Back to Journals » Cancer Management and Research » Volume 13
Antiemetic Efficacy of Adding Olanzapine 5 mg to Aprepitant, Palonosetron and Dexamethasone-Sparing After Day Two for Cancer Patients Receiving Anthracycline and Cyclophosphamide
Authors Suehiro M , Kojima Y , Takahashi M, Ito Y, Keira T , Ikegawa K, Minatogawa H, Tsugawa K, Tanaka T
Received 18 September 2020
Accepted for publication 15 December 2020
Published 17 February 2021 Volume 2021:13 Pages 1617—1624
DOI https://doi.org/10.2147/CMAR.S280995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Seema Singh
Marii Suehiro,1,* Yasuyuki Kojima,2,* Masaki Takahashi,3,* Yuka Ito,4 Takayuki Keira,1 Kiwako Ikegawa,1 Hiroko Minatogawa,1 Koichiro Tsugawa,2 Tsuneaki Tanaka1
1Department of Pharmacy, St. Marianna University School of Medicine Hospital, Kawasaki-shi, Kanagawa, 216-8511, Japan; 2Division of Breast and Endocrine Surgery, Department of Surgery, St. Marianna University School of Medicine Hospital, Kawasaki-shi, Kanagawa, 216-8511, Japan; 3Division of Medical Informatics, St. Marianna University School of Medicine Hospital, Kawasaki-shi, Kanagawa, 216-8511, Japan; 4Department of Pharmacy, Kawasaki Municipal Tama Hospital, Kawasak-shi, Kanagawa, 214-8525, Japan
*These authors contributed equally to this work
Correspondence: Marii Suehiro
Department of Pharmacy, St. Marianna University School of Medicine Hospital, 2-16-1 Sugao, Miyamae-Ku, Kawasaki-shi, Kanagawa, 216-8511, Japan
Tel +81-44-977-8111
Fax +81-44-975-0608
Email [email protected]
Purpose: Chemotherapy-induced nausea and vomiting (CINV) decrease patient quality of life (QOL). We evaluated the efficacy of adding 5 mg Olz to a three-drug steroid-sparing antiemetic regimen (aprepitant, palonosetron, and dexamethasone-sparing after day two) for breast cancer (BC) patients receiving anthracycline plus cyclophosphamide (AC) chemotherapy.
Patients and Methods: We retrospectively reviewed the records of 177 BC patients with no previous highly emetogenic chemotherapy history receiving AC plus the steroid-sparing three-drug regimen or the steroid-sparing four-drug regimen including Olz 5mg at our hospital between January 2012 and December 2018. The primary endpoint was complete response (CR), defined as no vomiting and no usage of rescue medication during the first AC cycle. We analyzed the odds ratio (OR) of the CR with 95% confidence interval (CI) in the three-drug group against the four-drug group. The OR was adjusted for types of anticancer drugs by the Cochran–Mantel–Haenszel (CMH) test. Secondary endpoints were incidences of nausea, anorexia, fatigue, and somnolence during the first cycle.
Results: Compared to the three-drug group, the four-drug group demonstrated high incidence of no vomiting (71% vs 95%), a similar incidence of no rescue medication usage (50% vs 51%), and a similar CR rate (45% vs 49%). The OR of the CR rate in the three-drug group against the four-drug group after CMH adjustment for drug type was 0.958 (95% CI, 0.46– 1.98). Compared to the three-drug group, the four-drug group demonstrated identical incidence of nausea (66%), but lower incidences of anorexia (78% vs 35%) and fatigue (86% vs 73%). The incidence of somnolence in the four-drug group was 49%. We did not have data of somnolence for the three-drug group in the records.
Conclusion: Adding 5 mg Olz to the steroid-sparing three-drug combination can reduce vomiting, anorexia, and fatigue, although there was no difference in CR rate.
Keywords: breast cancer, highly emetogenic chemotherapy, chemotherapy induced nausea and vomiting, antiemetics
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer among women and the leading cause of cancer-related death among women globally.1 Anthracycline combined with cyclophosphamide (AC) is the standard regimen for early and advanced BC.2–4 Chemotherapy-induced nausea and vomiting (CINV) decreases quality of life (QOL) and can negatively influence BC treatment outcome.5 AC is classified as a highly emetogenic chemotherapy (HEC) by several international guidelines.6–8 The recommended antiemetic treatment for CINV in patients receiving AC is a three- or four-drug combination of the neurokinin-1 receptor antagonist (NK1-RA), the serotonin 5-HT3 receptor antagonist (5-HT3-RA), and dexamethasone (Dex) without or with olanzapine (Olz).6–8
Olz is an antipsychotic that acts on multiple receptors, including dopamine receptors (D1, D2, and D3), serotonin receptors (5-HT2A, 5-HT2C, and 5-HT3), alpha1 adrenergic receptors, muscarinic cholinergic receptors, and histamine type 1 receptors (H1).9,10 Antagonism of D2, 5-HT2C, and 5-HT3 receptors in particular may contribute to the antiemetic effect of Olz. Therefore, Olz has been the subject of several clinical trials investigating its efficacy as an antiemetic drug. A recent Phase III trial reported that Olz (10 mg) in a four-drug combination was superior to a standard three-drug combination for suppression of nausea and vomiting induced by HEC.11 In that study, however, the dose of Olz was 10 mg and patients receiving Olz experienced significantly more severe sedation on day two compared to those receiving placebo. Another recent Phase II dose-finding study reported that for patients receiving cisplatin, complete response (CR; no emesis and no use of rescue medications) in the delayed phase (24–120 h after the start of cisplatin treatment) was achieved in 78% (80% CI: 70.3–83.8, P = 0.01) of the 10 mg Olz arm and 86% (80% CI: 79.2–90.7, P < 0.001) of the 5 mg Olz arm (P value for H0: complete response rate ≤ 65%). In addition, the Olz 5 mg arm showed a lower incidence of somnolence than the 10 mg arm. Further, the four-regimen including 5 mg Olz was more tolerable, especially in patients of Japanese ethnicity.12 As a result of these studies, Olz 5 mg was approved as an antiemetic drug by the Ministry of Health, Labour and Welfare of Japan with public knowledge based-application in December 2017.
A recent phase III trial also found that antiemetic Dex administration on days two and three can be spared when combined with NK1-RA and palonosetron in HEC (the DEX-1 study).13 Dex has multiple adverse effects, such as insomnia, indigestion/epigastric discomfort, agitation, increased appetite, weight gain, and diminished bone density.14,15 Moreover, dose-dense epirubicin and cyclophosphamide, one of the AC regimens used to treat early-stage BC, increases the risk of pneumocystis pneumonia (PCP),16 and PCP is thought to be associated with steroid use.17 Steroid-sparing may therefore be useful for decreasing Dex-induced adverse events (AEs) in clinical practice. However, the therapeutic benefits of adding Olz (5 mg) to a standard three-drug steroid-sparing regimen (aprepitant, palonosetron, and Dex-sparing after day two) has not been established for AC-induced nausea and vomiting.
We hypothesized that the antiemetic efficacy of adding Olz 5mg to the standard three-drug combination would be superior to the standard three-drug combination in Japanese BC patients receiving AC.
Patients and Methods
This study was conducted in accordance with Declaration of Helsinki, and the institutional review board of St. Marianna University School of Medicine approved the present study (approval number 4881). The objectives and methods of the study as well as the handling of personal information were provided on the website of our hospital. The patients were explained that they could freely withdraw from the study at any time upon contacting us regarding the same. The ethics committee of St. Marianna University School of Medicine Hospital permitted to perform retrospective study without consent statements (opt-out method).
The eligibility criteria for this study were as follows: age ≥20 years and receiving the first cycle of AC treatment, Eastern Cooperative Oncology Group performance status (ECOG-PS) 0–2, absolute neutrophil count ≥1500 cells/mm3, aspartate aminotransferase and alanine aminotransferase ≤100 IU/L, blood bilirubin ≤2.0 mg/dL, and creatinine ≤1.5 mg/dL. We extracted the following baseline clinical information for analysis: age at the time of treatment, sex, body surface area (BSA), body mass index (BMI), inpatient or outpatient treatment setting, ECOG-PS, cancer stage, type of AC regimen and dose, antiemetic use, CINV symptoms, use of rescue medication, and bone marrow, liver, and kidney function.
Exclusion criteria were as follows: nausea or vomiting before the first AC cycle, use of other drugs that may prevent CINV such as antipsychotics, antidepressants, corticosteroids, or dopamine receptor antagonists, comorbidities that induced nausea and/or vomiting prior to the initiation of chemotherapy (symptomatic gastrointestinal disease, accumulation of ascitic fluid or pleural effusion, and brain metastases), administration of non-standard antiemetic treatment, pregnancy, uncontrolled diabetes mellitus (DM) during the study period (the use of Olz is contraindicated for patients with past or current DM history or HbA1c ≥ 6.5%), and records lacking AE data. Patients were excluded from data analysis if BC was recurrent, in stage IV. We considered that the physical or mental condition of patients with stage IV disease may affect the incidence of AEs.
Continuous variables are expressed as median [range] and categorical variables as frequency or proportion.
We reviewed the electronic medical records (EMRs) of our hospital between January 2012 and December 2018. Patient records were de-identified and analyzed anonymously.
In this study, the AC regimen included epirubicin (90 mg/m2) and cyclophosphamide (600 mg/m2) tri-weekly (EC), epirubicin (90 mg/m2) and cyclophosphamide (600 mg/m2) biweekly with pegfilgrastim support (dose-dense EC), and the FEC regimen consisted of epirubicin (100 mg/m2), cyclophosphamide (500 mg/m2), and 5-fluorouracil (500 mg/m2) triweekly.
In January 2018, we switched our standard antiemetic management for BC patients receiving the AC regimen from a three-drug combination to a four-drug combination following approval of Olz as an antiemetic drug by the Ministry of Health, Labour and Welfare of Japan. Previously (the three-drug combination), oral aprepitant (125 mg) was administered 60 minutes prior to chemotherapy, and intravenous palonosetron (0.75 mg) and Dex (12 mg for EC and FEC, 10 mg for dose-dense EC) were administered 15 minutes prior to chemotherapy for patients on day one, followed by oral aprepitant (80 mg) on day two and three. At present (the four-drug combination), all patients receive oral Olz (5 mg) before dinner on days one to four as a fourth drug (the approved usage of Olz in Japan). For rescue medication, oral prochlorperazine (5 mg) and alprazolam (0.4 mg) were prescribed for all patients.
During chemotherapy, major AEs including CINV symptoms were assessed and recorded routinely in medical charts by pharmacists and nurses at the chemotherapy center rather than by physicians at the outpatient clinic. On the day one of the second cycle, the pharmacists interviewed and recorded their AEs during the first cycle in the EMRs. We performed this study based on the data in the EMRs. Compliance of oral medications was checked by hospital pharmacists.
The primary endpoint of this study was CR rate to CINV management during the first cycle (21 days for EC and FEC, and 14 days for dd EC), where CR was defined as no vomiting and no usage of rescue medication for CINV. We analyzed CR rates according to age, BMI, and the type of chemotherapeutic regimen. Dose-dense EC is usually administered for relatively younger patients, and younger age is a risk factor for CINV. Therefore, we also analyzed CR rate by age and the type of chemotherapeutic regimen.
The secondary endpoints of this study were the incidences of nausea, anorexia, and fatigue during the first cycle. The incidence of somnolence was also evaluated in the four-drug group. All AEs were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Statistical Analysis
Initially, we planned to compare the CR rate between the three-drug group and the four-drug group by propensity score matching where “age”, “BSA”, “stage”, and “types of anticancer drugs” were included as matching covariates based on clinical findings. When we assessed the group difference in these covariates, however, only “types of anticancer drug” differed. Therefore, we used stratified analysis instead of the propensity score method. The group difference in CR during the first cycle is expressed as the odds ratio (OR) with 95% confidence interval (CI). The odds ratio was adjusted for types of anticancer drugs by the Cochran–Mantel–Haenszel (CMH) test. AEs are expressed as proportion of patients. A P < 0.05 (two-tailed) is considered statistically significant for all tests. All statistical analyses were performed using R version 3.5 (https://cran.r-project.org).
Results
Patient Characteristics
Figure 1 presents the patient recruitment and follow-up flow diagram of this study. From January 2012 to December 2018, a total of 206 potentially eligible patients were identified. Twenty-five patients were excluded and additional four patients were withdrawn from the analysis. Finally, a total of 177 patients were analyzed in this study.
 |
Figure 1 Patient recruitment and follow-up flow diagram. |
Patient characteristics are summarized in Table 1. There was no difference in median age between the three-drug and four-drug group (50 years [27–73 years] vs 49 years [27–74]). All patients were treated as outpatients. The proportion receiving the FEC regimen was lower in the four-drug group (50.5% vs 7.3%), while the proportion receiving the dose-dense EC regimen was lower in the three-drug group (0% vs 39%). Alternatively, use of the EC regimen did not differ between the two groups (49.5% vs 53.7%).
 |
Table 1 Patient Characteristics |
Primary Endpoints
The CR rate for the first cycle did not differ between the three-drug and four-drug groups (45.3% vs 48.8%; CMH-adjusted OR= 0.958, 95% CI: 0.46 to 1.98, p > 0.99). The incidence of no vomiting was higher in the four-drug group (70.5% vs 95.1%) while the incidence of no rescue medicine did not differ (49.5% vs 50.5%). We also analyzed CR rates according to age, BMI, and the type of chemotherapeutic regimen (Table 2). Among patients >60 years of age, CR rate was slightly higher in the four-drug group (60.9% vs 65.0%), but did not differ between groups among patients younger than 60 years. However, the CR rate of patients receiving dose-dense EC, which was not included in the three-drug group, was markedly lower than that of EC. Nonetheless, the CR rate was still higher in the four-drug group when comparing only patients receiving the EC regimen (46.8% vs 56.8%).
 |
Table 2 Complete Response Rate According to Age, Type of Regimen, and Body Mass Index |
Adverse Events
The incidences of all grades of nausea, anorexia, fatigue, and somnolence are shown in Table 3. There was no difference in nausea incidence between three- and four-drug groups (66.3% vs 65.9%). On the other hand, anorexia was less frequent in the four-drug group (77.9% vs 35.4%). The incidence of fatigue was higher in the three-drug group (86.3% vs.73.2%). The incidence of somnolence was 48.8% in the four-drug group and one of the excluded patients discontinued Olz due to grade 2 somnolence. Data on somnolence were not included in the EMRs for the three-drug group. Except for somnolence, there were no grade 3 or higher AEs in either group.
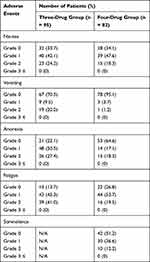 |
Table 3 Incidences of the Adverse Events |
Discussion
In this retrospective observational study, we confirm the antiemetic efficacy of the steroid-sparing four-drug combination consisting of Olz 5 mg, aprepitant, palonosetron, and Dex (sparing after day two) in Japanese BC patients receiving AC. While the CR rate was not superior to the conventional three-drug regimen, the no vomiting rate was markedly higher and anorexia rate lower in the four-drug group, suggesting reduced steroid-induced side effects and potentially enhanced QOL.
The CR rate has been used as an indicator of antiemetic efficacy in many clinical studies.12,13,18–20 A previous study by Yeo and colleagues reported a CR (defined as no vomiting and no use of rescue therapy) rate of 65.0% during the 120 h after starting the AC regimen among patients receiving the four-drug combination (10 mg of Olz and Dex sparing after day two).18 The rates of no vomiting and no use of rescue therapy were higher in the four-drug group than in the three-drug group (68.3% vs 40.0% and 91.7% vs 76.7%, respectively). Moreover, the rate of no nausea rate (defined as visual analogue scale < 5mm) was also higher in the four-drug group than in the three-drug group (58.3% vs 33.3%).18
On the other hand, the incidence of no vomiting was higher in the four-drug group than in the three-drug group, while the incidence of no rescue medicine did not differ in our study. The reason for the lack of a difference in rescue medicine usage is that 5 mg of Olz reduced vomiting but may not have reduced nausea.
There are several differences between the study by Yeo and our study including the assessments for nausea, the timing of Olz administration, the dose of Olz, the study setting and the type of 5-HT3-RA.
Several prospective clinical trials related to Olz have used a visual analog scale (VAS) to assess nausea, and the definition of “no nausea” in many clinical trials can vary (ie, VAS < 5 mm or VAS = 0 mm).11,18,19 On the other hand, in our study, all AEs were assessed according to the CTCAE, version 4.0. For example, VAS=5 mm would be grade ≥1 in the CTCAE and would not result in a determination of no nausea (since our study defined grade 0 as “no nausea”). The differences in assessment methods may be one reason why the difference in nausea could not be expressed.
However, Yeo et al reported that the presence of CTCAE grade 0–1 nausea was also higher in the four-drug group than in the three-drug group (98.3% vs 85.0%).18 The differences in the timing of administering Olz may affect the rate of nausea suppression. Olz was administered before chemotherapy in Yeo’s study and after in our study. The median time to the first episode of vomiting after the initiation of chemotherapy was 26.5 h in the standard (APR + Ondansetron + Dex) arm in Yeo’s study.18 Nausea might have been uncontrolled if Olz was administered after chemotherapy because the time for Olz to reach its maximum plasma concentration is 4.5 h.21
Another possible reason for our results is the dose of Olz. The dose of Olz was 10 mg in Yeo’s study and 5 mg in our study. Suthinee et al assessed the efficacy of APR or 10 or 5 mg of Olz plus ondansetron and Dex for CINV prophylaxis in patients receiving HEC (AC or cisplatin-based regimen).19 The rate of no nausea (defined as a VAS score of “0”) was 43% of the patients receiving 10 mg of Olz, 37% in those receiving 5 mg of Olz and 33% of those in the APR group.19 In the AC regimen, the dose of 10 mg of Olz may be needed for the control of nausea.
In our study, the distribution of treatment regimens differed between the two groups, with the proportion receiving FEC markedly higher in the three-drug group and dose-dense EC exclusive to the four-drug group. The effect of the specific anticancer drugs may not be adjusted using “chemotherapeutic regimen” as the covariate in stratified analysis, so we used “types of anticancer drugs” instead as the covariate in stratified analysis.
Based on the results of the NSABP B-36 trial, FEC has been excluded as an option for adjuvant therapy,1 and has not been used as a perioperative therapy in recent years. Therefore, we could not compare the CR rate between the three-drug and four-drug group patients receiving FEC. We also could not compare the CR rate of patients receiving dose-dense EC between groups, although the CR rate was markedly lower among four-drug group patients receiving dose-dense EC compared to those receiving EC. The patients treated with dose-dense EC were administrated Dex 10 mg on day 1 due to the risk of PCP. According to a meta-analysis by Lemos Duarte and colleagues, dose-dense therapy can improve the disease-free survival of early BC patients with little impact on safety,22 and use of dose-dense EC by our hospital began in 2018. In the GIM2 study, incidence of grade 1/2 vomiting was numerically higher in the dose-dense EC group than EC or FEC groups.23 A lower initial Dex dose and more frequent usage of rescue medication for vomiting, even for low-grade vomiting, may account for the equivalent CR rates between groups in our study. Future studies examining the effect of adding Olz for patients receiving dose-dense EC are warranted.
One of the most common AEs of Olz is somnolence, but an increase in somnolence could not be assessed as there were no data for the three-drug group.
A retrospective study reported the antiemetic efficacy and safety of a non-steroid-sparing four-drug combination (5 mg of Olz and Dex from days 1 to 4).20 The incidence of all grades of somnolence in the four-drug group in our study was higher than that in a previous study (Grade 1: 34.3% vs 22%, Grade 2: 12.9% vs 0%).20 Also, in a study of Dex for prophylaxis against delayed emesis, cancer patients reported moderate to severe insomnia (45%);14 therefore, steroid-sparing may also enhance somnolence. In our study, only one patient self-interrupted due to grade 2 somnolence, while in most cases Olz was well tolerated.
Our study has the following limitations. 1) This is a single-center, retrospective observational study so applicability to the broader BC population is uncertain. In general, the assessment period of the study endpoint in most CINV trials is 120 h or 168 h after the start of chemotherapy. However, we set the study period to be during the first cycle. This retrospective study was based on data obtained in daily clinical practice, and the time of appearance of nausea was not clearly stated in all patients. The problem in this study period is that there is a possibility of accumulating nausea and vomiting such as anxiety-related nausea. Besides, we did not include nausea as a part of the primary endpoint, and we also could not assess the patients’ QOL. 2) We could not assess other possible risk factors for CINV, such as alcohol habits, as many such factors were not available in the records. 3) The AC regimen and Dex dose differed between the two groups, which could affect antiemetic treatment outcome. 4) The difference in approved palonosetron dose between Japan (0.75 mg) and other countries (0.25 mg) also limits applicability to other populations. In Japan, Saito et al conducted a phase III study comparing palonosetron 0.75 mg and granisetron 0.04 mg/kg for HEC.24 Based on the results of that study, 0.75 mg is the approved palonosetron dose in Japan rather than 0.25 mg recommended in international guidelines.6–8 5) We could not evaluate AEs other than fatigue and somnolence, such as hyperglycemia or PCP.
Both Olz and corticosteroids have hyperglycemic AEs, so steroid-sparing could reduce the risk of hyperglycemia. However, when used as a neoadjuvant or adjuvant treatment, the AC regimen is usually administrated for four cycles, so hyperglycemia may not be a problem as many BC patients are relatively young and have no comorbidity.
In contrast, PCP is a serious AE, especially in response to dose-dense therapy, and corticosteroids are thought to increase PCP risk.16,17 In a previous report, patients who developed PCP received a median corticosteroid dose of 16.4 mg/day prednisolone (PSL) equivalents [14.3–27.3 mg/day],17 while in our steroid-sparing regimen, Dex is reduced from 12.4 to 4.8 mg PSL equivalent/day. A reduction in PCP risk during the dose-dense EC regimen would provide further support for the clinical utility of the Dex-sparing four-drug antiemetic therapy.
Conclusion
Although there was no statistically significant difference in CR rate between the three-drug and four-drug antiemetic treatment groups, adding 5 mg Olz to the standard three-drug combination with steroid-sparing (Dex on day one only) reduced the incidence of vomiting, anorexia, and fatigue among BC patients.
Acknowledgments
The authors would like to thank Enago for the English language review.
Disclosure
M.S. reports grants from Shionogi, during the conduct of the study. Y.K. reports personal fees from Chugai pharmaceutical, personal fees from Eisai, Novartis pharma, Taiho pharma, Daiichi Sankyo, Pfizer, Eli Lilly, AstraZeneca, and Kyowa-Kirin, outside the submitted work. K.T. reports grants and personal fees from AstraZeneca, Chugai pharmaceutical co. Ltd, Eisai, Taiho pharmaceutical co. Ltd, Takeda pharmaceutical co. Ltd, Nippon Kayaku co. Ltd; grants from MSD K.K.; personal fees from Eli Lilly Japan K.K., Daiichi Sankyo co. Ltd, and Pfizer, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer. Version 1; 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
2. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785.
3. Peto R, Davies C, et al.; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444.
4. Fossati R, Confalonieri C, Torri V, et al. Cytotoxic and hormonal treatment for metastatic breast cancer: a systematic review of published randomized trials involving 31,510 women. J Clin Oncol. 1998;16(10):3439–3460.
5. Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24(27):4472–4478.
6. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Antiemesis. Version 1. 2019; 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.
7. Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(28):3240–3261.
8. Multinational Association of Supportive Care in Cancer. MASCC/ESMO antiemetic guideline 2016 with updates in 2019; 2020. Available from: https://www.mascc.org/assets/Guidelines-Tools/mascc_antiemetic_guidelines_english_v.1.5SEPT29.2019.pdf.
9. Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14(2):87–96.
10. Bymaster FP, et al. Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and α1-adrenergic receptors in vitro. Schizophr Res. 1999;37(1):107–122. doi:10.1016/S0920-9964(98)00146-7
11. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375(2):134–142. doi:10.1056/NEJMoa1515725
12. Yanai T, Iwasa S, Hashimoto H, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol. 2018;23(2):382–388.
13. Ito Y, Tsuda T, Minatogawa H, et al. Placebo-controlled, double-blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin-1 receptor antagonist and palonosetron in high emetogenic chemotherapy. J Clin Oncol. 2018;36(10):1000–1006.
14. Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. 2006;94(7):1011–1015.
15. Nakamura M, Ishiguro A, Muranaka T, et al. A Prospective observational study on effect of short-term periodic steroid premedication on bone metabolism in gastrointestinal cancer. Oncologist. 2017;22(5):592–600.
16. Waks AG, Tolaney SM, Galar A, et al. Pneumocystis jiroveci pneumonia (PCP) in patients receiving neoadjuvant and adjuvant anthracycline-based chemotherapy for breast cancer: incidence and risk factors. Breast Cancer Res Treat. 2015;154(2):359–367.
17. Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illnesses and prior corticosteroid therapy. Mayo Clin Proc. 1996;71(1):5–13.
18. Yeo W, Lai TK, Li L, et al. A randomized study of olanzapine-containing versus standard antiemetic regimens for the prevention of chemotherapy-induced nausea and vomiting in Chinese breast cancer patients. Breast. 2020;50:30–38.
19. Ithimakin S, Theeratrakul P, Laocharoenkiat A, et al. Randomized, double-blind, placebo-controlled study of aprepitant versus two dosages of olanzapine with ondansetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving high-emetogenic chemotherapy. Support Care Cancer. 2020;28(11):5335–5342.
20. Kawazoe H, Uozumi R, Murakami A, et al. Olanzapine plus aprepitant, palonosetron, and dexamethasone for nausea and vomiting in patients with breast cancer receiving anthracycline: a retrospective study. Sci Rep. 2018;8(1):16232.
21. Amamoto T, Irie S, Kumamoto M, et al. Effect of food on LU170053 (Olanzapine) bioavailability and the bioequivalence of different formulations. Rinshouiyaku. 1998;14(15):2717–2735.
22. Lemos Duarte I, Da Silveira N, Lima JP, et al. Dose-dense chemotherapy versus conventional chemotherapy for early breast cancer: a systematic review with meta-analysis. Breast. 2012;21(3):343–349.
23. Mastro LD, Placido SD, Bruzi P, et al. Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised Phase 3 trial. Lancet. 2015;385(9980):1863–1872.
24. Saito M, Aogi K, Sekine I et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol.2009;10(2):115–124.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
