Back to Journals » Infection and Drug Resistance » Volume 16
Antifungal Susceptibility and Biological Characteristics of Fonsecaea monophora Causing Cerebral Phaeohyphomycosis in Jinhua, China
Authors Bi W, Xu L, Zong L, Zhu Z , Xia X
Received 14 August 2023
Accepted for publication 4 November 2023
Published 9 November 2023 Volume 2023:16 Pages 7187—7195
DOI https://doi.org/10.2147/IDR.S425961
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Wenzi Bi, Licheng Xu, Laibin Zong, Zhiqiang Zhu, Xiaoping Xia
The Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, Zhejiang, People’s Republic of China
Correspondence: Xiaoping Xia, Tel +86579-89979999, Email [email protected]
Background: The management of cerebral abscesses caused by dark-pigmented Fonsecaea monophora in healthy individuals continues to be challenging due to no consensus on the therapeutic regimen. Due to the absence of an accurate identification method, Fonsecaea species are often misidentified due to indistinct morphology features.
Materials and Methods: An F. monophora strain from an immunocompetent host with cerebral abscess was collected and identified by ITS rDNA molecular sequencing. The ITS sequences of the isolate were compared with that of the other ten Chinese F. monophora isolates obtained from GenBank for difference comparison and phylogenetic analysis. Fluorescence, Gram stains, and medan lactate were used to observe the colonial morphology. Antifungal susceptibility testing was implemented to demonstrate the antibiotic susceptibility profile. Galleria mellonella larvae were used as a model to study virulence of F. monophora. Medical records and clinical data of the patient were collected and analyzed.
Results: Antifungal susceptibility testing indicated that triazole antifungal drugs possess remarkable antifungal effect against F. monophora, and satisfactory antifungal effect of itraconazole was corresponding to the drug susceptibility results. Compared with the GM test, the serum G test was found to be more sensitive. The virulence and melanization in G. mellonella models for F. monophora were observed, and the death rates of infected larvae were positively related to injected concentrations of fungus. The phylogenetic tree was constructed from the ITS sequences of the clinical isolate along with ten Chinese F. monophora isolates, revealing that there is high relatedness in F. monophora strains collected from China.
Conclusion: F. monophora is an important neurotropic dematiaceous fungus and increasingly causing disease in immunocompetent individuals by means of noninvasive ways. Fungal culture, stainings, and molecular methods could be utilized to identify the etiologic agent. Triazole antifungal drugs can be applied as empiric therapeutic agents for phaeohyphomycosis.
Keywords: phaeohyphomycosis, Fonsecaea monophora, DNA sequence analysis, antifungal susceptibility testing
Introduction
Phaeohyphomycosis (PHM) is a group of superficial and deep mycoses, including central nervous system (CNS) infections, pulmonary infections, cutaneous and subcutaneous infections, associated with high mortality and caused by dematiaceous fungi that have brown-pigmented cell walls due to the formation of melanin.1,2 Previously, fungal brain infections generally occurred in immunocompromised patients and the pathogenic fungi were various. However, over half of the cerebral phaeohyphomycosis cases were reported in immunocompetent patients in a comprehensive review.3 Considering that dematiaceous fungi are ubiquitous in the environment and their ability to cause invasive infections in immunocompetent and immunocompromised hosts, the propensity of this condition needs emphasis and cannot be ignored.4
The discovered etiological agents of PHM are mainly the genera Fonsecaea, Phialophora, and Cladophialophora.5 The genus Fonsecaea comprises several related species, and new species of the genus Fonsecaea have been gradually described recently based on the internal transcribed spacer (ITS) region sequence analysis. Among them, Fonsecaea monophora was recently segregated from Fonsecaea pedrosoi, one of the major etiologic agents of chromoblastomycosis. F. monophora was found to cause infections in many organ systems, including the brain, gallbladder, and lymph node in the human host, and its predominantly neurotropic character has aroused wide public concern in recent years.6 Cerebral phaeohyphomycosis was documented mostly in tropical regions,7 but it has not been reported in otherwise healthy patients, in Zhejiang province, China.
Here, an F. monophora clinical isolate was collected from an immunocompetent host with cerebral abscess diagnosed by computerized tomography, and it was investigated by ITS rDNA molecular sequencing, antifungal susceptibility testing, morphologic observation, and virulence study. The corresponding antifungal treatment regimen was also analyzed. This study is aimed at providing a better understanding of the pathogen characteristics and practical experience related to this sophisticated mycosis in China to achieve early diagnosis and efficacious treatment.
Materials and Methods
Strains and Morphology
A 61-year-old retiree patient presented to our outpatient department in April 2022 due to an intermittent headache of unknown cause. The stereotactic puncture drainage of the brain abscess was performed on April 28, 2022, and the pus was drained during surgery and sent for etiological examination. In the microbiology laboratory, the cerebral abscess specimen taken during surgery was stained by Gram and fungal fluorescence, in addition, it was inoculated on potato dextrose agar (PDA) and candida color culture medium at different temperatures (28◦C and 35◦C) for 2 weeks. The fungal culture were stained with fluorescence, Gram, and medan lactate to observe the colonial morphology.
Molecular Identification and Phylogenetic Analysis
DNA was extracted from one-week-old colonies scraped from the growth on PDA media using the protocol DNA Purification from Yeast Using the Gentra Puregene Yeast/Bact. Kit (QIAGEN, Germany). The ITS 1–5.8S rDNA-ITS 2 region of rDNA was amplified by polymerase chain reaction (PCR) using primers ITS-1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS-4 (5’-TCCTCCGCTTATTGATATGC-3’) which were specific for internal transcribed spacer element, corresponding to related studies.8 PCR assays were performed using a C1000 Touch Thermal Cycler (Bio-Rad, USA) and conducted with the following conditions: 95◦C for 3 min, followed by 25 cycles of 95◦C for 30s, 50◦C for 30s, and 72◦C for 30s and a final extension of 72◦C for 5 min. Amplicons were sent to Beijing Ruiboxingke Biotechnology Co., Ltd. (Beijing, China) for direct sequencing. Nucleotide sequences were compared on the GenBank database using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
The ITS sequences of the isolate were determined and compared with those of other ten Chinese F. monophora isolates (F. monophora strain CGMHD 0311, F. monophora strain CGMHD 0145, F. monophora strain BMU07666, F. monophora strain CMCCf D6h, F. monophora strain SUMS0012, F. monophora isolate IS00391, F. monophora isolate BMU07699, F. monophora isolate HZ0221, F. monophora strain 1750 and a F. monophora without definition) using Blast Program.
In vitro Antimicrobial Susceptibility Testing
Minimum inhibitory concentrations (MICs) were determined by broth microdilution method, according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI) M38-A2 protocol with modifications.9 Amphotericin B (AMB), 5’flucytosine (5FC), fluconazole (FLC), itraconazole (ITC), posaconazole (POS), voriconazole (VRC), caspofungin (CAS), and micafungin (MFG) were tested. Candida parapsilosis ATCC22019 was used as a quality control.
Galleria mellonella Larvae as a Model to Study Virulence of F. monophora
Larvae weighing 250–300 mg were selected (20 per strain) and maintained at 16◦C before use. Larvae infections were implemented referred to the previous study with modulations.10 Larvae were grouped and infected with 20 μL saline solution or conidia inoculum with concentrations of 1 × 108, 1 × 106, 1 × 105, 1 × 104 per mL via the last left proleg, respectively. After injection, larvae were incubated at 37◦C and observed daily. Larvae were considered dead and removed away when they failed to respond to touch. Graphs of the survival rate of larvae were generated using GraphPad Prism 5 software. Survival data were analyzed by log-rank comparisons of Kaplan–Meier survival curves. Statistical significance (P value) was calculated by SPSS 17.0, and a P value is less than 0.05 was considered statistically significant.
Clinical Data Analysis
Medical records and clinical data (patient age, gender, comorbidities, occupation, immunologic status, underlying medical conditions, clinical features, physical examination, laboratory results, antifungal administration, outcome, and prognosis) of the patient were collected and analyzed.
Result
Laboratory Examination results and Clinical Data
The patient was a 61-year-old retiree weighing 63kg and presented to our outpatient department in April 2022 due to an intermittent headache of unknown origin. He was otherwise healthy and without any other clinical signs of infection. Physical examinations were normal, in addition, meningeal irritation signs, neck stiffness, Kernig signs, and Brudzinski signs were all not detected.
The results of blood routine examination were as follows: haemoglobin 16.3 g/dl, leucocyte count 4.8×109/l (neutrophils 3.2×109/l, lymphocytes 1.3×109/l, monocyte 0.3×109/l, eosinophils 0.01×109/l, basophilic granulocyte, 0.01×109/l), C-reactive protein 1.7 mg/l. Biochemical parameters and troponin I (0.001ng/mL) are normal. The patient was tested negative for human immunodeficiency virus (HIV). Routine examination and biochemical test of cerebrospinal fluid were also normal, except for the increased cerebrospinal fluid protein (1060.79mg/l). The serum G test result was 117 μg/L which indicated the presence of fungal infection. The serum GM test result was normal (0.09 pg/mL).
Magnetic resonance imaging (MRI) revealed a space-occupying lesion which was highly suspected of a brain abscess in the right temporal lobe. In the stereotactic puncture drainage of the brain abscess, the pus was drained and sent for bacteriological examination.
The patient was commenced on intravenous liposomal amphotericin B 50mg/kg/day qd and oral itraconazole 200 mg/kg/day qd. Liver functions and renal functions were deviant after the five-day antifungal treatment. Considering the possibility of side effects of amphotericin B, itraconazole was selected for subsequent single-drug treatment.
Strain Identification Results
Attempts to identify the fungus were failed by the MALDI-TOF-MS system. Strain identity was verified by sequencing the ribosomal internal transcribed spacer (ITS), and the PCR product was found to be similar to the sequences of many F. monophora strains in the GenBank database (https://www.ncbi.nlm.nih.gov/). The sequence showed 99% homology with F. monophora strain 35,641 in GenBank (Sequence ID: MH382070.1) and was confirmed as F. monophora.
Morphology
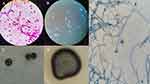
The cerebral abscess specimen was stained by Gram and fungal Fluorescence separately. Hypha and thick-walled spores could be found by microscopic observation (Figure 1A and B).
After culturing the cerebral abscess specimen for 7 days at 35°C, the evident colonies grew at a slow speed on the PDA media (Figure 1C). The colonies grew at a slow speed and grew better at 35°C than at 28°C. Velvety fungal spores, black hypha, typical morphology with conidiogenesis process, and dry colonies with friable texture were noted after 14 days (Figure 1D). Hyphae were dense at the dark-brown periphery of the colony, and convex central protrusions were grayish-white. The fungus had an olive colored septate hyphae with branches and multiple elliptic conidia were arranged at the apex of the conidiophores by microscopic observation after lactophenol cotton blue staining of culture (Figure 1E).
Antifungal Susceptibility Testing
Table 1 demonstrates the antibiotic susceptibility profile of the clinical strain. This strain had high MIC values in response to many antifungal drugs. The results indicated that VRC, POS, and ITC had the strongest anti-F. monophora effect. MFG and FLC had poor antifungal effects, and fungal growth was still observed at high concentrations.
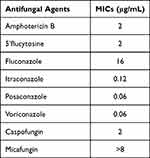 |
Table 1 Fungal Susceptibility |
Pathogenicity and Virulence of F. monophora in G. mellonella Larvae
Before injecting, healthy G. mellonella larvae were typically creamy (Figure 2A). Four groups of larva were injected with 1 × 104, 1 × 105, 1 × 106, and 1 × 108 per mL melanized strain conidia separately, and were incubated at 37°C. The survival condition of the infected larvae was monitored daily.
Melanization, comprising the synthesis and deposition of melanin at the infection position, showed up as black change on the cream-colored larvae (Figure 2B). Black larvae were completely melanized and died soon (Figure 2C). All larvae which were injected with 1 × 108 per mL melanized strain conidia, were all killed within 24 hours. The melanized strain showed lower virulence to G. mellonella at the inoculation of 1 × 104 conidia per insect. The death rates of infected larvae were positively related to injected concentrations of F. monophora (Figure 3). Distinct death rates of infected larvae were observed between groups with different injected concentrations of fungus (P<0.001), and the death rates of infected larvae were positively correlated with injected concentrations of fungus. However, no significant difference was found between the infection with 1 × 104 conidia per insect and saline solution (P > 0.05).
 |
Figure 3 Survival curves of G. mellonella infected with 20 μL saline solution or conidia inoculum with different concentrations of 1 × 108, 1 × 106, 1 × 105, 1×104 per mL. |
Sequence Analysis and Phylogenetic Analysis
The ITS regions of the F. monophora clinical isolate were amplified to produce approximately 600 bp fragments, and sequenced. The ITS sequences were compared with those of other ten Chinese F. monophora isolates obtained from GenBank for difference comparison and phylogenetic analysis (Figure 4). ITS tree revealed the phylogenetic relationship among the genus Fonsecaea, obtained by neighbor-joining analysis using Mega 7.0.8 The numbers given on the branches are the frequencies with which a given branch appeared in 1000 bootstrap replications. F. monophora strain 1750 and F. monophora isolate BMU07699 were used as outgroups (Figure 5).
 |
Figure 4 Comparison of the internal transcribed spacer (ITS) sequence of the F. monophora clinical isolate with those of the other ten Chinese F. monophora isolates. |
 |
Figure 5 Neighbor-Joining tree was constructed based on the internal transcribed spacer (ITS) sequences of the genus Fonsecaea isolated from China. |
Discussion
Since 2004, F. monophora was segregated from F. monophora by De Hoog et al, more F. monophora were isolated and identified by rDNA ITS sequencing. There is a significant difference in the pathogenicity of the two, with F. monophora being predominantly neurotropic in the human host. Cindy Fransisca et al re-evaluated twenty isolates recovered from chromoblastomycosis patients which were originally identified as F. pedrosoi, and 17 strains were verified to be F. monophora by rDNA ITS sequence.11 Finally, they found twenty strains were F. monophora, only four strains corresponded to F. pedrosoi.
The most frequent manifestation of cerebral phaeohyphomycosis is solitary brain abscess which could cause severe headache.12 Such serious fungal infections are generally associated with severe immunocompromised status. Otitis media and trauma resulting in the implantation of pathogenic fungus into the skin may be the cause of the disease.13 Considering that dematiaceous fungi which are the main pathogens for phaeohyphomycosis distribute in the environment, especially in the soil, several studies connected phaeohyphomycosis with the patients’ occupation. However, in our study, the patient was retired and did not engage in related agriculture. In addition, he had neither tympanitis nor a history of trauma, and was immunocompetent. Cerebral phaeohyphomycosis was mostly documented in tropical regions and immunocompromised hosts. However, in this study, we found that F. monophora could cause severe cerebral abscesses in immunocompetent individuals by means of noninvasive ways. The study provided extremely significant insights to realize the infected groups and infected way of F. monophora.
Inflammatory indicators of serum in this study were within normal ranges. The G test result was higher than the upper limit of the normal range, in contrast, the serum GM test result was normal. The possible reasons could be that substrates for the detection of GM test and G test were different, and there were differences in metabolic regulation and influencing factors between the substrates. Hence, the combined detection of two tests could improve the diagnosis rate of invasive fungal disease, and the serum G test was found to be more sensitive for the diagnosis of phaeohyphomycosis.
Early and rapid diagnosis are crucial for the effective treatment of invasive fungal diseases. The direct results of etiological diagnosis provide a basis for empirical and early treatment. MRI only revealed a space-occupying lesion in the right temporal lobe, nevertheless, gram staining and fungal fluorescence staining of the cerebral abscess specimen provided evidence for fungal infection immediately. Given the fact that pathogen cultures have a significant turnaround time which often delays the diagnosis, the development of molecular diagnostics for more rapid identification is necessary and promising. The results indicated that voriconazole, posaconazole and itraconazole had remarkable anti-F. monophora effect. In agreement with previous literature,14–16 the satisfactory antifungal effect of itraconazole was corresponding to the drug susceptibility results in this study. Taking many factors into consideration, including remarkable antifungal effects and relatively low side effects, triazole antifungal drugs can be applied as empiric therapeutic drugs for phaeohyphomycosis.17
Although the G. mellonella infection model is still in its infancy, G. mellonella has become increasingly applied as a substitute host to study infectious diseases and the virulence of the pathogen in recent years.18,19 In this study, it has been shown that the virulence and melanization in G. mellonella models for F. monophora, and the death rates of infected larvae were positively related with injected concentrations of fungus. Experimental conditions and operations often differ between individual research labs and need standardized operating procedures to reduce the differences. We constructed the phylogenetic tree from the ITS sequences of the clinical isolate along with ten Chinese F. monophora isolates, revealing that there is high relatedness in F. monophora strains collected from several regions in China.
In conclusion, our study presents one confirmed F. monophora-caused cerebral abscess case. F. monophora is an important neurotropic dematiaceous fungus and increasingly causing disease in immunocompetent individuals by means of noninvasive ways. Fungal culture, stainings, and molecular methods were utilized to identify the etiologic agent. The study is crucial on account of the increased incidence of cerebral infections by F. monophora and triazole antifungal drugs can be applied as empiric therapeutic drugs for phaeohyphomycosis.
Ethics Approval and Consent for Publication
This study has been reviewed and approved by the Research Ethics Committee of the Fourth Affiliated Hospital of Zhejiang University School of Medicine (1018-2-J1). The consent form was signed by the patient before surgery and the patient consented to publish the clinical details. In addition, written informed consent was obtained.
Funding
No funding was granted.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Arcobello JT, Revankar SG. Phaeohyphomycosis. Semin Respir Crit Care Med. 2020;41(1):131–140. doi:10.1055/s-0039-3400957
2. Pouran L, Naser TM, Mohammad R, et al. Disseminated phaeohyphomycosis caused by Cyphellophora ludoviensis: a novel case report from Iran. Australas J Dermatol. 2020;61(3):250–252. doi:10.1111/ajd.13262
3. Revankar SG, Sutton DA, Rinaldi MG. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin Infect Dis. 2004;38(2):206–216. doi:10.1086/380635
4. Takei H, Goodman JC, Powell SZ. Cerebral phaeohyphomycosis caused by ladophialophora bantiana and Fonsecaea monophora: report of three cases. Clin Neuropathol. 2007;26(1):21–27. doi:10.5414/npp26021
5. Qiu Y, Zhang J, Tang Y, et al. Case report: fever- pneumonia- lymphadenectasis- osteolytic- subcutaneous nodule: disseminated chromoblastomycosis caused by phialophora. J Infect Chemother. 2019;25(12):1031–1036. doi:10.1016/j.jiac.2019.05.002
6. He Y, Zheng HL, Mei H, et al. Phaeohyphomycosis in China. Front Cell Infect Microbiol. 2022;12:895329. doi:10.3389/fcimb
7. Surash S, Tyagi A, De Hoog GS, et al. Cerebral phaeohyphomycosis caused by Fonsecaea monophora. Med Mycol. 2005;43(5):465–472. doi:10.1080/13693780500220373
8. Lim SW, Suh MK, Kang GS, et al. Molecular phylogenetics of Fonsecaea strains isolated from chromoblastomycosis patients in South Korea. Mycoses. 2011;54(5):e415–e420. doi:10.1111/j.1439-0507.2010.01937
9. Coelho RA, Brito-Santos F, Figueiredo-Carvalho MHG, et al. Molecular identification and antifungal susceptibility profiles of clinical strains of Fonsecaea spp. isolated from patients with chromoblastomycosis in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2018;12(7):e0006675. doi:10.1371/journal.pntd.0006675
10. Liu Y, Huang X, Liu H, et al. Increased virulence of albino mutant of Fonsecaea monophora in Galleria mellonella. Med Mycol. 2019;57(8):1018–1023. doi:10.1093/mmy/myz007
11. Fransisca C, He Y, Chen Z, et al. Molecular identification of chromoblastomycosis clinical isolates in Guangdong. Med Mycol. 2017;55(8):851–858. doi:10.1093/mmy/myw140
12. Ochiai H, Kawano H, Minato S, et al. Cerebral phaeohyphomycosis: case report. Neuropathology. 2012;32(2):202–206. doi:10.1111/j.1440-1789.2011.01244.x
13. Litchevski V, Goldschmidt A, Nass D, et al. Cerebral phaeohyphomycosis in an immunocompetent patient: a case report and literature summary. Clin Neurol Neurosurg. 2014;124:179–181. doi:10.1016/j.clineuro
14. Najafzadeh MJ, Badali H, Illnait-Zaragozi MT, et al. In vitro activities of eight antifungal drugs against 55 clinical isolates of Fonsecaea spp. Antimicrob Agents Chemother. 2010;54(4):1636–1638. doi:10.1128/AAC.01655-09
15. Shen Y, Jiang B, Zhang H, et al. Combination therapy for an elderly patient with chromoblastomycosis caused by Fonsecaea monophora: a case report. Ann Transl Med. 2022;10(2):114. doi:10.21037/atm-21-6119
16. Daboit TC, Magagnin CM, Heidrich D, et al. A case of relapsed chromoblastomycosis due to Fonsecaea monophora: antifungal susceptibility and phylogenetic analysis. Mycopathologia. 2013;176(1–2):139–144. doi:10.1007/s11046-013-9660-1
17. Al-Abdely HM, Alkhunaizi AM, Al-Tawfiq JA, et al. Successful therapy of cerebral phaeohyphomycosis due to Ramichloridium mackenziei with the new triazole posaconazole. Med Mycol. 2005;43(1):91–95. doi:10.1080/13693780400011104
18. Tsai CJ, Loh JM, Proft T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence. 2016;7(3):214–229. doi:10.1080/21505594.2015.1135289
19. Kaito C. Understanding of bacterial virulence using the silkworm infection model. Drug Discov Ther. 2016;10(1):30–33. doi:10.5582/ddt.2016.01020
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.