Back to Journals » Infection and Drug Resistance » Volume 16
Antimicrobial Resistance Pattern of Pseudomonas aeruginosa: An 11-Year Experience in a Tertiary Care Hospital in Makkah, Saudi Arabia
Authors Momenah AM , Bakri RA, Jalal NA , Ashgar SS, Felemban RF, Bantun F , Hariri SH, Barhameen AA, Faidah H, AL-Said HM
Received 30 March 2023
Accepted for publication 19 June 2023
Published 26 June 2023 Volume 2023:16 Pages 4113—4122
DOI https://doi.org/10.2147/IDR.S409726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Aiman M Momenah,1 Rafat Ahmed Bakri,2 Naif A Jalal,1 Sami S Ashgar,1 Rakan Fahad Felemban,3 Farkad Bantun,1 Sumyya H Hariri,1 Abeer A Barhameen,1 Hani Faidah,1 Hamdi M AL-Said1
1Department of Microbiology, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia; 2University Medical Center, Umm Al-Qura University, Makkah, Saudi Arabia; 3Department of Laboratory and Blood Bank, Al-Noor Specialist Hospital, Makkah, Saudi Arabia
Correspondence: Aiman M Momenah, Department of Microbiology, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia, Tel +966503501449, Email [email protected]
Purpose: Pseudomonas aeruginosa (P. aeruginosa) is a common causative pathogen in healthcare settings and displays increasing levels of resistance to common antimicrobial drugs. Its capacity to resist has been reported in multiple locations across the world. This study evaluates current levels of antibiotic resistance and seeks to understand antibiotic resistance patterns in the context of the clinical isolates of P. aeruginosa.
Methods: All clinical isolates were cultured at 37 °C for 24 h in different media: blood sheep agar, McConkey agar, and cystine–lactose–electrolyte‐deficient agar (CLED), bacterial identification and antibiotic susceptibility patterns were determined using the Vitek‐2 (bioMérieux) automated system.
Results: In total, there were 61,029 patient specimens, of which 5534 were identified as non-duplicated P. aeruginosa clinical isolates, most being from males aged over 60 years. The research findings revealed that the maximum antibiotic resistance associated with P. aeruginosa isolates was found in colistin (97%), which was followed by piperacillin/tazobactam (75.8%). The maximum resistance rates in P. aeruginosa isolates were found in relation to cefepime (42.7%,) which was followed by ciprofloxacin (34.3%).
Conclusion: The antibiotic resistance rate during the first six years of the research period was notably higher than in the last years, due to the application of infection control protocols and strict policies to control antibiotic prescriptions in all Saudi hospitals.
Keywords: Pseudomonas aeruginosa, antimicrobial drug, drug resistance, cefepime
Introduction
In 1882, Gessard became the first person to successfully isolate P. aeruginosa from green pus.1 This bacterium is classified as Gram-negative and aerobic, and it takes the form of a rod without spore formation.2 “It is capable of growing at temperatures ranging from 4°C to 42°C, but its optimal growth temperature is 37°C”.3
Soil, decomposing organic matter, vegetation, and water are all environments favorable to pseudomonas. However, moist settings in hospital environments (eg, food, cut flowers, sinks, toilets, floor mops, equipment for respiratory therapy and dialysis, disinfectant solutions) are propitious to this bacterium as well.4 Indeed, being of an opportunistic nature, pseudomonas is frequently encountered in hospitals, where it can easily be transferred from the surrounding environment to human beings. Although the bacterium does not usually affect healthy people, it can infect people with a weak immune system, especially those receiving treatment with antibiotics ineffective against pseudomonas.5
Individuals admitted to the hospital with conditions like burns, cancer, and cystic fibrosis are vulnerable to infection with P. aeruginosa, which can give rise to endocarditis, pneumonia, and infections affecting the urinary tract, central nervous system, eye wounds, ears, skin, and musculoskeletal system.6 The bacterium proliferates in excessively crowded or improperly disinfected hospital settings, which is why it is considered to have an opportunistic character.5 However, knowledge remains limited about how exactly the pathogenicity of P. aeruginosa manifests. The bacterium achieves human tissue colonization and immune response evasion through its effective production of virulence factors. Another important property is strong adherence to a range of surfaces and hydrolysis of different biocompatible materials.7
Statistics published by the National Healthcare Safety Network (NHSN) for 2015–2017 revealed that, in the US, P. aeruginosa was among the top three pathogenic bacteria of highest prevalence associated with cases of catheter-associated urinary tract infection (CAUTI) and ventilator-associated pneumonia (VAP).8 Similarly, high prevalence has been found in other countries as well. For instance, Diekema et al reported that P. aeruginosa was the third greatest source of bloodstream infections, based on the data gathered from locations in 45 countries within the context of a SENTRY antimicrobial surveillance program.9 In Saudi Arabia, P. aeruginosa is also highly prevalent and becoming more and more resistant to antibiotics.10–12 Yezli et al found that 11% of all infections developed in the hospital were caused by P. aeruginosa and Gram-negative isolates accounted for 31% of all nosocomial isolates. More specifically, 13–25% of wound infections, 16% of respiratory infections, 6–16% of urinary tract infections (UTIs), and 7–11% of bacteremia were caused by P. aeruginosa isolates.7
The resistance displayed by P. aeruginosa to several antibiotics is a major obstacle in the treatment of infections caused by this bacterium. Multi-drug resistant (MDR) strains of P. aeruginosa have emerged recently owing to excessive antibiotic usage, especially in hospitals. This has had ramifications not only in terms of heightened morbidity and death rates, but also extended hospitalization and greater healthcare expenditure. Furthermore, MDR P. aeruginosa remains difficult to treat. Given these considerations, it is essential to monitor antibiotic efficacy closely to better understand antibiotic sensitivity patterns as well as to simplify the formulation of preliminary therapy, particularly in cases of critical illness.13
The purpose of the present work was to gain insight into the patterns and alterations in the antibiotics to which P. aeruginosa has become resistant in the last decade. To that end, data were gathered from cases of P. aeruginosa infections treated at a tertiary hospital with a capacity of 560 beds in western Saudi Arabia, namely, Alnoor General Hospital. Such a line of inquiry was motivated by the assumption that antibiotic treatment and prescription practices can be better understood and managed based on analysis of the modifications in the patterns of antibiotic resistance over an interval of a decade.
Materials and Methods
Ethics Statement
The Research Ethics and Advisory Committees of the College of Medicine at Umm Al-Qura University provided ethical approval for this study to take place (reference no HAPO-02-K-012-2022-02-946).
Study Design
This retrospective study was carried out in a tertiary hospital in Makkah, Saudi Arabia (with 560 beds) to examine the vulnerability patterns of all P. aeruginosa isolates. This study was carried out over an extended 11-year period from January 2011 to December 2021. Altogether, 86,631 clinical specimens were collected from various hospital departments, including urology, intensive care unit (ICU), and surgery units. Moreover, the samples collected included specimens of blood, pus, urine, sputum, swabs (ie, ear, catheter, urethral, nasal, throat, central venous puncture), bodily fluid (ie, semen, urethral discharge, pleural fluid) and these samples were sent to the microbiology laboratory for processing. A majority of the samples were cultured on sheep blood agar medium and incubated for 24–48 hours at 37°C in order to isolate P. aeruginosa. Additionally, Cystine Lactose Electrolyte Deficient agar (CLED) plates were used to inoculate the urine samples, after which they were incubated at 37°C for 24 hours. After the bacteria had grown on the solid medium, a Vitek-2 (bioMérieux) automated system was employed for identification purposes and to determine the antibiotic susceptibility testing of all P. aeruginosa isolates.
Subsequently, 0.5% sterile NaCl was added to the bacterial suspensions and the concentration was modified at 0.5–0.63 McFarland using the VITEK Densi CHEK (BioMérieux).
For purposes of identification, the GN-21341 cards were employed, after which the antibiotic susceptibility of the P. aeruginosa isolates could be examined using AST-N291 cards. Moreover, quality control was important, and thus the reference strain P. aeruginosa ATCC 27853 was employed. A number of antibiotics were tested in this examination, including piperacillin/tazobactam, ceftazidime, cefepime, imipenem, gentamicin, ciprofloxacin, and colistin. The interpretation of the MIC results based on Clinical Laboratory Standard Institute (CLSI) guidelines.
Statistical Analysis
A database was created to store important data, such as the total number of patients, specimen type, and antibiograms. The antibiotic susceptibilities were recorded as a percentage of all P. aeruginosa isolates. Chi-square tests were carried out to compare the antibiotic susceptibility and sample types of different isolates from different years. Additionally, the analyses were all two-tailed. A p-value below 0.05 was determined to be statistically significant. Finally, Prism version 7 was employed to perform the statistical analysis.
Results
Patient Demographics
The current data collection study was conducted retrospectively over an 11-year period from January 1st, 2011, to December 30th, 2021. 61,029 clinical samples were gathered in total, of which 5534 (9.1%) revealed P. aeruginosa (Table 1). The proportion of samples in which P. aeruginosa was isolated significantly declined over the course of the study from 9.8% (2011) to 5.2% (2021) (p < 0.05).
 |
Table 1 Demographic Data for P. aeruginosa Detection Throughout the 11-Year Study Duration |
Overall, male subjects exhibited a greater prevalence of infection with P. aeruginosa than their female counterparts. The proportions of men and women with a positive result for this bacterium in 2011 were 53.8% and 41.7%, respectively; equivalent data for 2021 were 63.8% and 36.2%, respectively.
Throughout the duration of the study, in-patients’ samples positive for P. aeruginosa (4644) outnumbered those from out-patients (890, p < 0.05) (Table 1). The frequency of the former over the 11-year study duration was between 74.6% and 88.8%. The number of in-patients detected in the initial two years of the study, ie 87.4% (2011) and 87.8% (2012), was greater than in the final two years, ie 74.7% and 74.6%.
When evaluated in terms of patients’ age, the majority of instances of P. aeruginosa were isolated in those over 60 years (Figure 1). In all age-ranges, more in-patient than out-patient cases were documented (p < 0.01). The least frequency of P. aeruginosa was noted in patients aged 10 years or less.
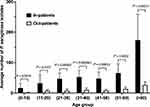 |
Figure 1 Proportions of the various sample types from which P. aeruginosa was isolated over the 11-year study duration. |
Sample Demographics
During the entire study period, positive isolations of P. aeruginosa were most frequently identified in the wound (39.6%) and sputum (31.2%) specimens, followed by urine (8.7%) and blood (5.9%) (Figure 2). Irrespective of the sample origin, the mean P. aeruginosa isolate percentages were elevated in in-patient specimens as opposed to those from out-patients, eg 98% of sputum samples were obtained from in-patients; the remaining 2% were from out-patients.
 |
Figure 2 Distribution of P. aeruginosa isolates according to sample origin over the 11-year study duration. |
The marked decrease in P. aeruginosa isolates identified in specimens from the last two years of the study period, 2020 and 2021, compared to the initial two years, 2011 and 2012, is shown in Table 2.
 |
Table 2 Distribution of P. aeruginosa Isolates According to Sample Origin with Respect to Year |
Anti-Microbial Resistance Profiles of P. aeruginosa
In total, over the course of the study, 5534 positive cultures for P. aeruginosa isolates were identified.
- Mean rates of resistance to sample drugs from different classes of anti-microbial agents, listed highest percentage first, were: cefepime, 42.7%; ciprofloxacin, 34.3%; imipenem, 30.5%; ceftazidime, 29.2%; gentamicin, 26%; piperacillin/tazobactam, 24.2%; and colistin, 3.1% (Figure 3).
- The rates of resistance were equivalent regardless of whether the samples were obtained from in-patients or out-patients (Figure 4).
- The temporal data demonstrated a rise in the resistance trend for all the anti-microbials under investigation early in the study period and subsequently, a steep decline (Figure 5).
- The rate of resistance during the initial two years of the research period, 2011 and 2012, was notably higher than in the last two years, 2020 and 2021 (p < 0.01). This can be illustrated by cefepime, which had resistance rates in these time periods of 46.5% and 12.5%, respectively, exhibiting a fall of 34%, and gentamicin, for which the equivalent resistance rates were 39% and 6.5%, respectively, which indicated a reduction of 32.5% (Figure 6).
 |
Figure 3 Average rates of resistance for the antibiotics tested amongst isolates of P. aeruginosa. |
 |
Figure 4 Average rates of resistance for the antibiotics tested with respect to in-patient or out-patient sample source. |
 |
Figure 5 Schematic demonstrating the evolution of resistance to anti-microbial agents over the study period, 2011–2021. |
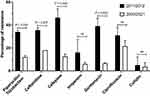 |
Figure 6 Antibiotic resistance rates of P. aeruginosa recognized during the initial (2011 and 2012) and final (2020 and 2021) two years of the study. |
Discussion
As described by Ahmed et al, Pseudomonas aeruginosa is the most commonly isolated non-fermenting bacterium from clinical samples, representing a substantial problem in treating both community-acquired and nosocomial infection.14 To achieve the best clinical outcome, analyzing and selecting the first-line of antibiotic defense is critical. The potential of antibiotic resistance is of great importance, and the World Health Organization (WHO) report (2015) recommends that continuous surveillance of antimicrobial resistance (AMR) is conducted to both identify the infection caused by resistant organisms and to monitor the emergence of resistant strains.15 As noted by Ahmed and Farah et al, 2019 in the Kingdom of Saudi Arabia, even against the backdrop of a need for more study of resistance trends, only a few such studies of P. aeruginosa have been conducted.16,17 Herein, we provide data and analysis that fills the void in the current understanding of AMR patterns. To meet this aim, we retrospectively completed an analysis of AMR in P. aeruginosa samples obtained over an eleven-year period (2011 to 2021) from a tertiary hospital in Saudi Arabia. Our longer-term study enables the analysis of variation in the patterns of resistance over a long period. In contrast, other studies have been cross-sectional in design, consequently providing resistance data at a single time point.
The prevalence rate of 9.1% of P. aeruginosa (5543 isolates from 61,029 total clinical isolates) identified in our study is comparable to that of Ahmed, who reported a rate of 8.7% in a survey conducted in Makkah, Saudi Arabia.16 However, both of these values are substantially lower than the prevalence rates described17,18, They reported rates of 24% and 23.8%, respectively, in their Saudi-based studies. The rates identified in two Iraqi sites by Al-Derzi (2012) were substantially lower – compared to the current study – at 5.2% (Mosul study) and 2.1% (Duhok study). These apparent location-related variances may be attributed to a number of possible causes; clinical specimen type, the population from which the samples were collected, the treating hospital, and the geographic location.5
In our analysis, a comparison of the rate of detection of P. aeruginosa isolates from the initiation date (2011/2012) and the endpoint of our study (2020/2021) identified a significant decrease in isolates, with a drop from 10% to 5%, respectively. There are a number of policy areas which may have led to this decrease. During this time, the Ministry of Health in Saudi Arabia established the specialized body, the “Health Facilities Infection Control General Department”, with a remit to address the issue of hospital-acquired infection and AMR. Also, in 2020 the Ministry of Health introduced a policy preventing the sale of antibiotics without a prescription.19 In addition, the decrease in the number of isolates identified in 2020/2021 may also be attributed to the global Covid-19 pandemic and the reduced number of individuals traveling during this time.20
When investigating the prevalence of P. aeruginosa within the in- and outpatient settings, our findings concurred with those of Farah et al, who found that, in their Saudi-based study, P. aeruginosa was one of the most frequently identified isolates in inpatients. Specifically, in intensive care units, P. aeruginosa is noted as one of the leading causes of hospital-acquired infection.17
Sputum and wound swab samples account for 70% of the P. aeruginosa identified in our study. However, Javiya et al, in their Gujarat, India, study identified urine, pus, and sputum as the primary sources of isolates, with each accounting for 27%, and endotracheal secretion contributing 14% of the P. aeruginosa isolates.21 Once again there are a number of possible contributory factors which led to these differences, specifically the study period, patient population and size, and geographic location. To the best of our knowledge, no other recent studies in Saudi Arabia have determined and reported on the distribution of P. aeruginosa in clinical samples.
When analysing the association between gender and P. aeruginosa infection rates, our findings concurred with those of a number of other researchers. Specifically, we found that the number of cases in males is significantly higher than in females. Senthamarai et al study found that over three quarters (76.6%) of P. aeruginosa isolates were identified in male patients.22 Earlier,5 had proposed that occupational incidents are more likely to occur in males, a theory extended to include infection via outdoor activity, personal habits, work, and exposure to sources of contamination, such as soil, water, and other sources of infection by.22
Our data did not fully agree with that of study.23 We found that P. aeruginosa isolates exhibited high levels of resistance to a panel of antibiotics, whereas the work presented by Ahmad et al demonstrated generally lower levels of resistance. Specifically, we recorded 42% for cefepime, 34.3% for ciprofloxacin, 30.5% for imipenem, 29.2% for ceftazidime, 26% for gentamicin, 24% for piperacillin/tazobactam and 3.1% to colistin, while the other study showed 36.7% for imipenem, 16.7% for ceftazidime, 13.3% to ciprofloxacin, and 10% to cefepime.23 The observed variance may be a consequence of the study period, sample size, and patient population, as well as geographic location.21
One prominent area of agreement between our study and others – conducted in Saudi Arabia and in other regions – is the high resistance level to ciprofloxacin. Our study, Ahmed’s study,16 and studies by24 in Iran, and25 in Turkey, produced rates of 34%, 24.1%, 58%, and 48.9%, respectively. The high level of resistance found in P. aeruginosa isolates to ciprofloxacin may be a consequence of the use of this antibiotic driving high levels of resistance in the bacterium. As a consequence, both clinical and economic outcomes are affected.16
Similarly, high resistance levels were observed in P. aeruginosa to cefepime; in our study, 42% resistance, and in Ibrahim’s Saudi Arabian study, 67% resistance.18 Therefore, we propose that earlier overuse of this antibiotic may have induced a rapid re-emergence of cefepime resistance in P. aeruginosa.26
Our study demonstrates a relatively low (24%) level of resistance to piperacillin-tazobactam, while other Saudi-based studies have shown substantially higher resistance levels of 38% to this antibiotic.16 These variances may result from multiple factors, including total numbers of isolates assessed and screening duration, and may also represent setting-specific antibiotic usage and selective pressure.27
Conclusion
Our study revealed that colistin was the most effective antimicrobial agent against P. aeruginosa isolates and the greatest resistance was seen with cefepime, and the results showed a significant decrease in the resistance to several antibiotics, including piperacillin/tazobactam, cefepime, ceftazidime, and gentamicin, especially in the period from 2017 to 2021, this due to application of infection control protocols and strict policies to control antibiotic prescriptions in all Saudi hospitals.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kanj SS, Sexton DJ, Calderwood SB, Hall KK. Epidemiology, microbiology, and pathogenesis of Pseudomonas aeruginosa infection. UpToDate. 2021.
2. Wilson MG, Pandey S. Pseudomonas Aeruginosa. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
3. Green AE. Investigating the Genetic Basis of Preservative Resistance in an Industrial Pseudomonas Aeruginosa Strain [Doctoral dissertation]. Cardiff University; 2017.
4. Murray PR, Rosenthal KS, Pfaller MA. Medical Microbiology.
5. Al-Derzi NA. Pattern of resistance to Pseudomonas infection in the North of Iraq: emphasis on the potential role of a combination antibiogram. Iraqi J Commun Med. 2012;11:193–198.
6. Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67(3):159–173. doi:10.1111/2049-632X.12033
7. Yezli S, Shibl AM, Livermore DM, Memish ZA. Prevalence and antimicrobial resistance among gram-negative pathogens in Saudi Arabia. J Chemother. 2014;26(5):257–272. doi:10.1179/1973947814Y.0000000185
8. Weiner-Lastinger LM, Abner S, Edwards JR, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National healthcare safety network, 2015–2017. Infect Control Hosp Epidemiol. 2020;41(1):1–18. doi:10.1017/ice.2019.296
9. Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2019;63(7). doi:10.1128/AAC.00355-19
10. Al Johani SM, Akhter J, Balkhy H, El-Saed A, Younan M, Memish Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann Saudi Med. 2010;30(5):364–369. doi:10.4103/0256-4947.67073
11. Alhumaid S, Al Mutair A, Al Alawi Z, et al. Antimicrobial susceptibility of gram-positive and gram-negative bacteria: a 5-year retrospective analysis at a multi-hospital healthcare system in Saudi Arabia. Ann Clin Microbiol Antimicrob. 2021;20(1):43. doi:10.1186/s12941-021-00450-x
12. Said KB, Alsolami A, Khalifa AM, et al. A multi-point surveillance for antimicrobial resistance profiles among clinical isolates of gram-negative bacteria recovered from Major Ha’il Hospitals, Saudi Arabia. Microorganisms. 2021;9(10):2024. doi:10.3390/microorganisms9102024
13. Al-Orphaly M, Hadi HA, Eltayeb FK, et al. Epidemiology of multidrug-resistant pseudomonas aeruginosa in the middle east and North Africa region. mSphere. 2021;6(3). doi:10.1128/mSphere.00202-21
14. Sid Ahmed MA, Hassan AAI, Abu Jarir S, et al. Emergence of multidrug- and pandrug- resistant Pseudomonas aeruginosa from five hospitals in Qatar. Infect Prev Pract. 2019;1(3–4):100027. doi:10.1016/j.infpip.2019.100027
15. World Health Organization. Global Action Plan on Antimicrobial Resistance. World Health Organization; 2015.
16. Ahmed O. Incidence and antibiotic susceptibility pattern of Pseudomonas aeruginosa isolated from inpatients in two tertiary hospitals. Clin Microbiol. 2016;5:2.
17. Farah SM, Alshehri MA, Alfawaz TS, Alasmeri FA, Alageel AA, Alshahrani DA. Trends in antimicrobial susceptibility patterns in King Fahad Medical City, Riyadh, Saudi Arabia. Saudi Med J. 2019;40(3):252–259. doi:10.15537/smj.2019.3.23947
18. Ibrahim ME. High antimicrobial resistant rates among gram-negative pathogens in intensive care units. A retrospective study at a tertiary care hospital in Southwest Saudi Arabia. Saudi Med J. 2018;39(10):1035–1043. doi:10.15537/smj.2018.10.22944
19. MH. World antibiotic awareness week; 2020. Available from: https://www.moh.gov.sa/en/HealthAwareness/HealthDay/2020/Pages/HealthDay-2020-11-18-24.aspx.
20. Jokhdar H, Khan A, Asiri S, Motair W, Assiri A, Alabdulaali M. COVID-19 mitigation plans during Hajj 2020: a success story of zero cases. Health Secur. 2021;19(2):133–139. doi:10.1089/hs.2020.0144
21. Patel J, Javiya V, Ghatak SB. Antibiotic susceptibility patterns of Pseudomonas aeruginosa at a tertiary care hospital in Gujarat, India. Indian J Pharmacol. 2008;40(5):230–234. doi:10.4103/0253-7613.44156
22. Senthamarai S, Suneel A, Sivasankari S, et al. Resistance pattern of Pseudomonas aeruginosa in a tertiary care hospital of Kanchipuram, Tamilnadu, India. J Clin Diagn Res. 2014;8:DC30–DC32. doi:10.7860/JCDR/2014/7953.4388
23. Ahmad S, Alotaibi M, Mohmmed S. Antibiotic sensitivity pattern of clinical isolates of Pseudomonas aeruginosa at a tertiary care hospital in Saudi Arabia. Dhaka Univ J Pharm Sci. 2020;19:77–82. doi:10.3329/dujps.v19i1.47821
24. Savaş L, Duran N, Savaş N, Önlen Y, Ocak S. The prevalence and resistance patterns of Pseudomonas aeruginosa in intensive care units in a university hospital. Turk J Med Sci. 2005;35:317–322.
25. Golshani Z, Mohammad Ahadi A, Sharifzadeh A. Antimicrobial susceptibility pattern of Pseudomonas aeruginosa isolated from patients referring to hospitals. J Arch Hyg Sci. 2012;1:1.
26. Akhabue E, Synnestvedt M, Weiner MG, Bilker WB, Lautenbach E. Cefepime-resistant Pseudomonas aeruginosa. Emerg Infect Dis. 2011;17(6):1037–1043. doi:10.3201/eid/1706.100358
27. Khan MA, Faiz A. Antimicrobial resistance patterns of Pseudomonas aeruginosa in tertiary care hospitals of Makkah and Jeddah. Ann Saudi Med. 2016;36(1):23–28. doi:10.5144/0256-4947.2016.23
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
