses.12,13,36 Little is known about the effects of flavonoids on the regulation of antitumor immune responses. It has been reported that wogonin was the dominant flavonoid in Scutellaria ocmulgee leaf extract and that the oral administration of S. ocmulgee leaf extract significantly inhibited intratumoral TGF-β1 level and Treg cell frequency in F344 rats transplanted subcutaneously with F98 gliomas.37 Thus, we were interested in understanding whether flavonoids from Radix Tetrastigmae could inhibit Treg in LLC mouse model. Our study showed that flavonoids from Radix Tetrastigmae significantly decreased Tregs in mice (Figure 5), suggesting that flavonoids from Radix Tetrastigmae may have a regulatory role in the antitumor immunity by downregulating Tregs. It has been reported that Baicalin, a flavonoid from Huangqin (Scutellaria baicalensis Georgi), promotes Treg development.38 Thus, different flavonoids may have opposite effects on Treg development.
TGF-β has been shown to play an important role in the induction, maintenance, and function of Tregs.28,39 Further studies demonstrated that TGF-β induced COX2 expression.40 COX2 regulates PGE2 expression, which was shown to induce FoxP3 expression.41 Thus, the COX2-PGE2 pathway is involved in the induction, maintenance, and function of Tregs.29 Our analysis revealed that flavonoids from Radix Tetrastigmae significantly decreased the serum levels of TGF-β1, PGE2, and COX2 in mice (Figure 6). These results therefore suggest that flavonoids from Radix Tetrastigmae may suppress the expression of TGF-β, PGE2, and COX2, and thus inhibit Treg development.
High-performance liquid chromatography analysis showed that quercetin and kaempferol were present in total flavonoids from Radix Tetrastigmae. It has been reported that both quercetin and kaempferol have anticancer function.42–44 A recent study indicated that quercetin could suppress Treg expansion.45 Therefore, it is possible that quercetin in flavonoids from Radix Tetrastigmae may play a major role in the inhibition of Treg and tumor growth. Further studies, however, will be needed to understand the compositions and their functions in total flavonoids from Radix Tetrastigmae for cancer treatment.
Acknowledgments
This work was supported by a grant (Y12H270055 to ZF) from the Natural Science Foundation of Zhejiang Province, People’s Republic of China; a “Taishan Scholar” Special Fund (109, 2012 to JZ) from Shandong Government, People’s Republic of China; and a grant from National Institute of Health (R01HL116626 to DF).
Disclosure
The authors report no conflicts of interest in this work.
References
Babu BV, Konduru NK, Nakanishi W, Hayashi S, Ahmed N, Mitrasinovic PM. Experimental and theoretical advances in functional understanding of flavonoids as anti-tumor agents. Anticancer Agents Med Chem. 2013;13(2):307–332. | |
Wei A, Wu G, Xiong C, Zhou D, Cai Y, Ruan J. [Flavonoids with special B-ring from Macrothelypteris viridifrons and their anti-proliferative effects on tumor cell]. Zhongguo Zhong Yao Za Zhi. 2011;36(5): 582–584. Chinese. | |
Kim YA, Kim H, Seo Y. Antiproliferative effect of flavonoids from the halophyte Vitex rotundifolia on human cancer cells. Nat Prod Commun. 2013;8(10):1405–1408. | |
Szliszka E, Czuba ZP, Jernas K, Król W. Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Int J Mol Sci. 2008;9(1):56–64. | |
Han J, Sun M, Cui Y, et al. Kushen flavonoids induce apoptosis in tumor cells by inhibition of NF-kappaB activation and multiple receptor tyrosine kinase activities. Phytother Res. 2007;21(3): 262–268. | |
Schindler R, Mentlein R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J Nutr. 2006;136(6):1477–1482. | |
Piantelli M, Rossi C, Iezzi M, et al. Flavonoids inhibit melanoma lung metastasis by impairing tumor cells endothelium interactions. J Cell Physiol. 2006;207(1):23–29. | |
Lotito SB, Frei B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J Biol Chem. 2006;281(48):37102–37110. | |
Yao H, Xu W, Shi X, Zhang Z. Dietary flavonoids as cancer prevention agents. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29(1):1–31. | |
Russo P, Del Bufalo A, Cesario A. Flavonoids acting on DNA topoisomerases: recent advances and future perspectives in cancer therapy. Curr Med Chem. 2011;9(31):5287–5293. | |
Romagnolo DF, Selmin OI. Flavonoids and cancer prevention: a review of the evidence. J Nutr Gerontol Geriatr. 2012;31(3):206–238. | |
Banerjee A, Vasanthakumar A, Grigoriadis G. Modulating T regulatory cells in cancer: how close are we? Immunol Cell Biol. 2013;91(5):340–349. | |
Galgani M, Di Giacomo A, Matarese G, La Cava A. The Yin and Yang of CD4(+) regulatory T cells in autoimmunity and cancer. Curr Med Chem. 2009;16(35):4626–4631. | |
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609): 1057–1061. | |
Dranoff G. The therapeutic implications of intratumoral regulatory T cells. Clin Cancer Res. 2005;11(23):8226–8229. | |
Watanabe MA, Oda JM, Amarante MK, Cesar Voltarelli J. Regulatory T cells and breast cancer: implications for immunopathogenesis. Cancer Metastasis Rev. 2010;29(4):569–579. | |
Oleinika K, Nibbs RJ, Graham GJ, Fraser AR. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol. 2013;171(1):36–45. | |
Ménétrier-Caux C, Curiel T, Faget J, Manuel M, Caux C, Zou W. Targeting regulatory T cells. Target Oncol. 2012;7(1):15–28. | |
Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202(7): 919–929. | |
Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. | |
Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648. | |
Zhang L, Dermawan K, Jin M, et al. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin Immunol. 2008;129(2):219–229. | |
Feng Z-Q, Ni K-F, He Y, et al. [Experimental study on effect of Tetrastigma hemsleyanum Diels et Gilg flavone on inducing apoptosis of SGC-7901 cell line in vitro.] Chin J Clin Pharmacol Ther. 2006;11: 669–672. Chinese. | |
Liu B, Yang J, Ma Y, Yuan E, Chen C. Antioxidant and angiotensin converting enzyme (ACE) inhibitory activities of ethanol extract and pure flavonoids from Adinandra nitida leaves. Pharm Biol. 2010;48(12):1432–1438. | |
Chen L, Ding L, Yu A, et al. Continuous determination of total flavonoids in Platycladus orientalis (L.) Franco by dynamic microwave-assisted extraction coupled with on-line derivatization and ultraviolet-visible detection. Anal Chim Acta. 2007;596(1):164–170. | |
Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31(4):229–234. | |
Sriarj W, Aoki K, Ohya K, Takahashi M, Takagi Y, Shimokawa H. TGF-β in dentin matrix extract induces osteoclastogenesis in vitro. Odontology. Epub December 24, 2013. | |
Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29(9):429–435. | |
Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase-2/ prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65(12):5211–5220. | |
Knekt P, Järvinen R, Seppänen R, et al. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am J Epidemiol. 1997;146(3):223–230. | |
Le Marchand L. Cancer preventive effects of flavonoids – a review. Biomed Pharmacother. 2002;56(6):296–301. | |
Ferry DR, Smith A, Malkhandi J, et al. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996;2(4):659–668. | |
Jinushi M. The role of innate immune signals in antitumor immunity. Oncoimmunology. 2012;1(2):189–194. | |
Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18(1):11–18. | |
Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257(1):56–71. | |
Kadagidze ZG, Chertkova AI, Slavina EG. [Regulatory T-cells and their role in antitumor immune response]. Vopr Onkol. 2009;55(3): 269–277. Russian. | |
Dandawate S, Williams L, Joshee N, et al. Scutellaria extract and wogonin inhibit tumor-mediated induction of T(reg) cells via inhibition of TGF-β1 activity. Cancer Immunol Immunother. 2012;61(5): 701–711. | |
Yong X, Xiao YF, Luo G, et al. Strategies for enhancing vaccine-induced CTL antitumor immune responses. J Biomed Biotechnol. 2012;2012:605045. | |
Ogawa C, Tone Y, Tsuda M, Peter C, Waldmann H, Tone M. TGF-β-mediated Foxp3 gene expression is cooperatively regulated by Stat5, Creb, and AP-1 through CNS2. J Immunol. 2014;192(1):475–483. | |
Neil JR, Johnson KM, Nemenoff RA, Schiemann WP. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29(11):2227–2235. | |
Baratelli F, Lin Y, Zhu L, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175(3):1483–1490. | |
Camargo CA, da Silva ME, da Silva RA, Justo GZ, Gomes-Marcondes MC, Aoyama H. Inhibition of tumor growth by quercetin with increase of survival and prevention of cachexia in Walker 256 tumor-bearing rats. Biochem Biophys Res Commun. 2011;406(4):638–642. | |
Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N, Burman AC. Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med Chem. 2009;9(2):138–161. | |
Xie F, Su M, Qiu W, et al. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int J Mol Sci. 2013;14(11):21215–21226. | |
Kim MG, Jung Cho E, Won Lee J, et al. The heat-shock protein-70-induced renoprotective effect is partially mediated by CD4+ CD25+ Foxp3 + regulatory T cells in ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2014;85(1):62–71. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.