Back to Journals » Infection and Drug Resistance » Volume 16
Antimicrobial Susceptibility of E. coli Isolates from Intra-Abdominal Infections in the Asia-Pacific Region: Trends in Ciprofloxacin, Ceftriaxone, Cefepime, and Piperacillin/Tazobactam Susceptibility
Authors Tsai CH, Chen YC, Chen PY, Lai CC , Tang HJ, Chuang YC, Chen CC , Ho CH , Hsu WY, Chang TH
Received 6 June 2023
Accepted for publication 15 August 2023
Published 25 August 2023 Volume 2023:16 Pages 5599—5611
DOI https://doi.org/10.2147/IDR.S422203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Chia-Hung Tsai,1,* Yu-Chin Chen,2,* Po-Yu Chen,3 Chih-Cheng Lai,4,5 Hung-Jen Tang,1 Yin-Ching Chuang,6 Chi-Chung Chen,6,7 Chung-Han Ho,6,8 Wei-Yun Hsu,9,* Tu-Hsuan Chang9,*
1Department of Internal Medicine, Chi Mei Medical Center, Tainan, Taiwan; 2Department of Pediatrics, Chi Mei Medical Center, Chiali, Tainan, Taiwan; 3Department of Nursing, Min-Hwei College of Health Care Management, Tainan, Taiwan; 4Division of Hospital Medicine, Department of Internal Medicine, Chi Mei Medical Center, Tainan, Taiwan; 5School of Medicine, College of Medicine, National Sun Yat-sen University, Kaohsiung, Taiwan; 6Department of Medical Research, Chi Mei Medical Center, Tainan, Taiwan; 7Department of Food Science, National Chiayi University, Chiayi, Taiwan; 8Department of Information Management, Southern Taiwan University of Science and Technology, Tainan, Taiwan; 9Department of Pediatrics, Chi Mei Medical Center, Tainan, Taiwan
*These authors contributed equally to this work
Correspondence: Tu-Hsuan Chang; Wei-Yun Hsu, Department of Pediatrics, Chi Mei Medical Center, Tainan, Taiwan, Tel +886-2-281-2811 Ext 57109, Fax +886-6-220-3706, Email [email protected]; [email protected]
Purpose: To investigate the antibiotic susceptibility of Escherichia coli isolates in patients diagnosed with intra-abdominal infections (IAIs) in the Asia-Pacific region.
Patients and Methods: This study was conducted at 50 medical hospitals across 9 countries/regions as part of the Study for Monitoring Antimicrobial Resistance Trends (SMART) surveillance program from 2014 to 2018. Nonduplicate isolates of aerobic and facultative gram-negative bacilli were collected and processed for further antimicrobial susceptibility testing.
Results: A total of 10,709 isolates were collected, with E. coli (n=4737, 44.2%) being the leading pathogen causing IAIs, followed by Klebsiella pneumoniae (n=2429, 22.7%) and Pseudomonas aeruginosa (n=931, 8.7%). Community-associated (CA) E. coli isolates generally exhibited higher susceptibility rates for most antibiotics than hospital-associated (HA) isolates. In countries/regions other than Hong Kong, South Korea, and Singapore, HA isolates displayed lower susceptibility rates for multiple classes (≥ 4) of antibiotics. Among the commonly used antibiotics in IAIs, the overall susceptibility rate for ciprofloxacin was low, with an average of 41.3%. Ceftriaxone susceptibility rates in all selected countries were below 80% starting in 2018, ranging from 23.3% to 75.8%. The cefepime susceptibility rates varied across regions, with consistently reduced susceptibility ranging from 45.5% to 57.8% in India, Thailand, and Vietnam. Piperacillin/tazobactam demonstrated effectiveness against E. coli isolates in almost all countries except India, with a downward trend observed in the Philippines and Taiwan. Carbapenems remained effective against more than 90% of E. coli isolates, except in India.
Conclusion: Prudent use of fluoroquinolones and ceftriaxone is advised when treating both CA and HA IAIs in the Asia-Pacific region. The low susceptibility rate of cefepime in India, Thailand, and Vietnam needs careful consideration in its administration. Moreover, the increase in nonsusceptibility to piperacillin/tazobactam in the Philippines and Taiwan poses a potential risk that should be closely monitored.
Keywords: intra-abdominal infection, E. coli, antibiotic susceptibility, Asia-Pacific
Introduction
Intra-abdominal infections (IAIs) refer to infections that occur within the abdominal cavity, involving organs such as the gallbladder, stomach, intestines, appendix, and peritoneum.1,2 The severity of IAIs can vary from mild intramural inflammation without anatomic disruption to diffuse peritonitis, severe sepsis, and even death.2,3 Empirically applied broad-spectrum antibiotics are often used initially, followed by targeted therapy based on culture results and clinical response.4 Proper antibiotic selection, along with adequate source control, is essential in improving survival among patients with IAIs.5,6
Beta-lactam-based regimens are commonly used in empirical therapy of nonsevere or community-associated (CA) IAIs.7 However, the increasing prevalence of extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales worldwide has raised questions about the effectiveness of this approach.8,9 Another concern arises from regional variations, with Asia exhibiting the highest ESBL prevalence in Escherichia coli and Klebsiella pneumoniae, approximately 40%, followed by the Middle East.8 In contrast, the prevalence in the South Pacific remains relatively low at approximately 10%.8,9 In patients with beta-lactam allergy, while fluoroquinolone-based therapy is equally effective for treating IAIs compared with beta-lactam-based therapy, increasing resistance has been reported in previous studies.10,11 In Korea, ciprofloxacin resistance is as high as 50%, while in the Philippines, Singapore, Thailand, and Vietnam, fluoroquinolone resistance rates vary considerably, ranging from 20% to 50%.11 Therefore, empirical antibiotic choice should be dependent on the regional epidemiology of antibiotic resistance.
The Study for Monitoring Antimicrobial Resistance Trends (SMART) is a global surveillance program initiated in 2002.9 This program collects longitudinal data on the antibiotic susceptibility of gram-negative bacteria from clinical isolates, allowing for the early identification of changes in the resistance patterns of community- or hospital-acquired organisms. With its consistent data collection and wide geographical coverage, SMART serves as a crucial source for monitoring global trends in antibiotic susceptibility.
Antimicrobial susceptibility monitoring of bacteria that cause IAIs is important to guide antibiotic therapy and to identify emerging trends in resistance. The microbial composition of IAIs is complex, involving both aerobic and anaerobic bacteria, with gram-negative bacteria such as E. coli, K. pneumoniae, and Bacteroides fragilis being commonly identified.12,13 E. coli is frequently implicated as the primary pathogen in IAIs. In this study, the main objective was to analyze the trend of antibiotic susceptibility among E. coli isolates obtained from patients diagnosed with IAIs in the Asia-Pacific region as part of the SMART program. Additionally, we sought to investigate potential differences in antibiotic susceptibility patterns between CA and hospital-associated (HA) E. coli isolates in the region. This comparison is particularly important, as it will provide valuable insights into the selection of empirical antibiotics in various healthcare settings.
Materials and Methods
Bacterial Isolates and Definitions
In this study, each participating site adhered to the protocols established by the SMART program, as described in a previous study.14 In brief, this study was conducted at 50 medical hospitals across 9 countries in the Asia-Pacific region as part of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2014 to 2018. The participating regions included the Hong Kong Special Administrative Region of China (n=4), India (n=8), Malaysia (n=4), the Philippines (n=4), Singapore (n=2), South Korea (n=7), Taiwan (n=9), Thailand (n=5), and Vietnam (n=7). Nonduplicate isolates of aerobic and facultative gram-negative bacilli were collected from patients diagnosed with IAIs. Clinical specimens were obtained from intra-abdominal abscess drainage fluid, gastrointestinal tissues, paracentesis, or peritoneal fluid aspiration, and deep wound cultures were obtained intraoperatively. Isolates obtained within 48 hours of hospital admission were classified as CA IAIs, while those collected more than 48 hours after admission were classified as HA IAIs.
Antimicrobial Susceptibility Testing
All isolates were sent to a central laboratory (International Health Management Associates, Inc., Schaumburg, IL) for further processing. Confirmation of isolates was performed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonics, Billerica, MA). Antimicrobial susceptibility testing was conducted using the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method. Minimum inhibitory concentration (MIC) breakpoints, as defined by CLSI M100-S33 standards, were used to determine the susceptibility of isolates to antimicrobial agents.15 The antibiotics tested from 2014 to 2018 included amikacin, ceftazidime, ciprofloxacin, ceftriaxone, ertapenem, cefepime, levofloxacin, imipenem, and piperacillin/tazobactam. Antibiotics that were not tested in consecutive years during the study period, such as meropenem, ceftolozane/tazobactam, and colistin, were excluded from our analysis.
Statistical Analysis
We compared the percentages of susceptible isolates between the two subgroups using chi-square tests. If the application conditions of the chi-square test were not met, Fisher’s exact tests were used instead. To examine the trend of annual rates of various β-lactamases among Enterobacterales isolates, we used the Mantel‒Haenszel chi-square test for trend. We considered a p value of 0.05 to be statistically significant. All analyses were performed using SPSS for Windows (version 26.0, SPSS, Chicago, IL, USA).
Ethical Approval
This study was conducted with approval from the Institutional Review Boards and Ethics Committees of Chi Mei Medical Center (Approval No. 10,908-E02). The requirement for written informed consent was waived by the Ethics Committees because this research involved in vitro antimicrobial susceptibility surveillance of bacterial isolates, posing no more than minimal risk to the subjects. Additionally, the links to limited clinical information required for the subjects in this study were removed.
Results
Between 2014 and 2018, a total of 10,709 isolates were collected. The top ten most frequently isolated pathogens are listed in Table 1. Among them, E. coli (n=4737, 44.2%), K. pneumoniae (n=2429, 22.7%), and Pseudomonas aeruginosa (n=931, 8.7%) were the most commonly recovered species, accounting for 75.6% of the total isolates. E. coli remained the leading pathogen causing IAIs in the Asia-Pacific region, but with some variability in annual counts. The annual distribution of E. coli isolates from 2014 to 2018 was as follows: 1002, 1074, 1057, 904, and 700, respectively.
 |
Table 1 Top ten Pathogens in Patients with Intra-Abdominal Infections (IAIs) from 2014 to 2018 in the Asia-Pacific Region |
Susceptibility to antibiotics that are commonly used to treat IAIs was examined. Detailed data can be found in Table 2. Overall, the highest susceptibility was observed for amikacin, with 97.3% of isolates being susceptible, followed by imipenem, with 96.2% of isolates being susceptible. The lowest susceptibility was observed for ciprofloxacin, with 41.3% of isolates being susceptible, followed by levofloxacin, with 48.7% of isolates being susceptible. When comparing susceptibility patterns based on the time of collection after admission, we found that CA isolates generally exhibited higher susceptibility rates for most antibiotics than HA isolates. In Hong Kong, higher susceptibility rates for CA isolates (93.2%) than HA isolates (75.5%) were observed only for cefepime (p < 0.05). Similar trends of cefepime susceptibility were observed in South Korea (CA vs HA: 87.9% vs 78.1%; p < 0.05). In Singapore, only levofloxacin showed a statistically significant difference between CA and HA isolates (CA vs HA: 71.4% vs 56.3%; p < 0.05). However, in the other six countries/regions, multiple classes (≥4) of antibiotics exhibited lower susceptibility rates in HA isolates. Carbapenems remained effective against more than 90% of isolates, except in India. However, in Malaysia (CA vs HA: 100% vs 95.3%), Thailand (CA vs HA: 98.4% vs 91.8%), and Taiwan (CA vs HA: 98.2% vs 94.2%), there was a significant decrease in susceptibility to ertapenem among HA isolates (p < 0.05).
 |
Table 2 Antibiotic Susceptibility Patterns and Percentage of Susceptible Isolates in Community-Associated (CA) and Hospital-Associated E. coli Isolates from the Asia-Pacific Region |
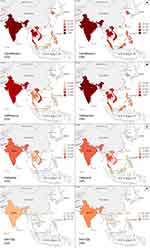
We observed significant differences in the susceptibility rates of four antibiotics (ceftriaxone, cefepime, ciprofloxacin, and piperacillin/tazobactam) between CA and HA isolates, as well as regional variations. To illustrate these findings more effectively, we depicted the visualized antimicrobial susceptibility pattern of E. coli isolates in Figure 1. This figure provides a clear context of the variations observed. India exhibited higher nonsusceptibility rates than other countries for all four antibiotics. Specifically, India showed susceptibility rates of 16.8%, 23.3%, 45.5% and 61.8% for ciprofloxacin, cefepime, ceftriaxone, and piperacillin/tazobactam, respectively; these rates were much lower than the susceptibility rates observed in other countries. In contrast, the remaining countries displayed a wide range of susceptibility rates for the same four antibiotics.
Among the four antibiotics depicted in Figure 1, ciprofloxacin exhibited the lowest susceptibility rate. We present the annual trend in Figure 2. Even among CA isolates, the susceptibility rate remained below 80%. Malaysia (47.6%) and Taiwan (54.1%) had the lowest CA susceptibility rates in 2018. For HA isolates, the susceptibility rate of ciprofloxacin in Hong Kong showed a wide range (11.8% to 80%) due to low case numbers. In 2018, Malaysia (27.8%), the Philippines (26.9%), South Korea (38%), Taiwan (47.2%), Thailand (7.4%), and Vietnam (8.7%) all showed the lowest susceptibility rates. The susceptibility rates of HA E. coli isolates in Thailand and Vietnam showed a continuous downward trend during the study period (p < 0.05).
Ceftriaxone susceptibility rates exhibited variability among regions and between CA and HA isolates, as shown in Figure 3. Until 2016, Hong Kong, Malaysia, the Philippines, and South Korea displayed relatively high ceftriaxone susceptibility rates in CA isolates, approximately 70–80%. In contrast, India had the lowest ceftriaxone susceptibility rates, ranging from 28.4% in 2014 to 9.4% in 2018. However, in 2018, ceftriaxone susceptibility rates were no longer higher than 80% in any of the countries. Similar trends were observed for HA isolates. India (13.6%), Taiwan (48.3%), and Vietnam (17.4%) showed the lowest ceftriaxone susceptibility rates in 2018. A significant downward trend was observed in both CA and HA isolates from the Philippines (p < 0.05).
In terms of cefepime susceptibility rates, Hong Kong, Malaysia, the Philippines, Singapore, South Korea, and Taiwan demonstrated relatively high susceptibility rates, ranging from 84.7% to 97.8% in CA isolates and 60% to 88.9% in HA isolates. On the other hand, India, Thailand, and Vietnam exhibited low susceptibility rates, ranging from 33.3% to 76.7% in CA isolates and 28.2% to 61.4% in HA isolates. Over the years, there were fluctuations in cefepime susceptibility rates among regions. A slight increase in cefepime susceptibility was found in HA isolates from India (p < 0.05). The trend is displayed in Figure 4.
Piperacillin/tazobactam is commonly used as a broader-spectrum alternative to penicillin antibiotics and is often employed before using carbapenems. Figure 5 shows that CA E. coli isolates remained more than 80% susceptible to piperacillin/tazobactam in almost all regions except for India. However, its performance was suboptimal against HA isolates in the Philippines (69.2%) and Vietnam (69.6%) in 2018, with a significant downward trend observed in the Philippines and Taiwan (p < 0.05).
Carbapenems are highly effective against gram-negative bacteria in the Asia-Pacific region. However, our data suggest a downward trend in susceptibility rates among CA isolates for ertapenem and imipenem in India; their susceptibility rates have both declined significantly since 2017 (p < 0.01). In 2017, the susceptibility rate of ertapenem was as low as 68.8%, while that of imipenem was only 70.8%.
Discussion
Our study presents regional antimicrobial susceptibility data for the most common pathogen that causes IAIs, E. coli, in the Asia-Pacific region between 2014 and 2018. In general, the susceptibility rates of commonly used antibiotics were significantly lower for HA isolates. Carbapenems remained highly effective. However, for antibiotics other than carbapenems, the susceptibility showed high regional variation.
Ciprofloxacin and levofloxacin, commonly used as first-line and empirical treatments in both community and hospital settings, showed the lowest susceptibility rates among all selected antibiotics in our study. The ciprofloxacin MIC in E. coli is mainly influenced by target mutations in specific residues, such as gyrA and parC.16 However, the spread of plasmid-mediated quinolone resistance genes and efflux pump mutants has also been reported.17 In a previous meta-analysis, India had the lowest ciprofloxacin susceptibility among E. coli isolates in Asian countries.18 However, in our analysis, Thailand and Vietnam also showed increased ciprofloxacin resistance. The spread of multiple local strains, such as ST1193 in Vietnam, may contribute to the prevalence of antibiotic resistance in different regions.19–21 These strains may carry mutations in the quinolone resistance-determining region and undergo clonal expansion, resulting in a higher number of resistant isolates in certain areas.
Earlier reports showed that the success rate of treating complicated IAIs with ceftriaxone plus metronidazole was similar to that of treating IAIs with ertapenem.22 However, ceftriaxone is not currently a good choice due to the increasing prevalence of CTX-M-producing E. coli, which are an important cause of healthcare-associated infections in the Asia-Pacific region.23,24 Our previous work identified CTX-M-1 (CTX-M-15, CTX-M-55) and CTX-M-9 (CTX-M-14, CTX-M-27) as the major groups of ESBLs between 2015 and 2018 in the Asia-Pacific region.25 ST131 is considered the most prevalent and clinically important sequence type associated with CTX-M ESBLs.26 These findings may partly explain the increasing ceftriaxone resistance observed in the Asia-Pacific region, considering the widespread prevalence of ESBLs among E. coli isolates.
Cefepime is a broad-spectrum antibiotic with activity against common gram-negative and gram-positive bacteria in IAIs. It is an important option for HA IAIs due to its coverage of P. aeruginosa. However, we consistently observed reduced cefepime susceptibility in certain countries for both CA and HA E. coli isolates, particularly in India, Thailand, and Vietnam. Multiple resistance mechanisms can contribute to cefepime resistance, including the production of CTX-M and co-occurrence with other β-lactamases as well as loss of outer membrane proteins.27,28 Previous SMART surveillance data have shown that the co-occurrence of plasmid-mediated AmpC beta-lactamase/ESBLs among carbapenemase-negative E. coli is common in Taiwan and Vietnam.25 The in vitro antimicrobial susceptibility rates to cefepime in these isolates are low, with rates as low as 19.6%. While this finding may partially explain the observed low in vitro susceptibility rates to cefepime, further examination is needed to confirm this hypothesis.
Piperacillin/tazobactam is commonly used as an empirical therapy for suspected healthcare-associated infections because it is effective against a broad range of organisms, including ESBL-producing Enterobacterales and P. aeruginosa. However, significantly decreased susceptibility has been observed in HA isolates from Taiwan and the Philippines. In our data, 12 HA isolates from the Philippines were analyzed for ESBL genes, and one isolate was found to carry the OXA-48-producing gene, while another carried both NDM-7 and KPC-2. On the other hand, no carbapenemase-producing genes were detected in piperacillin/tazobactam-resistant isolates from Taiwan. The prevalence of carbapenemase-producing genes has remained stable in previous SMART surveillance, indicating that dissemination of carbapenemase-producing clones is an unlikely explanation for this trend.25 Recent studies suggest that hyperproduction of SHV and TEM-1 β-lactamases may contribute to β-lactam/β-lactamase inhibitor resistance in E. coli.29,30 In previous work, we found that the TEM, CTX-M-15, and CMY-2 β-lactamases were the most commonly identified among our piperacillin/tazobactam-resistant E. coli isolates.25 Although we could not measure their enzyme activity in our study, these isolates are known to potentially express substantial amounts of β-lactamase, which could explain their resistance to piperacillin/tazobactam.
There are several limitations to this study. First, some demographic data were incomplete, and there were gaps in some years, which may affect the representativeness of the trends. Second, the data were collected before 2019, and the trend and susceptibility patterns may have changed due to the COVID-19 pandemic. Third, in India, where the lowest susceptibility rate was observed for all selected antibiotics, the lack of ESBL and carbapenemase data in the current database calls for further studies to investigate the underlying mechanisms.
Conclusion
Prudent use of fluoroquinolones and ceftriaxone is advised when treating both CA and HA E. coli infections causing IAIs in the Asia-Pacific region. The low susceptibility rate of cefepime in India, Thailand, and Vietnam needs careful consideration in its administration. Moreover, the increase in nonsusceptibility to piperacillin/tazobactam in the Philippines and Taiwan poses a potential risk that should be closely monitored.
Acknowledgments
C.H. Tsai and Y.C. Chen contributed equally to this work and shared first authorship for this study, while W.Y. Hsu and T.H. Chang contributed equally and served as co-corresponding authors for this study. Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, by a research grant from Chi Mei Medical Center, Taiwan (CMOR11202, CMNCKU11101, CMFHR11181, CMFHR11107, CCFHR11202), and by the National Science and Technology Council (NSTC 112-2314-B-384-003), Taiwan.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sartelli M, Chichom-Mefire A, Labricciosa FM, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12(1):1–34.
2. Sartelli M, Cristini F, Coccolini F, Labricciosa FM, Siquini W, Catena F. A proposal for a classification guiding the selection of appropriate antibiotic therapy for intra-abdominal infections. Antibiotics. 2022;11(10):1394. doi:10.3390/antibiotics11101394
3. Lopez N, Kobayashi L, Coimbra R. A comprehensive review of abdominal infections. World J Emerg Surg. 2011;6(1):1–10.
4. Surat G, Vogel U, Wiegering A, Germer C-T, Lock JF. Defining the scope of antimicrobial stewardship interventions on the prescription quality of antibiotics for surgical intra-abdominal infections. Antibiotics. 2021;10(1):73. doi:10.3390/antibiotics10010073
5. Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/SIS-E/WSIS/AAST global clinical pathways for patients with intra-abdominal infections. World J Emerg Surg. 2021;16(1):1–48. doi:10.1186/s13017-020-00345-w
6. Peeters P, Ryan K, Karve S, et al. The impact of initial antibiotic treatment failure: real-world insights in patients with complicated, health care-associated intra-abdominal infection. Infect Drug Resist. 2019;Volume 12:329–343. doi:10.2147/IDR.S184116
7. Sy CL, Chen P-Y, Cheng C-W, et al. Recommendations and guidelines for the treatment of infections due to multidrug resistant organisms. J Microbiol Immunol Infect. 2022;55(3):359–386. doi:10.1016/j.jmii.2022.02.001
8. Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A review of ten years of the study for monitoring antimicrobial resistance trends (SMART) from 2002 to 2011. Pharmaceuticals. 2013;6(11):1335–1346. doi:10.3390/ph6111335
9. Paterson DL, Rossi F, Baquero F, et al. In vitro susceptibilities of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: the 2003 study for monitoring antimicrobial resistance trends (SMART). J Antimicrob Chemother. 2005;55(6):965–973. doi:10.1093/jac/dki117
10. Mavros MN, Theochari NA, Kyriakidou M, Economopoulos KP, Sava JA, Falagas ME. Fluoroquinolone-based versus β-lactam-based regimens for complicated intra-abdominal infections: a meta-analysis of randomised controlled trials. Int J Antimicrob Agents. 2019;53(6):746–754. doi:10.1016/j.ijantimicag.2019.01.004
11. Ko WC, Hsueh PR. Increasing extended-spectrum beta-lactamase production and quinolone resistance among Gram-negative bacilli causing intra-abdominal infections in the Asia/Pacific region: data from the Smart Study 2002-2006. J Infect. 2009;59(2):95–103.
12. Jean SS, Lee YL, Liu PY, Lu MC, Ko WC, Hsueh P-R. Multicenter surveillance of antimicrobial susceptibilities and resistance mechanisms among Enterobacterales species and non-fermenting Gram-negative bacteria from different infection sources in Taiwan from 2016 to 2018. J Microbiol Immunol Infect. 2022;55(3):463–473. doi:10.1016/j.jmii.2021.07.015
13. Liu J, Zhang L, Pan J, et al. Risk factors and molecular epidemiology of complicated intra-abdominal infections with carbapenem-resistant Enterobacteriaceae: a multicenter study in China. J Infect Dis. 2020;221(Supplement_2):S156–S163. doi:10.1093/infdis/jiz574
14. Karlowsky JA, Lob SH, Raddatz J, et al. In vitro activity of imipenem/relebactam and ceftolozane/tazobactam against clinical isolates of gram-negative bacilli with difficult-to-treat resistance and multidrug-resistant phenotypes-study for monitoring antimicrobial resistance trends, United States 2015–2017. Clin Infect Dis. 2021;72(12):2112–2120. doi:10.1093/cid/ciaa381
15. Humphries RM, Ambler J, Mitchell SL, et al. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol. 2018;56(4):e01934–17. doi:10.1128/JCM.01934-17
16. van der Putten BC, Remondini D, Pasquini G, Janes VA, Matamoros S, Schultsz C. Quantifying the contribution of four resistance mechanisms to ciprofloxacin MIC in Escherichia coli: a systematic review. J Antimicrob Chemother. 2019;74(2):298–310.
17. Hansen LH, Jensen LB, Sørensen HI, Sørensen SJ. Substrate specificity of the OqxAB multidrug resistance pump in Escherichia coli and selected enteric bacteria. J Antimicrob Chemother. 2007;60(1):145–147. doi:10.1093/jac/dkm167
18. Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G. Ciprofloxacin resistance in community-and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2015;15:1–16.
19. Nguyen Q, Nguyen TTN, Pham P, et al. Genomic insights into the circulation of pandemic fluoroquinolone-resistant extra-intestinal pathogenic Escherichia coli ST1193 in Vietnam. Microb Genom. 2021;7(12). doi:10.1099/mgen.0.000733
20. Uchida Y, Mochimaru T, Morokuma Y, et al. Geographic distribution of fluoroquinolone-resistant Escherichia coli strains in Asia. Int J Antimicrob Agents. 2010;35(4):387–391. doi:10.1016/j.ijantimicag.2009.12.005
21. Tabut P, Yongyod R, Ungcharoen R, Kerdsin A. The distribution of mobile colistin-resistant genes, carbapenemase-encoding genes, and fluoroquinolone-resistant genes in Escherichia coli isolated from natural water sources in upper Northeast Thailand. Antibiotics. 2022;11(12):1760. doi:10.3390/antibiotics11121760
22. Yellin AE, Hassett JM, Fernandez A, et al. Ertapenem monotherapy versus combination therapy with ceftriaxone plus metronidazole for treatment of complicated intra-abdominal infections in adults. Int J Antimicrob Agents. 2002;20(3):165–173. doi:10.1016/S0924-8579(02)00160-7
23. Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother. 2017;72(8):2145–2155. doi:10.1093/jac/dkx146
24. Rossolini G, D’andrea M, Mugnaioli C. The spread of CTX‐M‐type extended‐spectrum β‐lactamases. Clin Microbiol Infect. 2008;14:33–41. doi:10.1111/j.1469-0691.2007.01867.x
25. Chen YC, Chen WY, Hsu WY, et al. Distribution of β-lactamases and emergence of carbapenemases co-occurring Enterobacterales isolates with high-level antibiotic resistance identified from patients with intra-abdominal infection in the Asia-Pacific region, 2015–2018. J Microbiol Immunol Infect. 2022;55(6 Pt 2):1263–1272. doi:10.1016/j.jmii.2021.07.007
26. Peirano G, Pitout JD. Molecular epidemiology of Escherichia coli producing CTX-M β-lactamases: the worldwide emergence of clone ST131 O25: H4. Int J Antimicrob Agents. 2010;35(4):316–321. doi:10.1016/j.ijantimicag.2009.11.003
27. Kakoullis L, Papachristodoulou E, Chra P, Panos G. Mechanisms of antibiotic resistance in important gram-positive and gram-negative pathogens and novel antibiotic solutions. Antibiotics. 2021;10(4):415. doi:10.3390/antibiotics10040415
28. Iredell J, Brown J, Tagg K. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ. 2016;352. doi:10.1136/bmj.h6420
29. Han MS, Park KS, Jeon JH, et al. SHV hyperproduction as a mechanism for piperacillin–tazobactam resistance in extended-spectrum cephalosporin-susceptible Klebsiella pneumoniae. Microb Drug ResisT. 2020;26(4):334–340. doi:10.1089/mdr.2019.0079
30. Schechter LM, Creely DP, Garner CD, et al. Extensive gene amplification as a mechanism for piperacillin-tazobactam resistance in Escherichia coli. MBio. 2018;9(2):e00583–18.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.