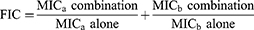Back to Journals » Infection and Drug Resistance » Volume 16
Antimicrobial Effect of Oxazolidinones and Its Synergistic Effect with Bedaquiline Against Mycobacterium abscessus Complex
Authors Gao T , Yao C, Shang Y , Su R , Zhang X, Ren W, Li S , Shu W, Pang Y , Li Q
Received 1 November 2022
Accepted for publication 6 January 2023
Published 14 January 2023 Volume 2023:16 Pages 279—287
DOI https://doi.org/10.2147/IDR.S395750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Tianhui Gao,1,* Cong Yao,1,* Yuanyuan Shang,1 Renchun Su,1 Xuxia Zhang,1 Weicong Ren,1 Shanshan Li,1 Wei Shu,2 Yu Pang,1 Qi Li2
1Department of Bacteriology and Immunology, Beijing Key Laboratory for Drug-Resistant Tuberculosis Research, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Institute, Beijing, People’s Republic of China; 2Clinical Center on Tuberculosis Control, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Institute, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qi Li; Yu Pang, Beijing Chest Hospital, Capital Medical University, No. 97, Machang, Tongzhou District, Beijing, 101149, People’s Republic of China, Tel/Fax +86 010 6954 6690 ; +86 10 8950 9366, Email [email protected]; [email protected]
Purpose: Unsatisfactory efficacies of currently recommended anti-Mycobacterium abscessus complex (MABC) treatment regimens have led to development of novel drugs to combat MABC infections. In this study, we evaluated in vitro antimicrobial activities of bedaquiline (BDQ) and four oxazolidinones against MABC isolates.
Methods: The resazurin microplate assay was performed to determine minimum inhibitory concentrations (MICs) of BDQ and four oxazolidinones, including tedizolid (TZD), sutezolid (SZD), delpazolid (DZD), and linezolid (LZD), against 65 MABC isolates. A checkerboard method was used to investigate efficacies of various antimicrobial drug combinations.
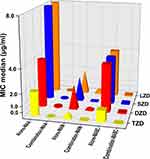
Results: BDQ MICs for MABC isolates ranged from < 0.031 to 1 μg/mL, while MIC50 and MIC90 values were 0.125 μg/mL and 0.25 μg/mL, respectively. TZD MIC50 and MIC90 values for MABC isolates were 1 μg/mL and 4 μg/mL, respectively, which were fourfold lower than corresponding LZD values (P < 0.001). DZD MIC90 values for MABC isolates was 8 μg/mL, which were 0.5-fold lower than corresponding LZD values (P < 0.01). MICs of BDQ, SZD, and LZD for M. abscessus subspecies massiliense isolates were significantly lower than corresponding MICs for M. abscessus subspecies abscessus isolates (P < 0.05). Notably, use of oxazolidinones (DZD, SZD, LZD, or TZD) with BDQ against MABC isolates led to reduction of the oxazolidinone median MIC range from 4 to 0.125 μg/mL to 1– 0.031 μg/mL.
Conclusion: These results demonstrated excellent BDQ inhibitory activity against MABC isolates. TZD exhibited stronger antimicrobial efficacy against MABC isolates as compared to efficacies of DZD, SZD, and LZD. Importantly, MICs of oxazolidinones were markedly decreased when they were combined with BDQ, thus suggesting that combinations of BDQ and oxazolidinones may be effective treatments for MABC infections.
Keywords: bedaquiline, tedizolid, oxazolidinones, susceptibility testing, Mycobacterium abscessus complex, delpazolid
Introduction
Increasing incidence of nontuberculous mycobacteria (NTM) infections has emerged as a major public health concern worldwide that has even eclipsed Mycobacterium tuberculosis (MTB) concerns in some countries.1,2 Despite the fact that NTM as a group is comprised of numerous species, human NTM diseases appear to be caused mainly by only two NTM species, Mycobacterium avium complex and Mycobacterium abscessus complex (MABC).3,4 MABCs are rapidly growing mycobacterial organisms that are frequently responsible for pulmonary infections, skin and soft tissue infections, and nosocomial infections.5 Three subspecies of MABCs are known: M. abscessus subsp. abscessus (MAA), M. abscessus subsp. massiliense (MAM), and M. abscessus subsp. bolletii.6 Owing to their impressive natural and acquired resistance to chemotherapeutic agents (especially to macrolides), MABC infections lead to high relapse rates and poor treatment outcomes for affected patients,7 thus highlighting an urgent need for new antibiotics to combat these infections.
Due to the lack of comprehensive clinical studies on therapeutic outcomes of patients with MABC infections, no standardized drug treatment regimens have yet been established for these patients. Nonetheless, current antibiotic regimens used for treatment of MABC infections rely on use of macrolides, amikacin, and various combinations of moxifloxacin, linezolid, or cefoxitin.8 However, the emergence of a combination of inducible and mutation-based macrolide resistance mechanisms has undoubtedly impaired the efficacies of macrolide-containing regimens. To address this growing concern, new antimicrobial agents with efficacy against MABC bacilli are urgently needed.
Oxazolidinones (OZLs), which represent a novel class of synthetic antimicrobial agents that exhibit promising bacteriostatic activity against many important human pathogens, act by inhibiting protein biosynthesis.9,10 The first member of a new generation of OZLs, linezolid (LZD), is known for its broad-spectrum in vitro antimicrobial activity against Gram-positive bacteria9 and remarkable antimicrobial activity against MABC that have inspired researchers to develop new OZLs.11 Recently, several novel OZLs have been identified, including sutezolid (SZD), delpazolid (DZD), and tedizolid (TZD), which have not yet been evaluated for in vitro activities against MABCs.
Meanwhile, bedaquiline (BDQ), a cornerstone multidrug-resistant tuberculosis treatment, has also been shown to be efficacious against multiple NTM species when used as a salvage treatment for pulmonary NTM infections.12,13 In fact, results of a recent study indicated that treatment of patients with disseminated MABC infections with BDQ-containing regimens achieved sustained mycobacterial culture negativity,14 highlighting the promise of BDQ as an effective treatment for NTM infection. In this study, we compared in vitro activities of four OZLs against MABC isolates and also confirmed in vitro anti-MABC synergistic effects of BDQ and OZLs.
Materials and Methods
Ethics Statement
This study was approved by the Ethics Committee of Beijing Chest Hospital, Capital Medical University (Approval No.: 2016KY005).
Bacterial Strains
A total of 65 MABC isolates obtained from clinical specimens were randomly selected from the Tuberculosis BioBank, which is maintained by the National Clinical Laboratory on Tuberculosis. The isolates were classified via multilocus sequence analysis as previously described15 and included 44 MAA and 21 MAM isolates.
The reference strain Mycobacterium abscessus ATCC 19977 was included as a control in all experiments. All bacterial specimens were stored at −80 °C and recovered on Löwenstein–Jensen (L-J) slants (Zhuhai, China) at 37 °C before performing drug susceptibility testing.
Antimicrobial Agents
BDQ, LZD, SZD, DZD, and tedizolid free-alkali were purchased from Shanghai Biochempartner Co., Ltd. (Shanghai, China). Antimicrobial compounds were all dissolved in dimethyl sulfoxide (DMSO) and stored at −40 °C.
Determination of Minimum Inhibitory Concentration (MIC)
MICs were determined for each antimicrobial compound according to Clinical and Laboratory Standards Institute (CLSI) guidelines16 using a broth microdilution method to generate serial dilutions of drugs in wells of 96-well microtiter plates in Mueller–Hinton broth. Individual MICs were determined for drugs within the following concentration ranges: 0.031–4 µg/mL for BDQ; 0.5–64 µg/mL for LZD, SZD, and DZD; and 0.125–16 µg/mL for TZD. MABC cultures were generated in L-J broth then bacterial cells were added to microtiter plate wells (105 CFU/well) for susceptibility testing. Following incubation at 37 °C for 72 h, 70 µL resazurin solution was added to each well then plates were incubated for another 24 h at 37 °C followed by reading and recording of assay results.
The MIC is defined as the lowest drug concentration to prevent a color change from blue to pink, while MIC50 and MIC90 values are defined as drug concentrations that inhibit growth of 50% and 90% of MABC isolates, respectively.
LZD breakpoints were applied as specified by CLSI guidelines (susceptible: ≤8 µg/mL; intermediate resistant: 16 µg/mL; resistant: ≥32 µg/mL).16 We performed a preliminary analysis of SZD, DZD, and TZD activities against MABCs based on reference LZD breakpoints, since no formally recognized breakpoints have been reported for SZD, DZD, or TZD.
Drug Interaction Analysis
The checkerboard method was used to evaluate the fractional inhibitory concentrations (FIC) of various combinations of BDQ and the four oxazolidinone agents. A total of 35 clinical MABC isolates, including 17 MAA and 18 MAM isolates, were randomly chosen for testing. FIC values were calculated using the following formula:
where “a” represents a single OZL agent and “b” represents BDQ. Synergy was defined as FIC ≤ 0.5, an additive effect was defined as 0.5 < FIC ≤ 1, a neutral effect was identified as 1 < FIC ≤ 4, and an antagonistic effect was defined as FIC > 4.17
Statistical Analysis
Median and interquartile ranges were applied to generate descriptive statistics. Each violin plot presents the distribution and probability density of the data. Data were compared by the chi-square test or Fisher’s exact test for categorical variables and the Mann–Whitney rank test for continuous variables. Statistical analysis was performed using SPSS v.26.0 (SPSS Inc., Chicago, IL). Differences were considered statistically significant for P < 0.05.
Results
BDQ Activity Against Mycobacterium abscessus
BDQ MICs for MABCs ranged from <0.031 to 1 µg/mL, with MIC50 and MIC90 values obtained of 0.125 µg/mL and 0.25 µg/mL, respectively. The BDQ MIC range for 44 MAA isolates was <0.031–1 µg/mL, with MIC50 and MIC90 values obtained of 0.125 µg/mL and 0.25 µg/mL, respectively. The BDQ MIC range for the 21 MAM isolates was 0.031–0.125 µg/mL, with MIC50 and MIC90 values obtained that were both 0.125 µg/mL (Table 1). The median BDQ MIC obtained for MAM isolates was 0.125 [0.063, 0.125] µg/mL as compared to the median BDQ MIC obtained for MAA isolates of 0.125 [0.063, 0.25] µg/mL. BDQ MIC distributions obtained for MAM isolates were significantly lower than those obtained for MAA isolates (P < 0.05) (Figure 1).
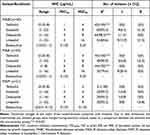 |
Table 1 In vitro Activity of Oxazolidinones and Bedaquiline Against Mycobacterium abscessus Clinical Isolates |
OZL Activities Against MABC Isolates
OZL MICs against the 65 MABC isolates, including 44 MAA isolates and 21 MAM isolates, are presented in Table 1 and Figure 2. TZD susceptibilities of MABC isolates were reflected by MIC50 and MIC90 values of 1 µg/mL and 4 µg/mL, respectively, which were generally fourfold lower than corresponding LZD values. Meanwhile, the median TZD MIC was 1 [0.5, 2] µg/mL versus the LZD MIC of 4 [2, 8] µg/mL (P < 0.001). MABC susceptibilities to DZD were reflected by MIC90 values of 8 µg/mL, respectively, that were generally 0.5-fold lower than corresponding LZD values (P < 0.01). No significant differences in TZD and DZD MIC distributions between MABC MAA and MAM subspecies were observed. MABC susceptibilities to SZD were similar to susceptibilities to DZD, as reflected by SZD MIC50 and MIC90 values of 4 µg/mL and 8 µg/mL, respectively, with lower MIC values obtained for MAM isolates than for MAA isolates.
Referring to the CLSI standard for LZD susceptibility breakpoints, MABC isolate TZD and DZD susceptibility rates were significantly greater than corresponding LZD susceptibility rates (100%: 84.6%, 98.5%: 84.6%, respectively; P<0.001). Overall, MAA isolates exhibited greater TZD and DZD susceptibility rates as compared to corresponding susceptibility rates of MAM isolates.
In vitro Interactions of BDQ with OZLs
A summary of in vitro interactions between BDQ and OZLs against MABC isolates is presented in Table 2 and Figure 3. Notably, a few drug combinations exhibited synergistic effects, which were most frequently noted for BDQ/DZD combinations, whereby a synergistic effect was observed for 10 (28.6%) of 35 BDQ/DZD-treated isolates as compared to only 2 (5.7%) of 35 isolates treated with BDQ/LZD (P < 0.05). For MAA isolates, a synergistic effect was noted in 7 (41.2%) of 17 isolates treated with BDQ/DZD, as compared to 0 (0%) of 17 isolates treated with BDQ/LZD (P < 0.05). Meanwhile, BDQ/TZD presented a similar synergistic effect as BDQ/LZD of MABC isolates (14.3%: 5.7%, P>0.05). By contrast, no synergistic antimicrobial effects on MABC isolates were observed for BDQ/SZD.
 |
Table 2 Number (%) of Isolates in vitro Interactions of Oxazolidinones Combined with Bedaquiline Against Mycobacterium abscessus |
As based on calculated FIC values, effects of combination BDQ/OZL treatments on MABCs were additive, with significant differences observed between MAA and MAM subspecies. For MAA isolates, median FIC values for DZD, LZD, TZD, and SZD were 0.508 [0.375, 0.516], 0.516 [0.508, 0.628], 0.508 [0.412, 0.524], and 0.531 [0.508, 0.550], respectively, as compared to respective values of 0.617 [0.524, 1.004], 0.742 [0.564, 0.995], 0.743 [0.549, 1.020], and 0.617 [0.550, 1.016] µg/mL that were obtained for MAM isolates. The average MAA FIC value for BDQ/OZL combinations was much lower than that obtained for MAM isolates (P < 0.01). Notably, treatment of MABCs with OZL in combination with BDQ significantly reduced the median MIC (P < 0.001) (Figure 4). For MABC isolates, when OZL treatments were combined with BDQ, median MIC range for DZD, SZD, LZD, and TZD decreased from 4 to 0.125 µg/mL and 1 to 0.031 µg/mL, respectively. For MAA isolates, the median MIC value decreased from 8 µg/mL to 0.063 µg/mL for SZD and LZD, decreased from 4 µg/mL to 0.125 µg/mL for DZD, and decreased from 2 µg/mL to 0.016 µg/mL for TZD when OZL treatments were combined with BDQ.
Discussion
Treatment of MABC infections is extremely challenging, due to intrinsic NTM antibiotic resistance. Thus, there is an urgent need for new, more effective antimicrobial agents. Within the context of drug development, reuse of existing agents has emerged as an attractive strategy to accelerate candidate drug selection and bring new hope to individuals infected with MABC. In this study, our results demonstrated that BDQ provided promising in vitro antimicrobial efficacy against MABC isolates, as consistent with results obtained for MABC isolates by Vesenbeckh and colleagues in Germany of BDQ MICs ranging from 0.12 µg/mL to 1.0 µg/mL.18 Similar results were obtained in a study by Brown-Elliott et al, which obtained MIC50 values of 0.06 µg/mL and 0.13 µg/mL for MAA and MAM, respectively, as compared with equivalent MIC50 values of 0.13 µg/mL obtained for both subspecies in our study.19 In addition, in a previous Chinese study of 218 clinical MABC isolates treated with BDQ, MIC50 and MIC90 values of 0.13 µg/mL and >16 µg/mL, respectively, were obtained, of which BDQ MICs for approximately one-fifth of MABC isolates were greater than 1.0 µg/mL.20 Although the precise explanation for extremely high MICs reported in the latter study is unclear, we speculate that misclassification of M. tuberculosis infections as NTM infections may have been responsible for observed extremely high MICs. Nevertheless, our results presented here indicate that BDQ is a promising therapeutic option for patients with MABC infections.
In addition, OZL derivatives exert antimicrobial activities against clinical MABC isolates. Although breakpoints for these new agents have not yet been established, our findings revealed that TZD exhibited greater antimicrobial efficacy than DZD, SZD, and LZD, as consistent with reported results obtained for multiple mycobacterial species, including M. tuberculosis, M. avium complex, and MABC.21,22 The potent activity of TZD against MABC isolates may be attributed to optimization of the A-ring C-5 substituent in TZD, which enhances the OZL interaction with the peptidyl transferase center binding site on the ribosome.23,24 Additionally, it is worth noting that prolonged use of LZD is associated with high rates of adverse events that have discouraged long-term use of this drug, while novel OZL derivatives such as TZD have been found to be safe and well tolerated in pre-clinical and clinical trials. Thus, the abovementioned results collectively indicate that TZD may be an effective antimicrobial treatment for MABC infections.
We also found that MAM isolates tended to be more susceptible than MAA isolates to BDQ, SZD, and LZD. Similarly, a recent study reported by Brown-Elliott and Wallace found that LZD MICs for MAA isolates were greater than MAM LZD MICs, as reflected by 2-fold-greater MIC50 values obtained for MAA isolates than for MAM isolates.21 Meanwhile, conflicting results obtained in other studies of in vitro susceptibilities of MABC subspecies to OZLs have shown that MAM isolates were more resistant than MAM isolates to TZD.25 It is possible that differences in results between studies may be due to results bias stemming from the limited number of MABC isolates in our study. However, diverse in vitro antimicrobial susceptibilities of predominant MABC isolates found in various regions may also explain these contradictory results. Given that NTM inhabits a variety of environmental niches, variable antibiotic exposure conditions over a long period of time may result in selection of mycobacteria with different drug resistance patterns, highlighting the urgent need to detect drug resistance early during treatment of MABC cases to ensure selection of effective drug regimens.
Another interesting finding of the present study was that we observed a dramatic reduction in OZL MICs that was mediated by concurrent BDQ treatment of all isolates tested, suggesting that BDQ synergizes with OZLs to exert antimicrobial effects against MABC isolates irrespective of subspecies. In line with our observation, BDQ plus clofazimine has been shown to exert a synergistic effect against MTB in log-phase and acid-phase metabolic states.26 Meanwhile, OZL binds to the 50S ribosomal subunit to inhibit initiation of bacterial protein synthesis as its main antimicrobial mechanism of action. Due to the well-known fact that protein synthesis is one of the most energy-consuming processes in the cell,27 we speculate that use of BDQ with TZD may enhance the bactericidal effect of TZD through targeting of ATP biosynthesis.
We acknowledge several obvious limitations of our study. First, no recommended breakpoints have yet been proposed for use in interpreting susceptibilities of MABC isolates to TZD, SZD, and DZD. Second, as demonstrated in previous studies, in vitro antibiotic efficacies are not always consistent with results obtained in in vivo experiments, warranting further investigations to validate our in vitro results. Nevertheless, results obtained in this study provide new insights to guide use of BDQ/OZL combinations for clinical management of MABC infections.
Conclusion
In conclusion, our data demonstrate that BDQ exhibited promising in vitro activity against MABC isolates. Additionally, TZD exhibited greater efficacy against MABC isolates as compared to efficacies of DZD, SZD, and LZD. Furthermore, MAM isolates were more susceptible than MAA isolates to BDQ, SZD, and LZD. Notably, a remarkable reduction in OZL MICs in all MABC isolates tested was mediated by BDQ exposure, thus suggesting that BDQ may synergize with OZLs to exert a stronger antimicrobial effect against MABC isolates irrespective of subspecies. Nevertheless, further research should be conducted using animal models to validate our results in vivo.
Acknowledgments
This study was supported by Research and Evaluation of the National Major Science and Technology Projects of China (2018ZX10722301) and the Beijing Hospitals Authority Clinical Medicine Development of Special Funding (ZYLX202122).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Lin C, Russell C, Soll B., et al. Increasing Prevalence of Nontuberculous Mycobacteria in Respiratory Specimens from US-Affiliated Pacific Island Jurisdictions. Emerg Infect Dis. 2018;24(3):485–491. doi:10.3201/eid2403.171301
2. Raats D, Brode SK, Mehrabi M, Marras TK. Increasing and More Commonly Refractory Mycobacterium avium Pulmonary Disease, Toronto, Ontario, Canada. Emerg Infect Dis. 2022;28(8):1589–1596. doi:10.3201/eid2808.220464
3. Jin P, Dai J, Guo Y, et al. Genomic Analysis of Mycobacterium abscessus Complex Isolates from Patients with Pulmonary Infection in China. Microbiol Spectr. 2022;10(4):e0011822. doi:10.1128/spectrum.00118-22
4. Santos A, Carneiro S, Silva A, Gomes JP, Macedo R. Nontuberculous Mycobacteria in Portugal: trends from the last decade. Pulmonology. 2022. doi:10.1016/j.pulmoe.2022.01.011
5. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An Official ATS/IDSA Statement: diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi:10.1164/rccm.200604-571ST
6. Yoshida M, Sano S, Chien JY, et al. A novel DNA chromatography method to discriminate Mycobacterium abscessus subspecies and macrolide susceptibility. EBioMed. 2021;64:103187. doi:10.1016/j.ebiom.2020.103187
7. Kasperbauer SH, De Groote MA. The Treatment of Rapidly Growing Mycobacterial Infections. Clin Chest Med. 2015;36(1):67–78. doi:10.1016/j.ccm.2014.10.004
8. Daley CL, Iaccarino JM, Lange C, et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: an Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Eur Respir J. 2020;56(1):2000535. doi:10.1183/13993003.00535-2020
9. Bozdogan B, Appelbaum PC. Oxazolidinones: activity, mode of action, and mechanism of resistance. Int J Antimicrob Agents. 2004;23(2):113–119. doi:10.1016/j.ijantimicag.2003.11.003
10. Diekema DJ, Jones RN. Oxazolidinones. Drugs. 2000;59(1):7–16. doi:10.2165/00003495-200059010-00002
11. Shaw KJ, Barbachyn MR. The oxazolidinones: past, present, and future. Ann N Y Acad Sci. 2011;1241:48–70. doi:10.1111/j.1749-6632.2011.06330.x
12. Ruth MM, Sangen JJN, Remmers K, et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother. 2019;74(4):935–943. doi:10.1093/jac/dky526
13. Chew KL, Octavia S, Go J, et al. In vitro susceptibility of Mycobacterium abscessus complex and feasibility of standardizing treatment regimens. J Antimicrob Chemother. 2021;76(4):973–978. doi:10.1093/jac/dkaa520
14. Gil E, Sweeney N, Barrett V, et al. Bedaquiline as Treatment for Disseminated Nontuberculous Mycobacteria Infection in 2 Patients Co-Infected with HIV. Emerg Infect Dis. 2021;27(3):944–948. doi:10.3201/eid2703.202359
15. Huang L, Li H, Ren W, et al. Highly Discriminative Genotyping of Mycobacterium abscessus Complex Using a Set of Variable Number Tandem Repeats in China. Front Microbiol. 2022;1:12. doi:10.3389/fmicb.2021.802133
16. Woods GL, Brown-Elliott BA, Conville PS, et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes.
17. Doern CD. When Does 2 Plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J Clin Microbiol. 2014;52(12):4124–4128. doi:10.1128/JCM.01121-14
18. Vesenbeckh S, Schönfeld N, Roth A, et al. Bedaquiline as a potential agent in the treatment of Mycobacterium abscessus infections. Eur Respir J. 2017;49(5):1700083. doi:10.1183/13993003.00083-2017
19. Brown-Elliott BA, Wallace RJ. In Vitro Susceptibility Testing of Bedaquiline against Mycobacterium abscessus Complex. Antimicrob Agents Chemother. 2019;63(2):e01919–18. doi:10.1128/AAC.01919-18
20. Pang Y, Zheng H, Tan Y, Song Y, Zhao Y. In Vitro Activity of Bedaquiline against Nontuberculous Mycobacteria in China. Antimicrob Agents Chemother. 2017;61(5):e02627–16. doi:10.1128/AAC.02627-16
21. Brown-Elliott BA, Wallace RJ. In Vitro Susceptibility Testing of Tedizolid against Nontuberculous Mycobacteria. J Clin Microbiol. 2017;55(6):1747–1754. doi:10.1128/JCM.00274-17
22. Kim TS, Choe JH, Kim YJ, et al. Activity of LCB01-0371, a Novel Oxazolidinone, against Mycobacterium abscessus. Antimicrob Agents Chemother. 2017;61(9):e02752–16. doi:10.1128/AAC.02752-16
23. Barbachyn MR, Ford CW. Oxazolidinone Structure–Activity Relationships Leading to Linezolid. Angew Chem Int Ed. 2003;42(18):2010–2023. doi:10.1002/anie.200200528
24. Locke JB, Finn J, Hilgers M, et al. Structure-Activity Relationships of Diverse Oxazolidinones for Linezolid-Resistant Staphylococcus aureus Strains Possessing the cfr Methyltransferase Gene or Ribosomal Mutations. Antimicrob Agents Chemother. 2010;54(12):5337–5343. doi:10.1128/AAC.00663-10
25. Wen S, Gao X, Zhao W, et al. Comparison of the in vitro activity of linezolid, tedizolid, sutezolid, and delpazolid against rapidly growing mycobacteria isolated in Beijing, China. Int J Infect Dis IJID off Publ Int Soc Infect Dis. 2021;109:253–260. doi:10.1016/j.ijid.2021.06.055
26. Kim S, Louie A, Drusano GL, et al. Evaluating the effect of clofazimine against Mycobacterium tuberculosis given alone or in combination with pretomanid, bedaquiline or linezolid. Int J Antimicrob Agents. 2022;59(2):106509. doi:10.1016/j.ijantimicag.2021.106509
27. Morita M, Gravel SP, Hulea L, et al. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle. 2015;14(4):473–480. doi:10.4161/15384101.2014.991572
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.