Back to Journals » Infection and Drug Resistance » Volume 16
Antimicrobial Resistance and Virulence Gene Profile of Clinical Staphylococcus aureus: A Multi-Center Study from Ethiopia
Authors Ibrahim RA , Berhe N, Mekuria Z, Seyoum ET , Balada-Llasat JM, Abebe T , Mariam SH, Tsige E , Fentaw Dinku S, Wang SH
Received 2 May 2023
Accepted for publication 12 July 2023
Published 25 July 2023 Volume 2023:16 Pages 4835—4844
DOI https://doi.org/10.2147/IDR.S419577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Rajiha A Ibrahim,1– 3 Nega Berhe,1 Zelalem Mekuria,3,4 Eyasu T Seyoum,3 Joan-Miquel Balada-Llasat,3,5 Tamrat Abebe,6 Solomon H Mariam,1 Estifanos Tsige,2 Surafel Fentaw Dinku,2 Shu-Hua Wang3,7
1Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia; 2Bacterial, Parasitic and Zoonotic Research Directorate, Ethiopian Public Health Institute, Addis Ababa, Ethiopia; 3Global One Health Initiative (GOHi), The Ohio State University, Columbus, OH, USA; 4Veterinary Preventive Medicine, Colleges of Veterinary Medicine, The Ohio State University, Columbus, OH, USA; 5Department of Pathology, College of Medicine, The Ohio State University, Columbus, OH, USA; 6Department of Microbiology, Immunology, and Parasitology, Schools of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 7Division of Infectious Diseases, Department of Internal Medicine, College of Medicine, The Ohio State University, Columbus, OH, USA
Correspondence: Rajiha A Ibrahim, Bacterial, Parasitic and Zoonotic Research Directorate, Ethiopian Public Health Institute, PO Box: 1242, Addis Ababa, Ethiopia, Tel +251920602640, Email [email protected]; [email protected]
Background: Staphylococcus aureus causes a wide range of infections from mild skin and soft tissue to severe life-threatening bacteremia. The pathogenicity of S. aureus infections is related to various bacterial surface components and extracellular proteins such as toxic-shock syndrome (TSS) toxin and Panton-Valentine leukocidin (PVL). In this study we determine the antimicrobial resistance of isolated strains and their virulence genes in Ethiopia.
Methods: A total of 190 archived S. aureus isolates from four Ethiopia Antimicrobial Resistance (AMR) Surveillance sites were analyzed. The identification of S. aureus was done by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF Biotyper) and antimicrobial susceptibility test (AST) was done using VITEK® 2. Multiplex PCR was used to detect mecA, mecC, pvl and spa genes and super-antigens (sea, seb, sec, seh and sej staphylococcal enterotoxins).
Results: A total of 172 isolates were confirmed as S. aureus, 9 (5.23%) were methicillin-resistant S. aureus (MRSA) and 163 (94.76%) were methicillin-susceptible S. aureus (MSSA). AST showed that 152 (88.4%) isolates were resistant to penicillin; 90 (52.32%) resistant to trimethoprim-sulfamethoxazole; and 45 (26.16%) resistant to tetracycline. A total of 66 (38.37%) isolates harbored at least one staphylococcal enterotoxin gene and 31 (46.96%) isolates had more than one. The most frequent enterotoxin gene encountered was seb 28 (16.28%). The TSST-1 gene was detected in 23 (13.37%). Presence of staphylococcal enterotoxin gene showed significant association with antibiotic resistance to cefoxitin, benzylpenicillin, oxacillin, erythromycin, clindamycin, tetracycline and SXT. The pvl gene was detected in 102 (59.3%) of isolates. Isolates from patients below 15 years of age showed significantly high numbers of pvl gene (P = 0.02). Presence of sej (P = 0.011) and TSST-1 (P < 0.001) genes were associated with the presence of pvl gene.
Conclusion: In this study, isolates were highly resistant to oral antibiotics and the pvl, seb, sea and TSST-1 genes were prevalent.
Keywords: MRSA, enterotoxin genes, Panton-Valentine leucocidin, PVL, Ethiopia
Introduction
Staphylococcus aureus is a part of the normal skin flora and commonly colonizes the nose, respiratory tract, and skin. S. aureus also causes a wide spectrum of infections ranging in severity from mild skin and soft tissue and osteomyelitis to severe life-threatening pneumonia and bacteremia.1
Infection with S. aureus is widespread worldwide, and different strains exhibit varying degrees of pathogenesis and virulence. Certain strains have the potential to spread quickly and be quite virulent. These strains may be associated with both community and healthcare infections worldwide.2 The pathogenicity of S. aureus infections is related to various bacterial surface components such as capsular polysaccharide and protein A (e.g., clumping factor and fibronectin binding protein), and extracellular proteins (e.g., coagulase, hemolysins, enterotoxins, toxic-shock syndrome [TSS] toxin, exfoliatins and Panton-Valentine leukocidin [PVL]).
In general, the precise roles of individual staphylococcal factors in invasive infections are difficult to assess, but PVL production has been linked to furuncles, cutaneous abscesses and severe necrotic skin infections.3,4 Presence of PVL is associated epidemiologically with community associated methicillin-resistant S. aureus (CA-MRSA) infections, and is responsible for increased virulence of the organisms.2 Toxic-shock syndrome (TSS) toxin and enterotoxins play a part in the pathogenesis of several important human diseases, including sepsis-related infections, pneumonia and toxic-shock syndrome.5 Staphylococcal enterotoxins also function as superantigens that promote the proliferation of T cells.6 Association of staphylococcal enterotoxins and a toxic-shock syndrome (TSS) toxin with multidrug antimicrobial resistance MRSA strains, has been reported including those resistant to quinolones or other antibiotics.7
S. aureus is an organism that has the potential to develop antimicrobial resistance rapidly. After penicillin was first introduced, significant resistance developed in less than 2 years.8,9 The discovery of methicillin was considered as a relief at first, however, it started to develop resistance after its introduction. Currently, penicillin resistance is reported from different studies at over 70% resistance.10,11
Horizontal gene transfer is the major mechanism of resistance in S. aureus resistance to methicillin and vancomycin.12 Endogenous resistance is acquired by natural mutation and selection under antimicrobial pressure, and is also known to play an important role in the clinical setting, providing a major route of resistance to antimicrobials such as fluoroquinolones, vancomycin (for the intermediate level of resistance), daptomycin, linezolid and more.12,13
In Ethiopia, studies on antimicrobial resistance (AMR) patterns of S. aureus were mostly based on traditional AST. The reported clinical MRSA prevalence in Ethiopia using the classical methods, shows a wide prevalence range between 17.5–97.0%.10,14,15 Currently, molecular detection of resistance and virulence genes is limited in clinical isolates in Ethiopia.16,17 This study therefore, aimed to fill the gap of limited information on the molecular characterization of virulence, antimicrobial resistance genes and other clinically important genes from archived clinical S. aureus isolates from four Ethiopia AMR Surveillance sites.
Materials and Methods
Sample Collection and Sites Description
This study was conducted using S. aureus isolates collected from the Ethiopia Antimicrobial Resistance Surveillance AMR sites. The surveillance was structured to connect sentinel surveillance systems in Ethiopia to a national reference laboratory at the Ethiopian Public Health Institute (EPHI). Each surveillance site includes laboratories that are either affiliated with or located within a reference hospital.18 In this study, a total of 190 archived human bacterial isolates (167 wound/pus, 8 blood, 6 ear swabs and 9 other body fluids) were used. These samples were collected from January 2016 to January 2019 at four active AMR Surveillance sites. The sites included different geographic areas in the country, these are Tikur Anbessa Specialized Hospital (TASH), Addis Ababa; Amhara Public Health Institute (APHI), Dessie; Ayder University Hospital (AUH), Mekele and the Clinical Bacteriology and Mycology National Reference Laboratory at EPHI, Addis Ababa. The isolates were initially identified using classical culture methods using biochemical tests such as catalase and coagulase at their respective AMR Surveillance sites. All isolates were transported and stored at −80°C at the National Reference Laboratory in EPHI. Aliquots of the specimen were shipped to the Ohio State University OSU for further phenotypic and genotypic characterization in February 2019.
Phenotypic Characterization of Staphylococcus aureus
All 190 S. aureus isolates were analyzed at OSU Wexner Medical Center using MALDI-TOF, Biotyper 2.0, Bruker, following the manufacturer’s protocol. Antimicrobial susceptibility test (AST) was performed using VITEK® 2 XL (bioMérieux version 08.01) and the result was interpreted following minimum inhibitory concentration (MIC) interpretation guide of Clinical Laboratory Standards Institution (CLSI) M100-S22 (2018). Based on the result of cefoxitin test, isolates were grouped into MRSA or MSSA.19
Nucleic Acid Preparation
Genomic DNA was extracted using a commercially available kit (QIAamp DNA Kits, Germany) following the manufacturer’s protocol. Extraction and PCR tests were done at OSU, Infectious Disease Epidemiology Molecular Laboratory (IDEML). In summary, bacterial colonies were harvested and suspended using molecular grade water in a micro centrifuge tube and centrifuged for 10 minutes at 7500 rpm. The supernatants were discarded and the bacterial pellets were re-suspended in 180 μL of lysis buffer. After multiple wash steps the DNA was eluted in 100 μL of molecular grade water and was stored at −20°C for further analysis.
Amplification of mecA, mecC, pvl and spa Gene and Enterotoxin Genes
For PCR amplification of mecA, mecC, pvl and spa genes, the illustra™ PuReTaq™ Ready-To-Go™ PCR Beads (GE Healthcare Bio-Sciences, USA) in 25 µL reaction were used following a previously described method.20 Briefly, PCR reactions were performed with an initial denaturation of 94°C for 5 min, 30 cycles of 94°C, 30 s; 59°C, 1 min and 72°C 1 min and a final extension at 72°C for 10 min.
Additionally, multiplex PCR reactions for the detection of six enterotoxin genes (sea, seb, sec, seh and sej) were performed according to the previously described protocol.21 The initial denaturation was 95°C for 10 min; followed by 15 cycles of 95°C, 1 min, 66°C, 45 s and 72°C, 1 min; then 20 cycles of 95°C, 1 min, 63°C, 45 s and 72°C, 1 min. The final extension was at 72°C for 2 min.
All PCR amplifications were done using SimpliAmp Thermal cycler (Applied Biosystems, Singapore). The PCR products were run in gel stained with ethidium bromide and the images were captured and visualized with UV light using AlphaImager instrument and FluorChem HD2 software. Product sizes were determined by comparison with a 100-bp gene ruler DNA ladder in gel reader. The following reference strains were used as positive control in PCR-amplifications of the tests: ATCC 14458, ATCC 13565, ATCC 25923, ATCC 23235 and ATCC 19095.
Statistical Analysis
Data were analyzed using STATA version 14.2. Cefoxitin screening by site and the prevalence of S. aureus by site were generated using summary tables. Logistic regression models were fitted to determine the association between antimicrobial resistance and staphylococcal enterotoxin genes of S. aureus, association between pvl and antimicrobial resistance and pvl and sample type.
Results
Socio-Demographic Characteristics of Participants
Type of infection and socio-demographic data were available for 124 of the 190 subjects. Among the 124 subjects, 78 (63%) were males and 46 (37%) were females. The ages ranged from 11 days to 84 years with a mean age of 26.5 years (standard deviation of the mean 20.21) and median age of 23 years. Most were in the age group of 0–14 years (39, 31.45%) (Table 1).
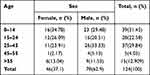 |
Table 1 Demographics of Study Participants |
Confirmation of S. aureus and MRSA
Out of 190 previously identified S. aureus isolates at site level, 172 isolates were confirmed as S. aureus using MALDI-TOF. Overall 18 (9.47%) could not be identified as S. aureus during confirmation. One of the isolates was not identified and the remaining 17 (8.94%) of the isolates were identified as other organisms. Eight (47.05%) were coagulase negative Staphylococcus spp., 5 (29.41%) were Enterococcus spp., 2 (11.76%) were Exiguobacterium aurantiacum and the remaining 2 (11.76%) were identified as Escherichia coli and Proteus mirabilis. From the 172 confirmed S. aureus, nine (5.23%) were MRSA. Five of the MRSA isolates were from AUH and four isolates were from EPHI (Table 2). All MRSA isolates were identified in the year 2018.
 |
Table 2 Methicillin-Resistant and Susceptible Staphylococcus aureus by Site |
Antimicrobial Resistance
Out of 172 S. aureus isolates tested for antimicrobial resistance, 152 (88.37%) were resistant to penicillin; 90 (52.32%) were resistant to trimethoprim sulfamethoxazole (SXT); 45 (26.16%) were resistant to tetracycline and 31 (18.02%) were resistant to clindamycin. None of the isolates were resistant to ceftaroline, vancomycin, linezolid and daptomycin.
Of the 11 isolates resistant to oxacillin, two were cefoxitin screening negative. Nine (5.23%) isolates were confirmed as MRSA (Table 3).
 |
Table 3 Antimicrobial Susceptibility Pattern of Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Strains to Different Antibiotics |
The antibiotic resistance of MRSA strains revealed that 8 (88.88%) were resistant to tetracycline and 5 (55.56%) resistant to SXT. Four (44.44%) of the isolate were resistant to ciprofloxacin, levofloxacin and erythromycin. All MRSA isolates were susceptible to vancomycin, linezolid, daptomycin and ceftaroline (Table 3).
A total of 163 (94.76%) were methicillin-susceptible S. aureus (MSSA); 143 (87.73%), 85 (52%) and 37 (22.69%) were resistant to benzylpenicillin, SXT and tetracycline respectively. Clindamycin and erythromycin showed 28 (17.17%) and 27 (16.56%) resistance. Other tested antimicrobial agents showed below 2% resistance (Table 3). Over all the percent resistance to antimicrobial agent is higher in MRSA than MSSA.
mecA, mecC, pvl and spa Gene Detection
All 172 isolates tested positive for the presence of spa gene whereas mecA gene was detected in nine of the isolates. None of the tested isolates were mecC positive. Out of all S. aureus isolates 102/172 (59.30%) possess the pvl gene.
None of the mecA positive isolates had the pvl gene. The overall MSSA isolates that were positive for pvl gene were 102/163 (62.57%). Of these 91/102 (89.22%) were from pus specimens. There was no significant association between specimen type and presence of pvl gene (Table 4). However, there was a significant increased number of pvl gene presence in the age 14 years and below (Table 4).
 |
Table 4 Association of the Presence of pvl Gene with Specimen Type and Age |
Presence of pvl was also associated with the presence of the enterotoxin genes. Specifically, presence of sej and TSST-1 gene were significantly associated with the presence of pvl gene (Table 5).
 |
Table 5 Association of Antimicrobial Resistance and Staphylococcal Enterotoxin Genes Among pvl Gene Positive Isolates |
Enterotoxin and Toxic Shock Syndrome Toxin Gene Detection
A total of 66 (38.37%) isolates harbored at least one staphylococcal enterotoxin gene (Table 6). Of these 31 (46.97%) isolates had more than one enterotoxin gene. Eleven of them had three different genes. The most frequent enterotoxin genes were seb 28 (16.28%) and sea 25 (14.53%), and sec 5 (2.90%) were the least frequent (Table 6). The toxic-shock syndrome toxin gene was detected in TSST-1 23 (13.37%). Isolates from EPHI and AUH had high numbers of enterotoxin genes. The distribution of enterotoxin gene varied across isolates identified from different specimen types. The highest number of enterotoxin genes was observed from isolates identified from ear swab 4/6 (66.67%) followed by blood 5/8 (62.50%) and pus 58/150 (38.00%). Of the 9 MRSA isolates 7 (77.78%) had at least one enterotoxin gene. One of them had three (sea, seb and TSST-1), another had two (sea and TSST-1) and the remainder had one staphylococcal enterotoxin gene each.
 |
Table 6 Staphylococcal Enterotoxin and Toxic-Shock Syndrome Toxin Genes Detection by Site |
The overall presence of staphylococcal enterotoxin gene showed significant association with cefoxitin, benzylpenicillin, oxacillin, erythromycin, clindamycin, tetracycline and SXT resistance. Staphylococcal seb had association with cefoxitin and tetracycline resistance. Presence of sea showed a significant association with rifampicin resistance. Cefixitin and SXT resistance also had association with presence of seh gene. Tetracycline resistance had association with presence of sej gene. Toxic shock syndrome toxin showed significant association with the oxacillin, erythromycin, clindamycin and tetracycline resistance (Table 7).
 |
Table 7 Association of Antimicrobial Resistance and Staphylococcal Enterotoxin Genes of S. aureus Isolates |
Discussion
In this study it was found that the majority of isolates were MSSA, and these were highly resistant to commonly used agents such as penicillin, SXT, tetracycline and clindamycin. Over one third of the isolates harbor at least one staphylococcal enterotoxin gene and the most frequent enterotoxin genes encountered were seb and sea genes.
Among the study age group, pediatric ages was higher in frequency and S. aureus infection was more prevalent in males. This is consistent with other studies that show S. aureus infection is more prevalent in males than females.1
Nine percent of isolates identified as S. aureus at the site level were found to be a different organism by MALDI-TOF. Comparative study of MALDI-TOF and the classical method indicates discrepancy in general and specifically with Streptococcus sp., and identification of Staphylococcus spp. also encountered some species level discrepancy.22,23 In this study almost half the discrepancy was in Staphylococcus species level. Misdiagnosis of S. aureus as other staphylococcus species was also reported in Southern Nigeria in large amounts, that is 85%.24
Compared with other studies conducted in Ethiopia, we only identified nine (5.2%) MRSA, that is fewer than the report of other studies in Ethiopia.10,15,17 Similar to our findings, other studies that used a molecular method also reported very low MRSA in Ethiopia.17,25 All MRSA isolates were identified in the year 2018. This may be because the number of S. aureus isolates were lower in the first two years. One of the limitations of the study was that the numbers of isolates were not proportional in every year because the study used stored isolates from the surveillance and the surveillance was strengthened and formally started by the first quarter of 2017 in four of the surveillance sites.18
All S. aureus isolates were susceptible to ceftaroline, linezolid, daptomycin and vancomycin in this study. These results were consistent with other studies conducted in East Africa.17,26 This is likely due to limited supply of ceftaroline, linezolid and daptomycin in clinical settings in Ethiopia. It is assuring to know that the isolates were all susceptible to vancomycin so that patients with severe invasive infections can still have access to effective antimicrobials.
Despite the susceptibility for IV drugs, very high percent resistance has been observed in oral administered drugs such as penicillin, SXT and tetracycline. Studies conducted in Ethiopia showed a similar resistance pattern for both SXT and tetracycline.17,27 These oral antibiotics are highly prescribed and can lead to resistance.
Eighteen percent resistance to both clindamycin and erythromycin was observed in this study, this is also similar with other studies conducted in Ethiopia.17 The resistance may be due to the presence of erythromycin ribosome methylase erm gene, which includes resistance to macrolides, lincosamides and streptogramin.28 Of the total clindamycin resistance, 90% of them are due to inducible clindamycin resistance (ICR). Similarly, other studies also reported higher inducible clindamycin resistance in Africa.14,29,30
Two isolates showed resistance to oxacillin but were susceptible to cefoxitine. Cefoxitine is the best indicator for the mecA gene and this was confirmed by the molecular characterization which showed that these isolates did not harbor mecA gene. It indicates that the resistance to oxacillin is mediated by other resistance mechanisms other than mecA gene.31 Gentamicin resistance was observed only in MRSA (22%) isolates.
Resistance to fluroquinolones was found to be 44% for MRSA isolates while it was 1.8% and 0.6% against ciprofloxacin and levofloxacin in MSSA strains, respectively. Similarly, the resistance for clindamycin and erythromycin was higher in MRSA at 44% while MSSA was 18%. Nitrofurantoin and rifampicin resistance also showed higher percentage in MRSA than MSSA, that is 89% of MRSA isolates are resistant to tetracycline. These findings (the higher rate of resistance to multiple classes of antibiotics) are consistent in other studies both in Africa and elsewhere.17,23,32,33 This might be due to the fact that MRSA strains often possess genes that express resistance to multiple classes of both β-lactam and non-β-lactam agents.34
In this research, the spa gene was detected in all isolates; however, other studies found that the change in primer binding sites resulted in up to 3% non-detection.35 A similar problem was handled by Votintseva et al, in 2014 using a new primer.36 Overall, from both MSSA and MRSA strains, virulence gene (pvl) prevalence was found to be 59.30%. There was a higher percentage on MSSA, at 62.57%. One explanation for this high percentage might be that the clinical cases were mainly cutaneous, because PVL is usually associated with furuncles, cutaneous abscesses and severe necrotic skin infections.3,4 The high percentage of PVL-positive strains are in agreement with previous studies conducted in other African countries indicating that there is a high prevalence of S. aureus strains presenting genes encoding pvl on this continent.4,17 Different studies have demonstrated that PVL is more prevalent in children and presence of PVL is associated with severe and life-threatening conditions for children below 14 years of age.34,37,38 In this study significantly high prevalence of pvl gene was observed in this age group. Infection prevention should be applied that considers children 14 and below in the study sites. The presence of pvl gene was also significantly associated with the presence of enterotoxin genes such as sej and TSST-1. In addition, the presence of pvl genes was associated with some antimicrobial resistance such as tetracycline and SXT. Similarly, a report from Egypt for PVL-positive S. aureus indicated high percent resistance to penicillin and SXT.39
Sixty-six (38.37%) of the isolates had at least one of the enterotoxin genes. Of these, 31 (46.97%) isolates had more than one enterotoxin gene and it is comparable to a previous study in Ethiopia.40 A study conducted in Sudan showed lower numbers of seb gene and no sea unlike our results.41 Most of the antimicrobial resistance including MRSA and presence of enterotoxin gene had significant association in this study. This was not the case in a study that was conducted in another African country.4
The most frequent enterotoxin gene encountered is seb however this is not consistent with other studies.40,41 The second most common enterotoxin gene in this study is sea and a different study put sea as the most common enterotoxin gene.40 The TSST-1 gene is the third most common toxin gene in this study, in other studies in Ethiopia, this gene is either detected in low amounts or was not detected at all.40,42 This indicates that isolates in this study were more virulent. S. aureus is among the most common bacterial pathogens for ear infections in Ethiopia.43,44 Presence of staphylococcal enterotoxin genes were suggested to be associated with otitis media. Since in this study isolates from ear samples showed slightly more enterotoxin genes, it may be suggestive of serious otitis media in the country.44,45 Studies in other places indicate that strains from bloodstream infection are highly virulent with more staphylococcal enterotoxin gene, however in this study this could not be demonstrated. This might be because the number of isolates from blood samples was small.1,46
Despite identification of some virulent factors from this study, it is limited by the retrospective nature of the isolates tested. Since specimens were not collected to include all sites of infection, some samples such as wound/pus are highly represented in this study. In addition, in this study no isolates were typed for molecular epidemiologic and evolutionary purposes. This is because robust genotyping with MLST and/or whole genome sequence (WGS) could not be performed due to budgetary constraints.
Conclusion
The level of MRSA detected from this study is lower than that reported by other studies in Ethiopia. Although complete susceptibility has been documented for antibiotics that are less prescribed, there is high resistance to the majority of commonly used antibiotics. Antibiotic usage regulation should be strengthened in the country. More than half of the isolates possessed the virulence gene pvl and about 40% of the isolates harbored at least one staphylococcal enterotoxin gene such as seb and sea genes. Isolates identified from children aged 14 years and below had high pvl gene and isolates from ear swabs had the highest enterotoxin gene prevalence. This highlights that isolates circulating in the study sites are highly virulent and need attention in regard to infection prevention specific to children and ears. This study improves the understanding of molecular determinants of S. aureus infections including virulent genes. Overall, there is a need to strengthen laboratory detection methods such as auto-method identification and detection for site level and molecular level testing capacity of the national reference laboratory.
Data Sharing Statement
We have presented the most important data with this report. However, upon reasonable request, additional data could be available from the corresponding author.
Ethics Approval and Consent to Participate
The study protocol was approved by EPHI Institutional Review Board (IRB) (Unique identifier: “EPHI-IRB-029-2017”). All isolates in this study were collected during bacteriological analysis in the clinical microbiology laboratory. Data analysis was anonymous and every step of the study did not identify patients in any way and consents were waived by the IRB.
Acknowledgments
I would like to acknowledge Ethiopia Antimicrobial Resistance Surveillance team at EPHI, APHI-Dessie, AUH and TASH.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Sustainable One Health Research Training Capacity (OHEART): Molecular epidemiology of zoonotic foodborne and waterborne pathogens in Eastern Africa. Funded by the NIH Fogarty International Center (D43TW008650), through the Global One Health initiative (GOHi). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript.
Disclosure
Dr Joan-Miquel Balada-Llasat reports grants, personal fees from BioMerieux, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–661. doi:10.1128/CMR.00134-14
2. Nimmo GR. USA300 abroad: global spread of a virulent strain of community-associated methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2012;18(8):725–734. doi:10.1111/j.1469-0691.2012.03822.x
3. Kobayashi SD, Malachowa N, Deleo FR. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol. 2015;185(6):1518–1527. doi:10.1016/j.ajpath.2014.11.030
4. Darboe S, Dobreniecki S, Jarju S, et al. Prevalence of Panton-Valentine Leukocidin (PVL) and antimicrobial resistance in community-acquired clinical staphylococcus aureus in an urban Gambian hospital: a 11-year period retrospective pilot study. Front Cell Infect Microbiol. 2019;9:1–7. doi:10.3389/fcimb.2019.00170
5. Li Z, Stevens DL, Hamilton SM, et al. Fatal S. aureus hemorrhagic pneumonia: genetic analysis of a unique clinical isolate producing both PVL and TSST-1. PLoS One. 2011;6(11):154.
6. Chuang-Smith ON, Schlievert PM. Staphylococcal enterotoxin C subtypes are differentially associated with human infections and immunobiological activities. mSphere. 2021;6(1). doi:10.1128/mSphere.01153-20
7. Ortega E, Abriouel H, Lucas R, Gálvez A. Multiple roles of staphylococcus aureus enterotoxins: pathogenicity, superantigenic activity, and correlation to antibiotic resistance. Toxins. 2010;2:2117–2131. doi:10.3390/toxins2082117
8. Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi:10.1038/nrmicro2200
9. Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111(9):1265–1273. doi:10.1172/JCI18535
10. Deyno S, Fekadu S, Astatkie A. Resistance of Staphylococcus aureus to antimicrobial agents in Ethiopia: a meta-analysis. Antimicrob Resist Infect Control. 2017;6(1). doi:10.1186/s13756-017-0243-7
11. Juayang AC, De Los Reyes GB, De La Rama AJG, Gallega CT. Antibiotic resistance profiling of Staphylococcus aureus isolated from clinical specimens in a tertiary hospital from 2010 to 2012. Interdiscip Perspect Infect Dis. 2014;2014:1–4. doi:10.1155/2014/898457
12. Pantosti A, Sanchini A, Monaco M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007;2(3):323–334. doi:10.2217/17460913.2.3.323
13. Guo Y, Song G, Sun M, Wang J, Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect Microbiol. 2020;10:1–11. doi:10.3389/fcimb.2020.00107
14. Mama M, Abdissa A, Sewunet T. Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethiopia. Ann Clin Microbiol Antimicrob. 2014;13(1). doi:10.1186/1476-0711-13-14
15. Dilnessa T, Bitew A. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus isolated from clinical samples at Yekatit 12 Hospital Medical College, Addis Ababa, Ethiopia. BMC Infect Dis. 2016;16(1). doi:10.1186/s12879-016-1742-5
16. Desta K, Aklillu E, Gebrehiwot Y, et al. High levels of methicillin-resistant Staphylococcus aureus carriage among healthcare workers at a teaching hospital in Addis Ababa Ethiopia: first evidence using mecA detection. Infect Drug Resist. 2022;15:3135–3147. doi:10.2147/IDR.S360123
17. Verdú-Expósito C, Romanyk J, Cuadros-González J, et al. Study of susceptibility to antibiotics and molecular characterization of high virulence Staphylococcus aureus strains isolated from a rural hospital in Ethiopia. PLoS One. 2020;15(3):1–17. doi:10.1371/journal.pone.0230031
18. Ethiopia AMR surveillance plan_final. Available from: https://www.ephi.gov.et/images/pictures/download2010/Ethiopia-AMR-Surveillance-Plan_Final.pdf.
19. Weinstein MP. M100-Performance Standards for Antimicrobial Susceptibility Testing.
20. Stegger M, Andersen PS, Kearns A, et al. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin Microbiol Infect. 2012;18(4):395–400. doi:10.1111/j.1469-0691.2011.03715.x
21. Løvseth A, Loncarevic S, Berdal KG. Modified multiplex PCR method for detection of pyrogenic exotoxin genes in staphylococcal isolates. J Clin Microbiol. 2004;42(8):3869–3872. doi:10.1128/JCM.42.8.3869-3872.2004
22. Risch M, Radjenovic D, Han JN, Wydler M, Nydegger U, Risch L. Comparison of MALDI TOF with conventional identification of clinically relevant bacteria. Swiss Med Wkly. 2010;140. doi:10.4414/smw.2010.13095
23. El-Bouri K, Johnston S, Rees E, et al. Comparison of bacterial identification by MALDI-TOF mass spectrometry and conventional diagnostic microbiology methods: agreement, speed and cost implications. Br J Biomed Sci. 2012;69(2):47–55. doi:10.1080/09674845.2012.12002436
24. Ayeni FA, Odumosu BT. False identification of other microorganisms as staphylococcus aureus in Southern Nigeria. Trop J Pharma Res. 2016;15(9):1941–1945. doi:10.4314/tjpr.v15i9.19
25. Eyasu T, Tesfu K, Daniel A, et al. Phenotypic and genotypic characterization of Staphylococcus aureus isolates recovered from bovine milk in central highlands of Ethiopia. Afr J Microbiol Res. 2015;9(44):2209–2217. doi:10.5897/AJMR2015.7562
26. Nyasinga J, Kyany’a C, Okoth R, et al. A six-member SNP assay on the iPlex MassARRAY platform provides a rapid and affordable alternative for typing major African Staphylococcus aureus types. Access Microbiol. 2019;1(3). doi:10.1099/acmi.0.000018
27. Mama M, Aklilu A, Misgna K, Tadesse M, Alemayehu E. Methicillin- and inducible clindamycin-resistant Staphylococcus aureus among patients with wound infection attending Arba Minch Hospital, South Ethiopia. Int J Microbiol. 2019;2019:1–9. doi:10.1155/2019/2965490
28. Mišić M, Čukić J, Vidanović D, et al. Prevalence of genotypes that determine resistance of staphylococci to macrolides and lincosamides in Serbia. Front Public Health. 2017;5:1–8. doi:10.3389/fpubh.2017.00200
29. Baguma A, Musinguzi B, Mpeirwe M, Bazira J. Clindamycin resistance among methicillin resistant Staphylococcus aureus isolated from human and respective household swine in Greater Kabale Region—South Western Uganda. Adv Infect Dis. 2019;09(04):285–294.
30. Kishk RM, Anani MM, Nemr NA, Soliman NM, Fouad MM. Inducible clindamycin resistance in clinical isolates of staphylococcus aureus in Suez Canal University Hospital, Ismailia. Egypt J Infect Dev Ctries. 2020;14(11):1281–1287. doi:10.3855/jidc.12250
31. Shah DA, Wasim S, Abdullah FE. Comparison of Oxacillin and Cefoxitin for the detection of mecA gene to determine Methicillin resistance in coagulase negative staphylococci (CoNs). J Coll Physicians Surg Pak. 2017;27(8):520–522.
32. Gitau W, Masika M, Musyoki M, Museve B, Mutwiri T. Antimicrobial susceptibility pattern of Staphylococcus aureus isolates from clinical specimens at Kenyatta National Hospital. BMC Res Notes. 2018;11(1):1–5. doi:10.1186/s13104-018-3337-2
33. Dhungel S, Rijal KR, Yadav B, et al. Methicillin-resistant Staphylococcus aureus (MRSA): prevalence, antimicrobial susceptibility pattern, and detection of mec A gene among cardiac patients from a Tertiary Care Heart Center in Kathmandu, Nepal Infect Dis. 2021;14:117863372110373.
34. Watkins RR, Holubar M, David MZ. Antimicrobial resistance in methicillin-resistant Staphylococcus aureus to newer antimicrobial agents. Antimicrob Agents Chemother. 2019;63(12). doi:10.1128/AAC.01216-19
35. Haggag MG, Aboelnour AE, Al-Kaffas M. MRSA screening and spa gene detection in isolates from healthcare workers at ophthalmology hospital in Egypt. Bull Natl Res Cent. 2019;43(1). doi:10.1186/s42269-019-0253-0
36. Votintseva AA, Fung R, Miller RR, et al. Prevalence of Staphylococcus aureus protein A (spa) mutants in the community and hospitals in Oxfordshire. BMC Microbiol. 2014;14(1):63. doi:10.1186/1471-2180-14-63
37. Iliya S, Mwangi J, Maathai R, Muriuki M, Wainaina C. Molecular detection of panton valentine leukocidin toxin in clinical isolates of Staphylococcus aureus from Kiambu County, Kenya. Int J Microbiol. 2020;2020:1–8. doi:10.1155/2020/3106747
38. Hardy C, Osei L, Basset T, Elenga N. Bone and joint infections with Staphylococcus aureus strains producing Panton-Valentine Leukocidin in French Guiana. Medicine. 2019;98(27):e16015. doi:10.1097/MD.0000000000016015
39. Sadat A, Shata RR, Farag AMM, et al. Prevalence and characterization of PVL-positive staphylococcus aureus isolated from raw cow’s milk. Toxins. 2022;14(2):1–17. doi:10.3390/toxins14020097
40. Seyoum ET, Mekonene TK, Woldetsadik DA, Zewudie BM, Gebreyes WA. Enterotoxin gene profile of staphylococcus aureus isolates recovered from bovine milk produced in central Ethiopia. J Infect Dev Ctries. 2016;10(2):138–142. doi:10.3855/jidc.6797
41. Yahya Ahmed M, Abdalbagi Ali H, Mohammed Taher Gorish B, et al. Molecular detection of Staphylococcal enterotoxins and mecA genes products in selected food samples collected from different areas in Khartoum State. Int J Microbiol. 2021;2021:1–9. doi:10.1155/2021/5520573
42. Tegegne DT, Mamo G, Waktole H, Messele YE. Molecular characterization of virulence factors in Staphylococcus aureus isolated from bovine subclinical mastitis in central Ethiopia. Ann Microbiol. 2021;71(1). doi:10.1186/s13213-021-01639-3
43. Molla M, Temesgen K, Seyoum T, Melkamu M. Surgical site infection and associated factors among women underwent cesarean delivery in Debretabor General Hospital, Northwest Ethiopia: hospital based cross sectional study. BMC Pregnancy Childbirth. 2019;19(1):1–10. doi:10.1186/s12884-019-2442-0
44. Demir D, Karabay O, Güven M, Kayabasoğlu G, Yilmaz MS. Do Staphylococcus aureus superantigens play a role in the pathogenesis of otitis media with effusion in children? Int J Pediatr Otorhinolaryngol. 2016;84:71–74. doi:10.1016/j.ijporl.2016.02.028
45. Xu J, Du Q, Shu Y, Ji J, Dai C. Bacteriological profile of chronic suppurative otitis media and antibiotic susceptibility in a tertiary care hospital in Shanghai, China. Ear Nose Throat J. 2021;100(9):NP391–6. doi:10.1177/0145561320923823
46. Deodhar D, Varghese G, Balaji V, et al. Prevalence of toxin genes among the clinical isolates of Staphylococcus aureus and its clinical impact. J Glob Infect Dis. 2015;7(3):97–102. doi:10.4103/0974-777X.162234
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
