Back to Journals » Infection and Drug Resistance » Volume 17
Antimicrobial Resistance Patterns of Staphylococcus Aureus Isolated at a General Hospital in Vietnam Between 2014 and 2021
Authors An NV , Hai LHL, Luong VH, Vinh NTH, Hoa PQ, Hung LV, Son NT , Hong LT, Hung DV, Kien HT, Le MN, Viet NH, Nguyen DH, Pham NV, Thang TB, Tien TV, Hoang LH
Received 25 September 2023
Accepted for publication 11 January 2024
Published 24 January 2024 Volume 2024:17 Pages 259—273
DOI https://doi.org/10.2147/IDR.S437920
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Nguyen Van An,1,* Le Ha long Hai,2,3,* Vu Huy Luong,4,5 Nguyen Thi Ha Vinh,5,6 Pham Quynh Hoa,7 Le Van Hung,5,7 Nguyen Thai Son,1 Le Thu Hong,1 Dinh Viet Hung,8 Hoang Trung Kien,9 Minh Nhat Le,10,11 Nguyen Hoang Viet,12 Duc Hoang Nguyen,13 Ngai Van Pham,14 Ta Ba Thang,15 Tran Viet Tien,16 Le Huy Hoang17
1Department of Microbiology, Military Hospital 103, Vietnam Military Medical University, Hanoi, Vietnam; 2Department of Clinical Microbiology and Parasitology, Faculty of Medical Technology, Hanoi Medical University, Hanoi, Vietnam; 3Department of Biochemistry, Hematology and Immunology, National Hospital of Dermatology and Venereology, Hanoi, Vietnam; 4Department of Laser and Skin Care, National hospital of Dermatology and Venereology, Hanoi, Vietnam; 5Department of Dermatology and Venereology, Hanoi Medical University, Hanoi, Vietnam; 6Department of General Planning, National hospital of Dermatology and Venereology, Hanoi, Vietnam; 7Department of Microbiology, Mycology and Parasitology, National hospital of Dermatology and Venereology, Hanoi, Vietnam; 8Department of Psychiatry, Military Medical 103, Vietnam Military Medical University, Hanoi, Vietnam; 9Department of Immunology, Vietnam Military Medical University, Hanoi, Vietnam; 10Tay Nguyen Institute of Science Research, Vietnam Academy of Science and Technology, VAST, Hanoi, Vietnam; 11Antimicrobial Resistance Research Center, National Institute of Infectious Disease, Tokyo, Japan; 12Molecular Pathology Department, Faculty of Medical Technology, Hanoi Medical University, Hanoi, Vietnam; 13Cardiovascular Laboratories, Methodist Hospital, Merrillville, Indiana, USA; 14Medical Testing Center, Medlatec Group, Hanoi, Vietnam; 15Respiratory Center, Military Hospital 103, Vietnam Military Medical University, Hanoi, Vietnam; 16Department of Infectious diseases, Military Hospital 103, Vietnam Medical Military University, Hanoi, Vietnam; 17Department of Bacteriology, National of Hygiene and Epidemiology, Hanoi, Vietnam
*These authors contributed equally to this work
Correspondence: Le Huy Hoang, Department of Bacteriology, National of Hygiene and Epidemiology, Hanoi, 100000, Vietnam, Tel + 84 977 803 986, Email [email protected]
Purpose: Staphylococcus aureus is a commensal bacteria species that can cause various illnesses, from mild skin infections to severe diseases, such as bacteremia. The distribution and antimicrobial resistance (AMR) pattern of S. aureus varies by population, time, geographic location, and hospital wards. In this study, we elucidated the epidemiology and AMR patterns of S. aureus isolated from a general hospital in Vietnam.
Methods: This was a cross-sectional study. Data on all S. aureus infections from 2014 to 2021 were collected from the Microbiology department of Military Hospital 103, Vietnam. Only the first isolation from each kind of specimen from a particular patient was analyzed using the Cochran–Armitage and chi-square tests.
Results: A total of 1130 individuals were diagnosed as S. aureus infection. Among them, 1087 strains were tested for AMR features. Most patients with S. aureus infection were in the age group of 41– 65 years (39.82%). S. aureus isolates were predominant in the surgery wards, and pus specimens were the most common source of isolates (50.62%). S. aureus was most resistant to azithromycin (82.28%), erythromycin (82.82%), and clindamycin (82.32%) and least resistant to teicoplanin (0.0%), tigecycline (0.16%), quinupristin-dalfopristin (0.43%), linezolid (0.62%), and vancomycin (2.92%). Methicillin-resistant S. aureus (MRSA) and multidrug-resistant (MDR) S. aureus were prevalent, accounting for 73.02% and 60.90% of the total strains respectively, and the strains isolated from the intensive care unit (ICU) had the highest percentage of multidrug resistance (77.78%) among the wards.
Conclusion: These findings highlight the urgent need for continuous AMR surveillance and updated treatment guidelines, particularly considering high resistance in MRSA, MDR strains, and ICU isolates. Future research focusing on specific resistant populations and potential intervention strategies is crucial to combat this rising threat.
Keywords: Staphylococcus aureus, antimicrobial resistance, methicillin-resistant S. aureus, multidrug resistance, Hanoi, Vietnam
Introduction
Antimicrobial resistance (AMR) poses a significant threat to global public health and was estimated to cause nearly 5 million deaths; in 2019, it directly led to 1.27 million deaths worldwide. Among the most predominant Gram-negative bacteria contributing to antibiotic resistance-related deaths are Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. Similarly, Gram-positive bacteria, notably S. aureus and Streptococcus pneumoniae are critical contributors to deaths associated with antibiotic resistance.1 Although S. aureus is a commensal bacterial species of the human body, it potentially causes various diseases, from skin and soft tissue infections to severe illnesses, such as bacteremia.2 Of particular note is the prominence of S. aureus, especially methicillin-resistant S. aureus (MRSA), as a leading cause of healthcare-associated infections. In 2019, MRSA emerged as the leading pathogen–drug combination of AMR, causing 13,800 and 121,000 deaths in European countries and worldwide, respectively.1,3,4 Importantly, the distribution and AMR of S. aureus infections were reported to vary according to population, time, geographical location, and hospital wards.5–8 Comprehensive AMR surveillance is crucial for the identification of trends and patterns in antimicrobial resistance and of emerging pathogens at various levels, from local hospital to provincial, national to global scales. AMR surveillance is not only creating and updating measures against antimicrobial resistance but also improving guidelines for treating bacterial infections. Recently, Vietnam has witnessed an increase in AMR due to distributing antibiotics without presciption, inappropriate use of antimicrobials, and misuse and overuse of antimicrobials.9 Despite this concerning trend, information about the AMR status of bacterial pathogens in Vietnam remains elusive. Therefore, the objective of the present study was to examine the AMR patterns of S. aureus strains obtained from Military Hospital 103, a general hospital in Hanoi, Vietnam from 2014 to 2021. This period has been selected to capture key developments and changes in healthcare practices that may influence AMR dynamics in Vietnam. Additionally, studying AMR in this specific context is vital, considering regional variations, healthcare practices, and unique challenges pertinent to the Vietnamese setting.
Materials and Methods
Study Setting and Design
This study was a cross-sectional study of data from a large medical center in Hanoi, Vietnam. Data were collected from January 2014 to December 2021 and included the patients’ sex and age, year of S. aureus isolation, antimicrobial susceptibility testing result, specimen type, and hospital ward. To eliminate bias on the findings, only the initial strain obtained from each type of specimen from a given patient was considered for inclusion in the analysis.
Specimen Collection and S. aureus Isolation
Trained healthcare staff collected samples from inpatients under clinical microbiology guidelines. The samples included blood; pus; urine; stool; sterile body fluids, such as cerebrospinal fluid, pleural fluid, synovial fluid, and ascitic fluid; specimens from the respiratory tract (SFRT), such as sputum, tracheal/bronchial secretion, bronchial lavage fluid; and specimens from genital tract, such as swabs from the urethral, vaginal, and vulva.10 Blood culture was conducted by using BACT/ALERT 3D (bioMérieux, France) and BD BACTEC FX40 (BD, USA). The other samples were inoculated onto suitable media, such as chocolate agar, blood agar, and brilliance UTI Clarity agar (Oxoid, England). Suspected bacteria that were beta-hemolytic on blood agar, Gram-positive, and catalase-positive were identified using an automatic identification instrument (Vitek-2 Compact system, bioMérieux, France).
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed by either Vitek 2 AST-GP67 card (bioMérieux, France) or by Kirby–Bauer disc diffusion method depending on availability of materials, according to the updated Clinical and Laboratory Standards Institute guidelines.11–18 Susceptibility testing to vancomycin was conducted by using Vitek 2 AST-GP67 card (bioMérieux, France). The vancomycin-resistant strains were confirmed by using Etest (bioMérieux, France). S. aureus (ATCC 25923), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853) were used as internal quality control strains for both bacterial culture and antimicrobial susceptibility testing.
Definition of Multidrug-Resistant (MDR) and Methicillin-Resistant S. aureus
Multidrug-resistant (MDR) S. aureus was defined as nonsusceptibility to at least one agent in three or more antimicrobial classes.18,19 MRSA was defined as cefoxitin resistance, which was determined by either Vitek 2 AST-GP67 card (bioMérieux, France) or Kirby–Bauer disc diffusion method.
Statistical Analysis
A chi-square test was conducted to compare the differences in proportion among groups. The Cochran–Armitage test was conducted to determine the significance of annual trends. P values <0.05 were considered statistically significant. R software version 4.2.1 and SPSS Statistics 25.0 (IBM Corp, NY, USA) were utilized to perform statistical analysis.
Results
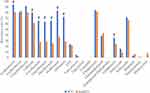
Table 1 shows the baseline characteristics of the study population. Over eight years, 1130 individuals were diagnosed as S. aureus infection, with a men-to-women ratio of 1.85:1 (734/396). The mean (standard deviation) age of patients was 47.55 years (21.10 years). The highest proportion of S. aureus infection was in the 41–65 years age group (39.82%), followed by the 16–40 years age group (32.74%). Conversely, patients in the 0–15 years age group accounted for the lowest proportion of S. aureus infection at 5.58%. S. aureus isolates were predominant in the surgery wards (48.32%) but had the lowest proportion in the infectious disease ward (10.71%). The highest proportion of S. aureus isolates was from pus specimens (50.62%), followed by blood and sterile body fluids (32.83%) and SFRT (13.45%). Finally, the stool and samples from the genital tract isolated only one strain each of S. aureus; these two strains were excluded from further analysis. A total of 1087 strains had antimicrobial susceptibility results. S. aureus was most resistant to azithromycin, erythromycin, and clindamycin (>82.0%) but was least resistant to vancomycin, tigecycline, linezolid, quinupristin-dalfopristin, and nitrofurantoin (<3.0%). Moreover, all tested S. aureus isolates were sensitive to teicoplanin. The proportion of S. aureus resistance to fluoroquinolones ranged from 30.48% (239/784, moxifloxacin) to 42.26% (71/168, norfloxacin). The resistance rate of S. aureus to tetracyclines varied widely from 0.16% (1/632, tigecycline) to 67.06% (456/680, tetracycline). The resistance rate of S. aureus to chloramphenicol was 42.96% (58/135), which was remarkably higher, compared with that to rifampin (5.91%, 42/711). The proportion of S. aureus resistance to gentamycin and trimethoprim-sulfamethoxazole (SXT) was relatively equal at 25.87% (215/831) and 22.11% (193/873), respectively. Over the study period, there were downward trends in the resistance rates of S. aureus to linezolid, doxycycline, and nitrofurantoin. In addition, we noted the AMR trends of S. aureus isolates during the study period. Linezolid resistance declined from 2.7% (1/37) in 2015 to 0.0% (0/152) in 2021. Doxycycline resistance started at 0.0% (0/38) in 2014 and gradually peaked at 25.35% (18/71) in 2017, before experiencing a sharp decrease to 0.0% (0/56) in 2021. A similar pattern was observed for nitrofurantoin resistance, which began at 0.0% (0/1) in 2014, peaked at 23.08% (3/13) in 2015, and plunged to 0.0% (0/154) in 2021. Conversely, SXT resistance significantly increased from 0.0% (0/3) in 2014 to 34.93% (51/146) in 2021 (P < 0.05) (Table 2). The rate of S. aureus resistance to most tested antibiotics, except erythromycin, chloramphenicol, rifampin, and minocycline, was higher in the strains isolated from patients in the intensive care unit (ICU) than those in the nonICU wards. Moreover, the proportion of S. aureus isolates that were resistant to azithromycin, cefoxitin, gentamycin, and fluoroquinolone was significantly higher in patients in the ICU than in those in nonICU wards (Figure 1). Among the types of specimens, SFRTs had S. aureus isolates (≥50.0%) that were highly resistant to 10 of 21 tested antibiotics, including three macrolide agents, five fluoroquinolone agents, clindamycin, and tetracycline. Pus, blood, and sterile body fluids had S. aureus isolates (≥50.0%) that were highly resistant to 5 of 21 tested antibiotics, including three macrolide agents, clindamycin, and tetracycline. Meanwhile, urine had S. aureus isolates (≥50.0%) that were highly resistant to 6 of 21 tested antibiotics, including three macrolide agents, clindamycin, tetracycline, and norfloxacin; all of these urine isolates (100%) were resistant to norfloxacin (Table 3). During the study period of eight years, the prevalence of the MDR S. aureus was 60.90% (662/1087). The highest percentage of MDR S. aureus isolates were from SFRTs (76.51%, 114/149), followed by urine and pus at 68.97% (20/29) and 59.35% (330/556), respectively. Moreover, the proportion of MDR strains was significantly higher in SFRTs than in blood and sterile body fluids, and pus. Among the hospital wards, the MDR rate was the highest in the ICU (77.78%, 98/126) and lower in the other wards (<60.0%). The MDR rate was the highest in the age group ≥66 years (66.95%, 160/239) and lowest in the age group 0–15 years (48.33%, 29/60) (Table 4). The overall proportion of MDR S. aureus substantially increased from 31.75% (20/63) in 2014 to 76.62% (118/154) in 2021 (P < 0.05), indicating a concerning trend (P value for trend < 0.05) (Figure 2A). Moreover, the rate of MDR S. aureus isolation from patients in the infectious disease, internal medicine, and surgery wards showed upward trends (Figure 2B). The proportion of MRSA isolates was the highest in SFRTs (79.69%, 102/128), followed by pus specimens (74.38%, 331/445); that for the other specimen types was from 68.0% (17/25) to 68.56% (205/299). The rate of MRSA isolation was the highest in the ICU (77.27%, 85/110), followed by infectious disease and surgery wards at 75.0% (75/100) and 75.94% (322/424), respectively; the proportion of MRSA isolates from internal medicine wards (65.78%, 173/263) was the least among hospital wards. Notably, the proportion of MRSA was the lowest in the age group ≥66 years (70.0%, 140/200) and was from 73.08% (38/52) to 74.83% (214/286) in the remaining age groups (Table 5). The prevalence of MRSA was 73.02% (655/897) over eight years. The proportion of MRSA significantly increased from 63.27% (31/49) in 2014 to 72.11% (106/147) in 2021 (P value for trend < 0.05) (Figure 2C). There were upward trends in the MRSA isolation rates in the surgery and infectious disease wards. On the contrary, the MRSA isolation rates in the ICU and internal medicine wards decreased during the study (Figure 2D). The MDR rate of the MRSA isolates was 75.75% (228/301), which was significantly higher than that of the methicillin- susceptible S. aureus (MSSA) isolates (17.54%, 30/171) (Figure 3). MRSA was most resistant to clindamycin, erythromycin, azithromycin, and clarithromycin (86.0% to 91.0%). Meanwhile, the proportion of MSSA resistance to these antibiotics ranged from 45.45% to 65.38%. The resistance rate of MRSA to fluoroquinolones ranged from 33.77% (179/530, moxifloxacin) to 57.14% (40/70, norfloxacin). Conversely, the proportion of MSSA resistance to fluoroquinolones ranged from 13.70% (10/73, ofloxacin) to 24.14% (35/145, moxifloxacin). The resistance rate of MRSA to quinupristin-dalfopristin, nitrofurantoin, linezolid, and minocycline was 0.21% (1/484), 0.21% (1/486), 0.75% (4/534), and 2.33% (1/43), respectively. Conversely, all MSSA strains were not resistant to these antibiotics. Compared with MSSA, MRSA had significantly higher proportions of resistance to azithromycin, erythromycin, clarithromycin, levofloxacin, ciprofloxacin, moxifloxacin, norfloxacin, ofloxacin, clindamycin, linezolid, and minocycline. Conversely, compared with MSSA, MRSA tended to have lower resistance rates to trimethoprim-sulfamethoxazole, chloramphenicol, gentamycin, and rifampin (Figure 4).
 |
Table 1 Distribution of Staphylococcus Aureus Isolated from 2014 to 2021 |
 |
Table 2 Resistance Rate to Common Antibiotics of Staphylococcus Aureus |
 |
Table 3 Resistance Rate to Common Antibiotics of Staphylococcus Aureus by Specimens |
 |
Table 4 Distribution of MDR Staphylococcus Aureus Among Types of Specimens and Hospital Wards |
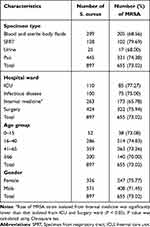 |
Table 5 Distribution of MRSA Among Types of Specimens and Hospital Wards |
Discussion
Although S. aureus is considered a part of human flora, it has been responsible for various diseases, ranging from mild diseases, such as skin and soft tissue infections to severe diseases such as bacteremia.2 S. aureus has been one of the most common pathogens causing infection in Vietnam and the global population.8,20,21 In 2019, S. aureus was reported to be the second leading pathogen causing deaths associated with AMR globally.1 In the present study, the total number of S. aureus isolates was 1130 for eight years and showed an increasing trend from 2014 to 2021. A study conducted by Diekema et al (2019) observed a similar increasing trend in S. aureus isolation, aligning with our findings.22 In contrast, studies by Mhondoro et al indicated fluctuations or decreased rates in S. aureus isolation over comparable periods. These differences may be attributed to variations in regional epidemiology, changes in diagnostic practices, or shifts in patient demographics.23 Moreover, the increase in the number of patients examined and treated in the study period, especially after our hospital was expanded in 2019, might contribute to the growing number of S. aureus infections. S. aureus colonizes the skin and nasal mucosa as a commensal bacterium, and around one-third of individuals are S. aureus carriers. Interestingly, among surgical patients, nosocomial-acquired infections were found to be more likely in S. aureus nasal carriers.24 The present study revealed that S. aureus was mainly isolated from pus and at surgical ward. The results were similar to previous studies, which reported a higher proportion of S. aureus isolated from pus was higher than from other specimens.25,26 Among the tested antibiotics in this study, the proportion of S. aureus resistance was the highest to macrolides (82.28–82.82%); this was similar to the results reported by the Viet Nam Resistance Network (VINARES), which surveyed AMR in 13 hospitals in 2016–2017. However, the rate of S. aureus resistance to SXT was lower in our study (22.11%) than in the report of VINARES (25.0%).8 The higher SXT resistance observed in our study compared to VINARES may be influenced by several factors. Differences in antibiotic prescribing practices, regional antimicrobial use policies, and accessibility to healthcare resources may contribute to variations in resistance rates. Additionally, variations in patient demographics and underlying health conditions, as well as differences in the prevalence of SXT-resistant strains within the local microbial population, could contribute to these disparities. It is essential to consider the specific context of each study, including the geographic location and characteristics of the patient population, when interpreting variations in antibiotic resistance. In contrast, the resistance rate to SXT and macrolides were considerably higher in the present study than in previous studies by Kot B et al in Poland (8.0% and 77.7%, respectively) and by Lee H et al in Korea (≤10.0% and ≤50.0%, respectively).6,20 Variances in regional antibiotic use policies and local resistance profiles may play a role. It is possible that differences in the prevalence of specific resistance mechanisms or the dissemination of resistant strains within different healthcare settings contribute to the observed distinctions. Notably, there was an upward trend in SXT-resistant S. aureus from 2014 to 2021. SXT had been widely used to treat a variety of infectious diseases caused by S. aureus. Increase in the consumption of SXT for the treatment of skin and soft tissue infections caused by community-acquired MRSA, as well as the spread of S. aureus strains harboring the trimethoprim resistance genes dfrA and dfrG, may have contributed to the increased SXT resistance rate.27–29 On the contrary, we observed downward trends in the resistance rates of S. aureus to linezolid, doxycycline, and nitrofurantoin. The decreased trend of these antibiotics can be influenced by various factors, including changes in prescribing practices, emerging resistance, and evolving medical guidelines. The rate of S. aureus resistance to fluoroquinolones in this study (32.57%; 1064/3267) was lower, compared with those in the report of VINARES (37.0%) and a previous study on a Poland population from 2015 to 2017 (approximately 83.0%), but was higher, compared with that in a study from Kenya from 2014 to 2016 (22.0%).6,8,26 In this study, the S. aureus isolates showed relatively less resistance to vancomycin, linezolid, quinupristin-dalfopristin, tigecycline, and teicoplanin. The finding was consistent with those of the Korea Global AMR Surveillance System (Kor-GLASS), which was conducted in six hospitals in 2016 to 2017 and showed that all S. aureus isolates were susceptible to vancomycin and teicoplanin and that nearly all isolates were susceptible to tigecycline, quinupristin-dalfopristin, and linezolid.20 Similarly, the China Antimicrobial Surveillance Network (CHINET) presented that most of the S. aureus strains isolated in 2018 were susceptible to vancomycin and linezolid, and the VINARES reported that the proportion of S. aureus with vancomycin resistance, including intermediate susceptibility results, was 2.0%.8,21 Similar to the reports of the VINARES and Kor-GLASS,7,8 the present study showed that among the hospital wards, the ICU had the highest proportion of S. aureus isolates that were resistant to most of the tested antibiotics, significantly higher MDR rate, and high MRSA rate. Patients in the ICU usually have critical illnesses, immunocompromisation, and underlying morbidities; They are often treated with broad-spectrum and multiple antibiotics. Furthermore, invasive devices, medical machines, and equipment such as mechanical ventilation, central venous catheters, and urinary catheters, are frequency used during the treatment course of these patients. These factors may cause the spread of AMR bacteria, especially MDR bacteria.25,30,31 Our data indicated significant differences in the proportion of S. aureus isolates with AMR among specimen types. The resistance rate of S. aureus isolated from SFRTs was higher, compared with that of strains isolated from other specimens. Isolates from SFRTs were highly resistant to 10 of 21 tested antibiotics, whereas isolates from the other types of specimens were highly resistant to 5 or 6 tested antibiotics. Notably, SFRTs had considerably higher proportion of MDR S. aureus strains, compared with those from blood and sterile body fluids, and pus, and had the highest rate of MRSA isolates among the specimen types. These findings were consistent with those of a previous study in the United States, which showed higher proportion of MRSA and MDR S. aureus strains from lower SFRTs than from blood, skin, and soft tissue.32 Moreover, in a previous study from Thailand, the MRSA rate was higher in sputum and endotracheal aspirates than in the other types of specimens, including blood, urine, pus/ wound, and biopsy.25 Previous studies showed that several biofilm-producing S. aureus strains were isolated from the lower SFRTs and biofilm formation was higher in MRSA isolates as compared to MSSA isolates; the AMR rate was higher in the biofilm-producing S. aureus strains than in the isolates that did not produce biofilm.33–37 Therefore, a high proportion of biofilm-forming S. aureus strains may account for the high AMR of the S. aureus isolates from SFRTs in our study. Previous reports indicated that the formation of bacterial biofilm enhances not only ability to resist antibiotics but also enable bacteria to survive in host cell; biofilm was reported increasing morbidity and mortality in hospitals worldwide.38,39 The data suggests the need for further studies concentrating on screening and characterizing biofilm of S. aureus to improve the effectiveness of treatment strategy for dealing S. aureus infections in our hospital. The proportion of MDR S. aureus was lower in the present study than in the previous study in Poland.6 The increase in AMR and in the proportion of MDR strains among S. aureus isolates had been associated with the transfer of mobile genetic elements that carry antibiotic resistance genes, the increased multidrug efflux pumps that extrude antibiotics from the cell by either acquisition or mutation among the S. aureus strains, and the misuse and overuse of antimicrobials.40–42 Notably, we observed an upward trend in the overall proportion of MDR S. aureus isolates from 2014 to 2021, consistent with the findings in the Poland population.6 Infections with MDR bacteria lead to prolonged hospital stay and increased mortality and treatment cost,43 indicating future challenges in treating S. aureus infections. To explore the reasons behind this trend, we considered antibiotic prescribing practices, infection control measures, and bacterial factors. Changes in broad-spectrum antibiotic use and the effectiveness of infection control measures, including potential community transmission. Acknowledging study limitations, we emphasized the need for further research, proposing molecular epidemiological studies to track resistance gene transmission and evaluate targeted interventions on MDR S. aureus prevalence. These steps are crucial for advancing our understanding and guiding strategies against the rise of MDR S. aureus. The proportion of MRSA in the present study was nearly equal to that in the report of VINARES (73.0%) but was considerably higher, compared to those reported by the CHINET (4277/6772, 63.16%) and by previous studies in Kenya from 2014 to 2016 (27.8%) and Thailand in 2017 (17%).8,21,25,26 Notably, our data indicated that the resistance rate to most of the tested antibiotics was remarkably higher for MRSA than for MSSA. Moreover, consistent with previous studies on populations from China, Ethiopia, and Vietnam,8,21,44 this study showed that the proportion of MDR was significantly higher among MRSA strains than among MSSA isolates. The observed resistance disparities between MRSA and MSSA have profound clinical implications. Limited treatment options for MRSA, in comparison to MSSA, underline the reliance on costlier, narrower-spectrum antibiotics with potential side effects. The emergence of multi-drug resistance in MRSA strains further accentuates the pressing need for innovative therapeutic strategies. Moreover, treatment delays in MRSA infections contribute to prolonged hospital stays, increased healthcare costs, and elevated risks of morbidity and mortality. Prolonged antibiotic use, particularly against multi-drug resistant MRSA strains, amplifies the susceptibility to secondary infections, impacting patient recovery and causing psychological distress.45–48 In addition to patient outcomes, stricter infection control measures are essential given the higher transmission risk associated with MRSA. Rapid diagnostic tests and robust interventions, including enhanced hand hygiene and isolation precautions, play a crucial role in preventing the spread of MRSA within healthcare settings. Since it was established, vancomycin had been the first choice for empiric and definitive treatment of MRSA infections.49 However, increase in vancomycin-resistant and -intermediate S. aureus strains led to the failure of vancomycin treatment of S. aureus infections.50,51 Notably, the surge in vancomycin resistance represents a critical clinical concern.52 The failure of vancomycin treatment for S. aureus infections raises alarming implications for patient outcomes, as it limits the available therapeutic options.53 This becomes particularly significant considering the historical reliance on vancomycin as a cornerstone in the management of MRSA infections. Notably, in 2017, the World Health Organization enlisted MRSA and vancomycin-resistant and -intermediate S. aureus in the second priority bacteria group that urgently needs new antibiotics for treatment.54 Interestingly, we observed an upward trend in the overall proportion of MRSA from 2014 to 2021 and a higher rate of vancomycin resistance in MRSA isolates than in MSSA isolates (Figures 2 and 4). Our data supported the urgent need for new antibiotics, comprehensive approaches to treatment, and infection control measures to prevent the spread of S. aureus, especially MRSA. While our study offers global comparisons of resistance patterns in S. aureus, it is essential to contextualize these findings within the specific framework of the local healthcare system and practices. Our study compared S. aureus resistance patterns across the world, but it’s important to see how these patterns apply to our local healthcare system. Different countries have different policies and ways of treating patients, which affect how much bacteria resist antibiotics. For example, some places use stronger antibiotics more often, or they might not be as strict with handwashing and cleaning. This can create unique antibiotic resistance patterns in each region. By exploring these local factors, we can not only refine the interpretation of our results but also contribute valuable insights for tailoring interventions and strategies to address antibiotic resistance effectively within our healthcare system. This localized perspective enhances the applicability and relevance of our findings, guiding the development of targeted measures to combat the challenges posed by antimicrobial resistance in our specific healthcare environment.
Conclusions
This study provided insights into the distribution and AMR patterns of S. aureus isolates in a hospital setting over an eight-year period. S. aureus infections were most prevalent in middle-aged patients and at the surgery ward, with the majority of isolates obtained from pus specimens. The AMR patterns of the S. aureus isolates revealed low rates of resistance to teicoplanin, tigecycline, and nitrofurantoin but high rates of resistance to azithromycin, erythromycin, and clindamycin that should be used more carefully. The prevalence of MDR S. aureus and MRSA were high and presented an increase trend. These findings emphasized the importance of infection control measures such as increase of hand hygiene rate, appropriate disinfection of medical equipment and hospital surfaces, decolonization of MRSA carriers in admitted patients, and preoperative decolonization, and the judicious use of antibiotics to prevent the spread of MRSA and MDR S. aureus strains in hospital settings. Our data also emphasized the need for further research, proposing molecular epidemiological studies to track resistance gene transmission and evaluate targeted interventions on MRSA and MDR S. aureus prevalence. These steps are crucial for advancing our understanding and guiding strategies against the rise of MRSA and MDR S. aureus.
Data Sharing Statement
Data can be made available upon request. For any inquiries, please contact the corresponding author.
Ethical Information
The ethics committee of Military Hospital 103 approved this study (Approval No. 35/CNChT- HĐĐĐ). The ethics committee waived the need for participants’ informed consent because the study was retrospective. Patients’ information was anonymized before being analyzed. The study was conducted following the principle of the Declaration of Helsinki.
Acknowledgments
We would like to thank the staff of the microbiology department of Military Hospital 103 for their contributions.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare no competing interests.
References
1. Antimicrobial Resistance C, Ikuta KS, Sharara F. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi:10.1016/S0140-6736(21)02724-0
2. Dayan GH, Mohamed N, Scully IL, et al. Staphylococcus aureus: the current state of disease, pathophysiology and strategies for prevention. Expert Rev Vaccines. 2016;15(11):1373–1392. doi:10.1080/14760584.2016.1179583
3. European Antimicrobial Resistance C. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Health. 2022;7(11):e897–e913. doi:10.1016/S2468-2667(22)00225-0
4. Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections - an overview. Infect Drug Resist. 2018;11:2321–2333. doi:10.2147/IDR.S177247
5. Tadesse S, Alemayehu H, Tenna A, et al. Antimicrobial resistance profile of Staphylococcus aureus isolated from patients with infection at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. BMC Pharmacol Toxicol. 2018;19(1):24. doi:10.1186/s40360-018-0210-9
6. Kot B, Wierzchowska K, Piechota M, Gruzewska A. Antimicrobial Resistance Patterns in Methicillin-Resistant Staphylococcus aureus from Patients Hospitalized during 2015–2017 in Hospitals in Poland. Med Princ Pract. 2020;29(1):61–68. doi:10.1159/000501788
7. Liu C, Yoon EJ, Kim D, et al. Antimicrobial resistance in South Korea: a report from the Korean global antimicrobial resistance surveillance system (Kor-GLASS) for 2017. J Infect Chemother. 2019;25(11):845–859. doi:10.1016/j.jiac.2019.06.010
8. Choisy M. Antimicrobial susceptibility testing results from 13 hospitals in Viet Nam: VINARES 2016–2017. Antimicrob Resist Infect Control. 2021;10(1):78. doi:10.1186/s13756-021-00937-4
9. World Health Organization. Antimicrobial resistance in Vietnam. https://www.who.int/vietnam/health-topics/antimicrobial-resistance.
10. Leber AL. Clinical Microbiology Procedures Handbook.
11. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100-S24.
12. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100-S25.
13. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100-S26.
14. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100-S27.
15. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100-S28.
16. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100-S29.
17. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100-S30.
18. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100-S31.
19. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
20. Lee H, Yoon EJ, Kim D, et al. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: first one-year report from Kor-GLASS. Euro Surveill. 2018;23:1.
21. Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38(12):2275–2281. doi:10.1007/s10096-019-03673-1
22. Diekema DJ, Pfaller MA, Shortridge D, Zervos M, Jones RN. Twenty-year trends in antimicrobial susceptibilities among staphylococcus aureus from the SENTRY antimicrobial surveillance program. Open Forum Infect Dis. 2019;6(Suppl 1):S47–S53. doi:10.1093/ofid/ofy270
23. Mhondoro M, Ndlovu N, Bangure D, et al. Trends in antimicrobial resistance of bacterial pathogens in Harare, Zimbabwe, 2012–2017: a secondary dataset analysis. BMC Infect Dis. 2019;19(1):746. doi:10.1186/s12879-019-4295-6
24. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–520. doi:10.1128/CMR.10.3.505
25. Waitayangkoon P, Thongkam A, Benjamungkalarak T, et al. Hospital epidemiology and antimicrobial susceptibility of isolated methicillin-resistant Staphylococcus aureus: a one-year retrospective study at a tertiary care center in Thailand. Pathog Glob Health. 2020;114(4):212–217. doi:10.1080/20477724.2020.1755550
26. Gitau W, Masika M, Musyoki M, Museve B, Mutwiri T. Antimicrobial susceptibility pattern of Staphylococcus aureus isolates from clinical specimens at Kenyatta National Hospital. BMC Res Notes. 2018;11(1):226. doi:10.1186/s13104-018-3337-2
27. Wood JB, Smith DB, Baker EH, Brecher SM, Gupta K. Has the emergence of community-associated methicillin-resistant Staphylococcus aureus increased trimethoprim-sulfamethoxazole use and resistance?: a 10-year time series analysis. Antimicrob Agents Chemother. 2012;56(11):5655–5660. doi:10.1128/AAC.01011-12
28. Nurjadi D, Olalekan AO, Layer F, et al. Emergence of trimethoprim resistance gene dfrG in Staphylococcus aureus causing human infection and colonization in sub-Saharan Africa and its import to Europe. J Antimicrob Chemother. 2014;69(9):2361–2368. doi:10.1093/jac/dku174
29. Coelho C, de Lencastre H, Aires-de-Sousa M. Frequent occurrence of trimethoprim-sulfamethoxazole hetero-resistant Staphylococcus aureus isolates in different African countries. Eur J Clin Microbiol Infect Dis. 2017;36(7):1243–1252. doi:10.1007/s10096-017-2915-x
30. El Mekes A, Zahlane K, Ait Said L, Tadlaoui Ouafi A, Barakate M. The clinical and epidemiological risk factors of infections due to multi-drug resistant bacteria in an adult intensive care unit of University Hospital Center in Marrakesh-Morocco. J Infect Public Health. 2020;13(4):637–643. doi:10.1016/j.jiph.2019.08.012
31. Timsit JF, Bassetti M, Cremer O, et al. Rationalizing antimicrobial therapy in the ICU: a narrative review. Intensive Care Med. 2019;45(2):172–189. doi:10.1007/s00134-019-05520-5
32. Styers D, Sheehan DJ, Hogan P, Sahm DF. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2. doi:10.1186/1476-0711-5-2
33. Samadi R, Ghalavand Z, Mirnejad R, Nikmanesh B, Eslami G. Antimicrobial resistance and molecular characteristics of methicillin-resistant staphylococcus aureus isolates from children patients in Iran. Infect Drug Resist. 2019;12:3849–3857. doi:10.2147/IDR.S229394
34. Senobar Tahaei SA, Stajer A, Barrak I, Ostorhazi E, Szabo D, Gajdacs M. Correlation between biofilm-formation and the antibiotic resistant phenotype in staphylococcus aureus isolates: a laboratory-based study in Hungary and a review of the literature. Infect Drug Resist. 2021;14:1155–1168. doi:10.2147/IDR.S303992
35. Yu F, Yang L, Pan J, et al. Prevalence of virulence genes among invasive and colonising Staphylococcus aureus isolates. J Hosp Infect. 2011;77(1):89–91. doi:10.1016/j.jhin.2010.07.019
36. Mazloomirad F, Hasanzadeh S, Sharifi A, Nikbakht G, Roustaei N, Khoramrooz SS. Identification and detection of pathogenic bacteria from patients with hospital-acquired pneumonia in southwestern Iran; evaluation of biofilm production and molecular typing of bacterial isolates. BMC Pulm Med. 2021;21(1):408. doi:10.1186/s12890-021-01773-3
37. Manandhar S, Singh A, Varma A, Pandey S, Shrivastava N. Biofilm producing clinical staphylococcus aureus isolates augmented prevalence of antibiotic resistant cases in tertiary care hospitals of Nepal. Front Microbiol. 2018;9:2749. doi:10.3389/fmicb.2018.02749
38. Mottola C, Matias CS, Mendes JJ, et al. Susceptibility patterns of Staphylococcus aureus biofilms in diabetic foot infections. BMC Microbiol. 2016;16(1):119. doi:10.1186/s12866-016-0737-0
39. Moormeier DE, Endres JL, Mann EE, et al. Use of microfluidic technology to analyze gene expression during Staphylococcus aureus biofilm formation reveals distinct physiological niches. Appl Environ Microbiol. 2013;79(11):3413–3424. doi:10.1128/AEM.00395-13
40. Costa SS, Viveiros M, Amaral L, Couto I. Multidrug Efflux Pumps in Staphylococcus aureus: an Update. Open Microbiol J. 2013;7:59–71. doi:10.2174/1874285801307010059
41. Haaber J, Penades JR, Ingmer H. Transfer of Antibiotic Resistance in Staphylococcus aureus. Trends Microbiol. 2017;25(11):893–905. doi:10.1016/j.tim.2017.05.011
42. Karakonstantis S, Kalemaki D. Antimicrobial overuse and misuse in the community in Greece and link to antimicrobial resistance using methicillin-resistant S. aureus as an example. J Infect Public Health. 2019;12(4):460–464. doi:10.1016/j.jiph.2019.03.017
43. Zhen X, Stalsby Lundborg C, Sun X, Zhu N, Gu S, Dong H. Economic burden of antibiotic resistance in China: a national level estimate for inpatients. Antimicrob Resist Infect Control. 2021;10(1):5. doi:10.1186/s13756-020-00872-w
44. Dilnessa T, Bitew A. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus isolated from clinical samples at Yekatit 12 Hospital Medical College, Addis Ababa, Ethiopia. BMC Infect Dis. 2016;16:398. doi:10.1186/s12879-016-1742-5
45. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–59. doi:10.1086/345476
46. Uematsu H, Yamashita K, Kunisawa S, Fushimi K, Imanaka Y. Estimating the disease burden of methicillin-resistant Staphylococcus aureus in Japan: retrospective database study of Japanese hospitals. PLoS One. 2017;12(6):e0179767. doi:10.1371/journal.pone.0179767
47. Inagaki K, Lucar J, Blackshear C, Hobbs CV. Methicillin-susceptible and methicillin-resistant staphylococcus aureus bacteremia: nationwide estimates of 30-day readmission, in-hospital mortality, length of stay, and cost in the United States. Clin Infect Dis. 2019;69(12):2112–2118. doi:10.1093/cid/ciz123
48. Zhen X, Lundborg CS, Zhang M, et al. Clinical and economic impact of methicillin-resistant Staphylococcus aureus: a multicentre study in China. Sci Rep. 2020;10(1):3900. doi:10.1038/s41598-020-60825-6
49. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. doi:10.1093/cid/ciq146
50. Casapao AM, Leonard SN, Davis SL, et al. Clinical outcomes in patients with heterogeneous vancomycin-intermediate staphylococcus aureus bloodstream infection. Antimicrob Agents Chemother. 2013;57(9):4252–4259. doi:10.1128/AAC.00380-13
51. Shariati A, Dadashi M, Moghadam MT, van Belkum A, Yaslianifard S, Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep. 2020;10(1):12689. doi:10.1038/s41598-020-69058-z
52. Guffey AA, Loll PJ. Regulation of resistance in vancomycin-resistant enterococci: the vanrs two-component system. Microorganisms. 2021;9:3
53. Lee AS, de Lencastre H, Garau J, et al. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers. 2018;4:18033. doi:10.1038/nrdp.2018.33
54. World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Secondary Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. World Health Organization; 2017.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.