Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Antidiabetic Effects of Physical Activity: How It Helps to Control Type 2 Diabetes
Authors Wake AD
Received 11 May 2020
Accepted for publication 1 July 2020
Published 19 August 2020 Volume 2020:13 Pages 2909—2923
DOI https://doi.org/10.2147/DMSO.S262289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Addisu Dabi Wake
Nursing Department, College of Health Sciences, Arsi University, Assela, Oromia, Ethiopia
Correspondence: Addisu Dabi Wake
Nursing Department, College of Health Sciences, Arsi University, P.O. Box: 393/04, Asella, Ethiopia
Tel +251 910 2867 66
Email [email protected]
Abstract: Despite the improvements in clinical care of the patients, research updates, and public health interventions, there is still an increase in the prevalence, incidence, and mortality because of diabetes mellitus (DM). DM is a public health problem in both developed and developing countries. It has increased alarmingly, putting this disease in the dimension of an epidemic. Diabetes is associated with several complications which increase the risk of many serious health problems on the other side. Therefore, this review was aimed to discuss the antidiabetic effects of physical activity (PA) on type 2 DM (T2DM) by summarizing the significant studies on this topic. This review found that several studies have recommended the utilization of PA for the effective management of T2DM. PA is a non-pharmacologic therapy which is a significant strategy for the management of T2DM and is an appropriate lifestyle modification approach to be practiced by these patients. The studies showed that PA has antidiabetic effects which are evidenced by its substantial role in improving the blood glucose (BG) levels of the individuals with T2DM where it helps them to control their levels of glucose in the blood. It plays a significant role in glycemic control of this disease by lowering the BG levels through possible mechanisms such as decreasing insulin resistance, increasing production of glucose transporter type 4 (GLUT-4), lowering visceral adipose tissue (VAT), increasing pancreatic β-cell functions, using glucose for energy, and so on. In turn, the controlled glycemia helps to prevent the complications associated with uncontrolled T2DM and this would further improve the overall health of the patients and the burden on the health professionals as well. Finally, this review concludes that PA is the cornerstone in the management of T2DM. It also suggests that more attention is needed to its significance in the prevention, glycemic control, and its role in the management of the morbidity and mortality associated with T2DM. Practical PA recommendations and suggestions for the future direction of research in this area are also provided.
Keywords: diabetes mellitus, physical activity, exercise, glycemic control, type 2 diabetes
Background
Diabetes is defined as a group of chronic diseases characterized by hyperglycemia.1 It is a chronic, progressive, partly understood metabolic condition mainly characterized by hyperglycemia.2 DM is a serious, chronic disease that happens either when the pancreas does not produce enough insulin or when the body cannot effectively use its produced insulin.3 Diabetes can be classified as type 1 DM (T1DM) (due to autoimmune b-cell destruction), T2DM (due to a progressive loss of β-cell insulin secretion frequently on the background of insulin resistance), gestational DM (GDM) (diabetes diagnosed in the second or third trimester of pregnancy), or specific types of diabetes because of other causes (monogenic diabetes syndromes, diseases of the exocrine pancreas, and drug- or chemical-induced diabetes).4
The socioeconomic status is a significant factor contributing to a rise of non-communicable diseases (NCD). This is because low socioeconomic groups consumeless fruit, vegetables, fish, and fibre than those of high socioeconomic status, whereas a high socioeconomic groups were found to be less physically active and consume more fats, salt, and processed food than individuals of a low socioeconomic status.5 T2DM has developed as a major chronic NCD in several countries.6 T2DM is a serious and common chronic disease resulting from a complex interaction of inheritance and environment besides other risk factors like obesity and sedentary lifestyle.7 It is the most frequent metabolic disorders, but thecauses remain mostly uncertain. Insulin resistance, the common underlying abnormality, is the outcome of an imbalance between energy intake and expenditure favoring nutrient-storage pathways, which evolved to maximize energy use and preserve an acceptable substrate supply to the brain.8 But the commonest reasons contributing to the pathophysiology of T2DM are impaired insulin secretion, resistance to tissue actions of insulin, or a combination of both.2 The adipokines, resistin may be raised in the plasma of the subjects with T2DM while this is expected to contribute to the impaired glucose tolerance and insulin resistance seen in T2DM.9 Besides, mitochondria dysfunction and reduced expression of GLUT-4 were recognized as the major factors of insulin resistance in this disease.10 And also, mitochondrial dysfunction is a consistent phenotype related with insulin resistance in multiple tissues.11
Diabetes has several associated complications, broadly divided into macrovascular complications and microvascular complications. The macrovascular complications include stroke, coronary artery disease, and peripheral arterial disease. Whereas the microvascular complications comprise retinopathy, diabetic nephropathy, and neuropathy.1 The DM leads to around a 2-fold higher risk of vascular diseases, independently from other conventional risk factors which are non-linearly linked for nondiabetic individuals.12 T2DM individuals also suffer from problems like dysregulation of an excess cardiovascular and metabolic functions, comprising dysglycemia, dyslipidemia, arterial hypertension, obesity, and a decreased cardiorespiratory fitness.13
Diabetes is growing to an alarming epidemic level.14 It is a pandemic of main public health significance that is unclear.15 Diabetes is a vital public health problem, which is one of the four prioritized NCD targeted for action by world leaders. Both the number of cases and the prevalence of diabetes have been progressively rising over the past few decades.3 The prevalence of diabetes has augmented in recent decades.16 Globally, in 2014, it was found that about 422 million adults were living with DM, compared to 108 million in 1980. The global prevalence of diabetes has almost doubled since 1980, where it was increased from 4.7% to 8.5% in the adult population.3 And also, the estimated prevalence of diabetes in adults was 4.0% in 1995, and this is expected to increase to 5.4% by the year 2025. The figure of adults with diabetes in the world will rise from 135 million in 1995 to 300 million in the year 2025 globally.17 The evidence showed that the global prevalence of diabetes for all age groups was predicted to be 2.8% in 2000, and 4.4% in 2030. While the projected figure of patients with diabetes is to increase from 171 million in 2000 to 366 million in 2030.18
Further, in 2015, the proportion of diabetes in adults aged 20–79 years was predicted to be 8.8%, and this was predicted to rise to 10.4% in 2040.19 And also, diabetes is on the rise globally, with a global proportion in adults being 8.8% of the world population in 2017, with the expectation of a further rise to 9.9% by 2045. This reflects that, in 2017, there were a population of 424.9 million patients with diabetes globally, with a predicted 48% rise to 628.6 million patients by 2045.20 T2DM is a major contributor to a very huge increase in the rate of NCD affecting developed as well as developing countries.2 The burden of diabetes-related complications and comorbidities are significant in Sub-Saharan Africans showing the urgent need for innovative public health approaches that prioritize the promotion of healthy lifestyles for prevention and early detection of T2DM.21
Overall, a significant rise in the prevalence of diabetes is proved in almost all regions of the world in recent decades. The rise in the figure of individuals with diabetes or with a longer duration of diabetes is probable to change the disease profile in several populations around the world, mainly because of a greater incidence of diabetes-related complications like kidney failure and peripheral arterial disease.22 Amongst the diseases, diabetes is one of the leading and increasing causes of hospital admission and disability.23 Elder patients with diabetes have been found to have a greater all-cause mortality rate than the general population.24 Disability from T2DM is increased globally and across the levels of development. In 2017, diabetes emerged as the fourth leading cause of disability worldwide.25 The prevalence of individuals with T2DM is rising in developed and developing countries, but developing countries in particular.26 T2DM and its related complications create a major global public health problem, affecting almost all populations in both economically developed and economically developing countries with high rates of diabetes-related morbidity and mortality.7 However, recent evidence has shown that the present understanding of the international burden and variation in diabetes associated complications is poor globally.22 Moreover, the high proportion of diabetes in adults also has significant social, financial, and development implications. There is a progressively urgent need for governments to implement policies to reduce the risk factors for T2DM and GDM and confirm a suitable access to management for all individuals living with diabetes.19 Diabetes is a chronic illness that needs ongoing pharmacological care and patient self-management to avoid acute and chronic complications. Diabetes care is complex, and needs several issues to be addressed, it is beyond just glycemic control.27
Despite the significant funds in research, clinical care, and public health interventions, there seems to be no sign of a decrease in the incidence, prevalence, and mortality because of T2DM.28 Physical inactivity has a major health consequence globally. Reducing or eliminating this unhealthy behavior could improve health significantly.29 To accomplish a good metabolic control in diabetes, keeping a long-term integration of lifestyle modifications and pharmacological management is essential.1,30 Several components of self-management involvements lead to clinically related progressin the behavior and clinical parameters.31 Those people on self-management involvement can be considered as willing volunteers in the majority of cases where they have either required an intervention or decided to take part.32 Self-management training is found to be effective in T2DM, especially in the short-term period,33 and the significance of exercise in the prevention and management of T2DM is more evident.6 Therefore, an exercise should be encouraged from diagnosis, as the individuals may be more agreeable to lifestyle modification. In order to improve an individual’s confidence in managing their diabetes with exercise, standard advice on exercise, and diabetes needs to be made available to health professionals and patients with diabetes.34
Physical Activity
“PA” has been used interchangeably with the term “exercise”.35 However, the term “PA” should not be mistaken with “exercise” because an exercise is a subcategory of PA.36 It is recommended that PA and exercise should not be used interchangeably.37 Regarding the definition, PA is any bodily movement formed by the skeletal muscles that results in energy expenditure above resting levels. The term is broadly including exercise, sport, and PA done as part of daily living, occupation, leisure, and active transport. Whereas, an exercise is defined as a planned, structured PA typically performed with the intent of improving the health and/or fitness.36,38
Exercise can be classified as mechanical and metabolic properties. Mechanical includes dynamic exercise, which causes movement of the limb and static exercise which results in no movement of the limb. However, the metabolic classification includes aerobic and anaerobic processes.39 Exercise could also be classified as aerobic and resistance exercise.40 The aerobic exercise comprises repeated and continuous movement of huge muscle groups,41 and it includes activities such as walking, cycling, jogging, and swimming, while resistance exercise includes activities like free weights, weight machines, body weight, or elastic resistance bands.40
Generally, PA is safe for almost everyone,41 and its key role in the prevention and management of NCD has had widespread recognition.42 It is broadly recognized as an effective strategy for the prevention and management of many chronic diseases.43 Moderate exercise following Roux-en-Y gastric bypass surgery was found to provide additional improvements in insulin sensitivity, glucose effectiveness, and cardiorespiratory fitness for obese individuals.44 The effects of exercise and leisure time PA have been extended from prevention to management of several components of metabolic syndrome and also for the mood and quality-of-life.45 Exercise training is found to improve body composition, cardiovascular, and metabolic outcomes in people with metabolic syndrome.46 As far as an exercise offers several health benefits,47 the adoption and maintenance of PA are essential for management of BG and total health in people with diabetes and prediabetes.48 Both aerobic and resistance exercise have beneficial effects for T2DM patients.49 Overall, evidence shows that all types of exercise are helpful for individuals with T1DM and T2DM.50 And also, people of all ages can benefit from doing PA where it may reduce all-causes of morbidities, and raise their lifespan through improving their quality-of-life.51,52
The Role of Physical Activity in the Management of T2DM
The primary prevention of T2DM could be accomplished through a non-pharmacologic intervention like PA which can be applied in a primary healthcare setting.53 The exercise improves BG levels in both diabetic and nondiabetic subjects.54 The use of PA breaks during sitting moderately reduces post-prandial glucose, insulin, and triacylglycerol, with a better glycaemic reduction in individuals with a high body mass index (BMI).55 The integration of dietary and PA is significant in diabetes improvement which may encourage to prevent or delay diabetes complications.56,57 And also, the combined intervention of supervised structured exercise training and caloric restriction are effective in improving metabolic health and decreasing an excess weight in obese patients with T2DM.58 The PA could result in an improved health status of T2DM patients and also decrease a burden of health professionals.59 Besides, rising a PA may also decrease the risk of progression from GDM to T2DM.60 Participating in PA prior to and during early pregnancy could prevent the development of GDM.61–63 This is because exercise helps to improve the glycemic levels of women with GDM,64–66 which could have the potential to protect the progression into T2DM. The PA may be used as a therapeutic tool in a variety of individuals who are with, or at risk for diabetes for its protecting effect against diabetes independently.67–69
PA plays a major role in the prevention and control of insulin resistance, prediabetes, GDM, T2DM, and diabetes related health complications.70 Exercise is used for the prevention,71 and treatment of T2DM72–78 by improving glycemic control.43,79–86 It is a comprehensive element of diabetes management.87,88 A randomized trial has reported that exercise performed for 30 minutes after meal eating may provide better improvements in glycemic control for people with T2DM.86 In these patients, 12 weeks of exercise training significantly improved oxygen uptake dynamics.89 And also, PA improves metabolic parameters in patients with pre-diabetes or T2DM while a low PA is related to an increased risk of incident T2DM.90 Even a single session of exercise can promote beneficial effects in T2DM individuals concerning blood pressure (BP) control, glycemia, carbohydrate oxidation during exercise, and fat oxidation after exercise.91 Generally, the varying types, intensities, and durations of exercise decreased BG levels in most people, while an exercise of longer duration is possibly most effective.92 Below is the summary of the effects of specific types of exercise such as the aerobic, resistance, and combined (aerobic plus resistant) exercise where each provides several health benefits for individuals with T2DM as they are proved by several studies.
Regarding aerobic exercise, the study found that aerobic exercise improves glycemic control,93–104 insulin sensitivity,105–107 insulin action,70 body composition,99 quality-of-life,108 physical capacity,99 nerve function,109 functional capacity,110 and cardiorespiratory fitness,101,111 reduces insulin resistance,97,112,113 insulin levels,96 lipid profile,97 BP,97,100 cardiovascular risk,106 hemoglobin A1c (HbA1c) levels,95,111 and waist circumstances,100 and modulates inflammatory cytokine levels113 and adipocytokines113 in T2DM patients.
The study also showed that resistance exercise improves glycemic control,97,104,114–130 insulin sensitivity,105,106,114,115,117,129 insulin responsiveness,116 insulin action,70 physical functions,131 and cardiopulmonary fitness,132 decreases insulin resistance,97 abdominal fat,114 BMI,132 insulin levels,118,132,133 triacylglycerol levels,118 lipid profile,97,105 body fat,132 HbA1c levels,119,132,133 BP,97 and cardiovascular risk,106 and increases GLUT-4 translocation in skeletal muscle,117 and strength119,120,128,131 in T2DM patients. Further, during resistance training, raised energy expenditure and excess post-exercise oxygen consumption in response to resistance training are also considered as other beneficial effects117 in these patients.
Concerning combined exercise, the study found that combined exercise also improves glycemic control,6,97,134–139 insulin sensitivity,139,140 body composition,137 functional capacity,137,138 strength,138 and vascular function,141 and reduces blood lipids,97,134,137 HbA1c levels,136,142,143 BP,97 and insulin resistance97,144 in T2DM patients. In fact, a systematic review and meta-analysis showed that combined exercise might be the most efficacious exercise modality to improve glycemic control and blood lipids in T2DM patients.134 A randomized trial also found that either the aerobic or resistance training alone improves glycemic control in T2DM, but the improvements are highest if they are combined.135 Similarly, the aerobic and resistant modality seems to have the ability to improve HbA1c values in T2DM patients, but the combined exercise form appears superior.142 AminiLari et al144 found that 12 weeks of combined exercise was more efficient in improving homeostasis model assessment of insulin resistance (HOMA-IR) and increasing serum omentin-1 among women with T2DM compared to aerobic and resistance exercises.
There are several trials evaluating the effect of PA/exercise in the management of T2DM. According to those findings, PA/exercise has a significant role in improvements of BG control, insulin sensitivity, and insulin resistance, reduction of BMI, HbA1c levels, and total cholesterol, etc.72,100,111,132,133,145–152 This section summarizes (Table 1) the evidence about the role of PA/exercise on the control of T2DM.
 |
Table 1 The Summary of Main Systematic Reviews with Meta-Analysis Covering the Role of Physical Activity/Exercise on T2DM Control |
The Physiological Effects of Physical Activity in T2DM
The mechanism of exercise in the improvement of T2DM is very complex and not completely known.75 During an exercise, there is a complex biological response because almost all organs and systems are involved in the interactions that lead to adaptations at the levels of genetic, metabolic, and neuromuscular.106 There are a few ways that PA could decrease BG levels.153 The beneath are a brief summary of PA which shows the proposed mechanisms through which it can help to lowers the BG levels. For instance, by decreasing insulin resistance, increasing production of GLUT-4, lowering VAT, increasing pancreatic β-cell functions, using glucose for energy, and so on (Figure 1).
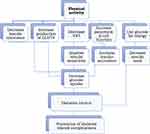 |
Figure 1 A short summary of the physiological effects of physical activity on type 2 diabetes. |
As explained above, evidence has shown that PA improves insulin sensitivity in T2DM patients.9,13,75,79,84,105–107,114,115,117,129,139,140,153–161 The increased insulin sensitivity makes the muscle cells better able to use any available insulin to take up glucose during, and after exercise. In fact, whenever muscles cells contract during exercise, the muscle cells are able to take up glucose and use it for energy, whether insulin is available or not.153 During PA, the production of GLUT-4 will be increased and this enhances the improvements of insulin sensitivity. The improved insulin sensitivity in turn increases glucose uptake and finally improves glycemic control.159 This improvement in insulin sensitivity contributes to improving glycemic control into the normal range.115,156 Bird and Hawley161 found that regular PA decreases the risk of insulin resistance and also improves the insulin sensitivity when people adhere to an exercise accordingly. However, the study showed that there is a dose–response relationship between exercise dose and the improvements in insulin sensitivity.157 A relatively modest single session of exercise in obese adults was even found to improve insulin sensitivity.158 In addition to this, even a single bout of brisk walking for 1 hour improves muscle insulin sensitivity.160
Further, evidence has shown that PA leads to an improvement in insulin action.70,85,162–164 This improvement in insulin action involves an enhanced sensitivity, and responsiveness of peripheral glucose uptake probably by muscle to insulin and also an increased inhibition of hepatic glucose production by insulin.163 Besides, the improved insulin action involves glucose transport, glycogen synthesis, and glycogen synthase activation as well as amino acid transport.164 An exercise also rises the rate of glucose uptake into the contracting skeletal muscles where this effect of exercise is identical to the action of insulin on glucose uptake. The mechanism through which both stimuli increase the skeletal muscle glucose uptake involves the translocation of GLUT-4 to the plasma membrane and transverse tubules.165 A major proposed mechanism involved in raising insulin stimulation of glucose uptake after exercise appears to be due to the exercise-related reduction in muscle glycogen content.164 Also, the improvement in mitochondrial muscle performance, and rises in muscle mass during resistance training may positively affect insulin responsiveness and glucose control.116
Furthermore, the studies showed that PA improves insulin resistance.10,72,73,97,112,113,144,166–169 The evidence presented that an accumulation of lipid in skeletal muscle is assumed to be associated to the development of insulin resistance and T2DM. Exercise was found to decrease accumulation of lipids in muscle which in turn reduces the resistance.105 The insulin resistance in individuals with T2DM can also be reduced via increasing the plasma levels of orexin-A and reducing in plasma levels of glucose and insulin by exercise.112 The finding showed that exercise training improves insulin resistance and decreases diacylglycerol and ceramides.167 A short-term aerobic exercise leads to reduced arterial stiffness in both common carotid and femoral arteries, while this decrease of stiffness was related with improvements of insulin resistance in T2DM patients.168 In a randomized trial, PA was found to reduce weight and insulin resistance in men.169 Also, regular exercise modulates several intracellular pathways improving insulin resistance and glucose uptake in the skeletal muscle of T2DM individuals.10
Moreover, several studies have shown that PA reduces VAT.35,140,154,158,170–176 Physical training reduces the VAT, which then leads to improvements in insulin sensitivity.170 Exercise also leads to a loss of abdominal subcutaneous and VAT and increased muscle density, while insulin sensitivity is improved related to these changes.140 Campos et al171 found that the magnitude of reduction in visceral fat is an independent predictor for insulin resistance. There were positive correlations between the visceral fat decrease and glucose metabolism. The systematic review and meta-analysis showed that a regular aerobic exercise decreases VAT and may also decrease the liver fat in adults with overweight/obesity and T2DM.173 The randomized trial showed that resistance training and aerobic training are equally effective in decreasing hepatic fat content among these patients.174 A systematic review and meta- analysis showed that aerobic training of moderate or high intensity has the greatest potential to decrease VAT in overweight males and females.172 Besides the evidence found that exercise decreases obesity and high-density lipoprotein concentration in the plasma by encouraging a loss of weight.35 Also, a reduction in systemic fatty acid uptake was associated with performing an exercise.158
Likewise, PA also improves pancreatic β-cell function in T2DM individuals.175–177 Two weeks of high-intensity interval or moderate intensity continuous training improved β-cell function in people with prediabetes or T2DM.175 Similarly, functional high intensity training was found to improve pancreatic β-cell function in adults with T2DM.177 The randomized trial demonstrated that 2 weeks of exercise training improves β-cell function.176 The baseline and training-induced changes in β-cell function may be a key determinant of training-induced improvements in glycemic control. An exercise is explained primarily by improving insulin secretion.178 Since the ectopic fat accumulation in the pancreas is a risk factor for T2DM, the study suggested that exercise training may help to decrease this ectopic fat, and in turn is used as an effective strategy to lower the risk of developing T2DM.175
Generally, an exercise improves the whole-body metabolic health in patients with T2DM. The adaptations to the skeletal muscles are crucial for this improvement, while an acute bout of exercise rises skeletal muscle glucose uptake.78 A resistance exercise reduces the requirements for insulin in overweight women with GDM.179 There is improvement in microvascular vasodilator, insulin signaling, and attenuates capillary rarefaction in skeletal muscle among T2DM patients performing an exercise. This exercise-induced modification then improves glucose and insulin delivery and also the uptake of glucose.180 The meta-analysis has reported that there were improvements in endothelial function in patients with T2DM performing aerobic or combined exercise.181 During exercise, the consumption of oxygen may rise by as much as 20-fold, and even higher rises may happen in the working muscles. To meet its energy needs during this condition, the skeletal muscle uses its own stores of glycogen and triglycerides, and also free fatty acids derived from the breakdown of adipose tissue triglycerides and glucose released from the liver at a higher increased rate.156
Several studies have shown that PA also lowers the HbA1c levels.79,95,111,119,132,133,136,142,143,146–151,182–184 This means that the effect of an exercise on the decrement of BG levels could be evidenced by the reduction of the biomarkers of glycemic control which is HbA1c levels. The term HbA1c refers to a glycated hemoglobin or glycosylated hemoglobin. It develops when hemoglobin, a protein within red blood cells that carries oxygen throughout the body, is combined with glucose in the blood. By measuring HbA1c, the health professionals are able to get an overall picture of what the average BG levels of the diabetic patients have been over a period of weeks/months. It is a measure of glucose control that is a result of glucose molecules attaching to hemoglobin for the life of the red blood cells which is 120 days. The amount of glucose that combines with this protein is directly proportional to the total amount of glucose that is in the system at that time. If the BG levels have been high in recent weeks, the HbA1c will also be higher. Umpierre et al,184 however, found that the decrement of HbA1c in T2DM patients during exercise is determined by the volume of an exercise.
Finally, several studies have recommended the utilization of PA for T2DM patients.13,57,68,85,122,154,155,185–190 The requirement for integrating regular exercise as a component of a patient’s lifestyle, including into daily activities, should be underlined.185 Exercise is frequently recommended in the management of diabetes because it can support and improve glycemic control.154 Regular exercise is recommended for patients with T2DM for effective control of BG levels.13 It is recommended for its valuable effects on glucose control and also for its abilities to delay the progression of other comorbidities which are common in individuals with diabetes.57 And also, regular exercise/PA is featured as a key component in management of T2DM patients in a current guideline established by the American College of Sports Medicine and American Diabetes Association. They prescribe a moderate-intensity aerobic exercise for 150 minutes each week.68 An exercise is also recommended for individuals who are at risk of developing or already have T2DM.186
It is commonly one of the primary management approaches recommended for people newly diagnosed with T2DM. Exercise is also a crucial component of obesity prevention if combined with other lifestyle modification.187 The evidence shows that exercise snacking is an effective approach to improve glycemic control in these patients. On the other hand, doing intensive interval exercise bouts immediately before breakfast, lunch and dinner had a better impact on postprandial and consecutive 24 hour glucose concentrations than did a single bout of moderate, continuous exercise undertaken before an evening meal.191 In obese T2DM patients, walking can be recommended as an adjunct therapy to diet management not only for body weight decrease but also for improvements of insulin sensitivity.155
Moreover, aerobic exercise and resistance exercise are recommended for diabetic people and must be adjusted to the physiological and metabolic limitations of each patient individually.85 Resistance training could be recommended in the early stage of T2DM, particularly for people with relatively poor glycemic control, because it is effective to control the glycemia.188 The study showed that a combined exercise is also recommended in the management of T2DM patients. Besides, 2–3 times per week, moderate-to-high intensity of the combined exercise is appropriate to be conducted for these patients.189 However, the recommendations and precautions of PA are varied depending on patients’ features and health status.48 Also, an exercise can be present with a significant challenge to glycemic control in diabetes because excessive glucose lowering can occur under certain circumstances which can enhance the threat of hypoglycemia; in other situations, hyperglycemia can be accentuated.57 Thus, those individuals who develop hyperglycemia after intensive exercise may require an injection of ultra-rapidly acting insulin at a time decided empirically.192
Conclusion
The burden of T2DM is significantly increasing worldwide. Because of this, a number of guidelines recommend the utilization of regular PA while it is the comprehensive component of diabetic management. It has been a long time since PA has been known to have beneficial effects for patients diagnosed with diabetes. It is found that exercise helps to improve the health status and decrease a burden on health professionals. The PA has antidiabetic effects due to its significant role in improving the BG levels of the individual with T2DM. It plays a substantial role in glycemic control of this disease by lowering the BG levels through the different possible mechanisms. Overall, PA plays an important role in the management approaches of T2DM patients because it offers several health benefits such as improving glycemic control, insulin sensitivity, body composition, quality-of-life, physical capacity, and cardiorespiratory fitness, reducing the obesity, insulin resistance, insulin level, lipid profile, VAT, BP, and cardiovascular risk, etc. However, PA is the most underutilized management of T2DM.
To achieve better benefits in the glycemic control of T2DM, the health professional should prescribe a structured frequency, volume, and intensity of training for patients diagnosed with T2DM. Also, the interaction of physician with patient is a major strategy in establishing a successful exercise programme for all patients with diabetes. Particularly, the multi-disciplinary team approach that comprise coordination among exercise physiologists, diabetes educators, the physician, the nurses, nutritionists, and the patient is usually the most effective way to create an individualized exercise regimen that provides benefits to the patient while avoiding potential harm. Further, PA is a tool, just like taking a medication or changing your diet in order to control the diabetes and it is so, so powerful. For most people with diabetes, it is a safe and highly recommended way to control diabetes and to reduce the risk of its complications. Therefore, the health professional should promote this message at every available opportunity in a clinical setting and public level. The patients should communicate their healthcare providers for special considerations needed to be known when they are working out and, primarily, it will be better if they test their BG levels before, after, and sometimes during exercise to observe how much their body responds to the exercise.
Finally, I will suggest that future study should be conducted on the duration, intensity, types of PA, and metabolic limitations that should be known to be prescribed considering all the characteristics of the patient. This is because I have seen that there is a limitation of literature on this information which could clearly identify it according to the patient characteristics. So, the findings of this study would be expected to encourage the utilization of exercise in an adjusted way to the physiological state of the T2DM patients.
Author Contributions
The author made a significant contribution to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; and agrees to be accountable for all aspects of the work.
Disclosure
The author declares no funding and no conflicts of interest for this work.
References
1. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. doi:10.2337/diaclin.26.2.77
2. Chaudhury A, Duvoor C, Reddy Dendi VS, et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol. 2017;8. doi:10.3389/fendo.2017.00006
3. World Health Organization. Global Report on Diabetes; 2016.
4. American diabetes association standards of medical care in diabetes—2018; 2018. Available from: https://diabetesed.net/wp-content/uploads/2017/12/2018-ADA-Standards-of-Care.pdf.
5. Allen L, Williams J, Townsend N, et al. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: a systematic review. Lancet Glob Health. 2017;5:e277–89. doi:10.1016/S2214-109X(17)30058-X
6. Oliveira C, Simões M, Carvalho J, Ribeiro J. Combined exercise for people with type 2 diabetes mellitus: A systematic review. Diabetes Res Clin Pract. 2012;98:187–198. doi:10.1016/j.diabres.2012.08.004
7. Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11:1185–1200. doi:10.7150/ijms.10001
8. Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576:51–60. doi:10.1038/s41586-019-1797-8
9. Cobbold C. Type 2 diabetes mellitus risk and exercise: is resistin involved? J Sports Med Phys Fitness. 2019;59. doi:10.23736/S0022-4707.18.08258-0.
10. Dos Santos JM, Moreli ML, Tewari S, Benite-Ribeiro SA. The effect of exercise on skeletal muscle glucose uptake in type 2 diabetes: an epigenetic perspective. Metabolism. 2015;64:1619–1628. doi:10.1016/j.metabol.2015.09.013
11. Gonzalez-Franquesa A, Patti M-E. Insulin Resistance and Mitochondrial Dysfunction. In: Santulli G, editor. Mitochondrial Dynamics in Cardiovascular Medicine. Cham: Springer International Publishing; 2017:465–520. doi:10.1007/978-3-319-55330-6_25
12. The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi:10.1016/S0140-6736(10)60484-9
13. Kemps H, Kränkel N, Dörr M, et al. Exercise training for patients with type 2 diabetes and cardiovascular disease: what to pursue and how to do it. A Position Paper of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol. 2019;26:709–727. doi:10.1177/2047487318820420
14. Kharroubi AT. Diabetes mellitus: the epidemic of the century. WJD. 2015;6:850. doi:10.4239/wjd.v6.i6.850
15. Bhutani J, Bhutani S. Worldwide burden of diabetes. Indian J Endocrinol Metab. 2014;18:868.
16. Heydari I, Radi V, Razmjou S, Amiri A. Chronic complications of diabetes mellitus in newly diagnosed patients. Int J Diabetes Mellit. 2010;2:61–63. doi:10.1016/j.ijdm.2009.08.001
17. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi:10.2337/diacare.21.9.1414
18. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi:10.2337/diacare.27.5.1047
19. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi:10.1016/j.diabres.2017.03.024
20. Standl E, Khunti K, Hansen TB, Schnell O. The global epidemics of diabetes in the 21st century: current situation and perspectives. Eur J Prev Cardiol. 2019;26(2_suppl):7–14. doi:10.1177/2047487319881021
21. Ekoru K, Doumatey A, Bentley AR, et al. Type 2 diabetes complications and comorbidity in Sub-Saharan Africans. EClinicalMedicine. 2019;16:30–41. doi:10.1016/j.eclinm.2019.09.001
22. Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3–16. doi:10.1007/s00125-018-4711-2
23. Naslafkih A, Sestier F. Diabetes mellitus related morbidity, risk of hospitalization and disability. J Insur Med. 2003;35:102–113.
24. Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care. 2002;25:471–475. doi:10.2337/diacare.25.3.471
25. Institute for Health Metrics and Evaluation (IHME). Findings from the Global Burden of Disease Study 2017. Seattle, WA: IHME; 2018. Available from: http://www.healthdata.org/sites/default/files/files/policy_report/2019/GBD_2017_Booklet.pdf.
26. van Dierena S, Beulensa JWJ, van der Schouwa YT, Grobbeea DE, Nealb B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(Suppl 1):S3–S8. doi:10.1097/01.hjr.0000368191.86614.5a
27. American Diabetes Association. Standards of medical care for patients with diabetes mellitus [published correction appears in Diabetes Care. 2003 Mar;26(3):972]. Diabetes Care. 2003;26 Suppl 1:S33–S50. doi:10.2337/diacare.26.2007.s33
28. Abdul Basith Khan M, Jawad Hashim M, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107–111. doi:10.2991/jegh.k.191028.001
29. Lee I-M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi:10.1016/S0140-6736(12)61031-9
30. Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. WJD. 2016;7:354. doi:10.4239/wjd.v7.i17.354
31. Heinrich E, Schaper N, de Vries N. Self-management interventions for type 2 diabetes: a systematic review. Eur Diabetes Nurs. 2010;7:71–76. doi:10.1002/edn.160
32. Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48:177–187. doi:10.1016/S0738-3991(02)00032-0
33. Norris SL, Engelgau MM, Venkat Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561–587. doi:10.2337/diacare.24.3.561
34. Kennedy A, Narendran P, Andrews RC, Daley A, Greenfield SM. Attitudes and barriers to exercise in adults with a recent diagnosis of type 1 diabetes: a qualitative study of participants in the Exercise for Type 1 Diabetes (EXTOD) study. BMJ Open. 2018;8:e017813. doi:10.1136/bmjopen-2017-017813
35. Taylor HL. Physical activity: is it still a risk factor? Prev Med. 1983;12:20–24. doi:10.1016/0091-7435(83)90164-0
36. WHO | Physical Activity. WHO; 2020. Available from: Available from: http://www.who.int/dietphysicalactivity/pa/en/.
37. Dasso NA. How is exercise different from physical activity? A concept analysis: DASSO. Nurs Forum. 2019;54:45–52. doi:10.1111/nuf.12296
38. Sigal RJ, Armstrong MJ, Bacon SL, et al. Physical activity and diabetes. Can J Diabetes. 2018;42:S54–63. doi:10.1016/j.jcjd.2017.10.008
39. Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. doi:10.1161/CIR.0b013e31829b5b44
40. Herriott MT, Colberg SR, Parson HK, Nunnold T, Vinik AI. Effects of 8 weeks of flexibility and resistance training in older adults with type 2 diabetes. Diabetes Care. 2004;27:2988–2989. doi:10.2337/diacare.27.12.2988
41. Physical Activity Guidelines Advisory Committee. Physical activity guidelines advisory committee report. Washington, DC, U.S. Department of Health and Human Services; 2008:683. Available from: https://health.gov/sites/default/files/2019-09/paguide.pdf.
42. Lobelo F, Rohm Young D, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137. doi:10.1161/CIR.0000000000000559
43. Seo DY, Ko JR, Jang JE, et al. Exercise as a potential therapeutic target for diabetic cardiomyopathy: insight into the underlying mechanisms. IJMS. 2019;20:6284. doi:10.3390/ijms20246284
44. Coen PM, Tanner CJ, Helbling NL, et al. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. J Clin Invest. 2015;125:248–257. doi:10.1172/JCI78016
45. Montesi L, Moscatiello S, Malavolti M, Marzocchi R, Marchesini G. Physical activity for the prevention and treatment of metabolic disorders. Intern Emerg Med. 2013;8:655–666. doi:10.1007/s11739-013-0953-7
46. Ostman C, Smart NA, Morcos D, Duller A, Ridley W, Jewiss D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:110. doi:10.1186/s12933-017-0590-y
47. Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441–447. doi:10.1016/j.pcad.2013.09.012
48. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–2079. doi:10.2337/dc16-1728
49. Thent ZC, Das S, Henry LJ. Role of exercise in the management of diabetes mellitus: the global scenario. PLoS One. 2013;8:e80436. doi:10.1371/journal.pone.0080436
50. Kourtoglou GI. Insulin therapy and exercise. Diabetes Res Clin Pract. 2011;93:S73–7. doi:10.1016/S0168-8227(11)70017-1
51. King AC, Guralnik JM. Maximizing the potential of an aging population. JAMA. 2010;304:1944. doi:10.1001/jama.2010.1577
52. Nicklett EJ, Semba RD, Xue Q-L, et al. Fruit and vegetable intake, physical activity, and mortality in older community-dwelling women. J Am Geriatr Soc. 2012;60:862–868. doi:10.1111/j.1532-5415.2012.03924.x
53. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of Type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi:10.1056/NEJM200105033441801
54. Adams P. The impact of brief high-intensity exercise on blood glucose levels. DMSO. 2013;113. doi:10.2147/DMSO.S29222.
55. Loh R, Stamatakis E, Folkerts D, Allgrove JE, Moir HJ. Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: a systematic review and meta-analysis. Sports Med. 2020;50:295–330. doi:10.1007/s40279-019-01183-w
56. Eriksson K-F, Lindgarde F. Prevention of Type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise The 6-year Malmo feasibility study. Diabetologia. 1991;34:891–898. doi:10.1007/BF00400196
57. Gulve EA. Exercise and glycemic control in diabetes: benefits, challenges, and adjustments to pharmacotherapy. Phys Ther. 2008;88:1297–1321. doi:10.2522/ptj.20080114
58. Zurlo F, Trevisan C, Vitturi N, et al. One-year caloric restriction and 12-week exercise training intervention in obese adults with type 2 diabetes: emphasis on metabolic control and resting metabolic rate. J Endocrinol Invest. 2019;42:1497–1507. doi:10.1007/s40618-019-01090-x
59. Armstrong MJ, Sigal RJ. Exercise as medicine: key concepts in discussing physical activity with patients who have type 2 diabetes. Can J Diabetes. 2015;39:S129–33. doi:10.1016/j.jcjd.2015.09.081
60. Bao W, Tobias DK, Bowers K, et al. Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern Med. 2014;174:1047. doi:10.1001/jamainternmed.2014.1795
61. Hopkins SA, Artal R. The role of exercise in reducing the risks of gestational diabetes mellitus. Womens Health (Lond Engl). 2013;9:569–581. doi:10.2217/WHE.13.52
62. Mottola MF, Artal R. Role of exercise in reducing gestational diabetes mellitus. Clin Obstet Gynecol. 2016;59:620–628. doi:10.1097/GRF.0000000000000211
63. Yu Y, Xie R, Shen C, Shu L. Effect of exercise during pregnancy to prevent gestational diabetes mellitus: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2018;31:1632–1637. doi:10.1080/14767058.2017.1319929
64. Sklempe Kokic I, Ivanisevic M, Biolo G, Simunic B, Kokic T, Pisot R. Combination of a structured aerobic and resistance exercise improves glycaemic control in pregnant women diagnosed with gestational diabetes mellitus. A randomised controlled trial. Women Birth. 2018;31:e232–8. doi:10.1016/j.wombi.2017.10.004
65. Sklempe Kokic I, Ivanisevic M, Kokic T, Simunic B, Pisot R. Acute responses to structured aerobic and resistance exercise in women with gestational diabetes mellitus. Scand J Med Sci Sports. 2018;28:1793–1800. doi:10.1111/sms.13076
66. Harrison AL, Shields N, Taylor NF, Frawley HC. Exercise improves glycaemic control in women diagnosed with gestational diabetes mellitus: a systematic review. J Physiother. 2016;62:188–196. doi:10.1016/j.jphys.2016.08.003
67. Dewan M. A prospective study of physical activity and its role in management and prevention of diabetes. J Exerc Sci Physiother. 2007;3:111–119.
68. Buresh R, Berg K. Exercise for the management of type 2 diabetes mellitus: factors to consider with current guidelines. J Sports Med Phys Fitness. 2018;58:510–524. doi:10.23736/S0022-4707.17.06969-9
69. Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose–response meta-analysis of prospective cohort studies. Diabetologia. 2016;59:2527–2545. doi:10.1007/s00125-016-4079-0
70. Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and Type 2 Diabetes: The American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–67. doi:10.2337/dc10-9990.
71. Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744–752. doi:10.2337/dc06-1842
72. Fedewa MV, Gist NH, Evans EM, Dishman RK. Exercise and Insulin resistance in youth: a meta-analysis. Pediatrics. 2014;133:e163–74. doi:10.1542/peds.2013-2718
73. Francesconi C, Lackinger C, Weitgasser R, Haber P, Niebauer J. Körperliche Aktivität und Training in der Prävention und Therapie des Typ 2 Diabetes mellitus. Wien Klin Wochenschr. 2016;128:141–145. doi:10.1007/s00508-015-0923-3
74. Hafstad AD, Boardman N, Aasum E. How exercise may amend metabolic disturbances in diabetic cardiomyopathy. Antioxid Redox Signal. 2015;22:1587–1605. doi:10.1089/ars.2015.6304
75. Yang D, Yang Y, Li Y, Han R. Physical exercise as therapy for type 2 diabetes mellitus: from mechanism to orientation. Ann Nutr Metab. 2019;74:313–321. doi:10.1159/000500110
76. Polikandrioti M, Dokoutsidou H. The role of exercise and nutrition in type II diabetes mellitus management. Health Sci J. 2009;3:6.
77. Ivy JL. Role of exercise training in the prevention and treatment of insulin resistance and non-insulin-dependent diabetes mellitus. Sports Med. 1997;24:321–336. doi:10.2165/00007256-199724050-00004
78. Stanford KI, Goodyear LJ. Exercise and type 2 diabetes: molecular mechanisms regulating glucose uptake in skeletal muscle. Adv Physiol Educ. 2014;38:308–314. doi:10.1152/advan.00080.2014
79. Amanat S, Ghahri S, Dianatinasab A, Fararouei M, Dianatinasab M. Exercise and Type 2 Diabetes. In: Xiao J, editor. Physical Exercise for Human Health. Singapore: Springer Singapore; 2020:91–105. doi:10.1007/978-981-15-1792-1_6
80. Kränkel N, Bahls M, Van Craenenbroeck EM, et al. Exercise training to reduce cardiovascular risk in patients with metabolic syndrome and type 2 diabetes mellitus: how does it work? Eur J Prev Cardiol. 2019;26:701–708. doi:10.1177/2047487318805158
81. Verboven M, Van Ryckeghem L, Belkhouribchia J, et al. Effect of exercise intervention on cardiac function in type 2 diabetes mellitus: a systematic review. Sports Med. 2019;49:255–268. doi:10.1007/s40279-018-1003-4
82. Yates T, Khunti K, Troughton J, Davies M. The role of physical activity in the management of type 2 diabetes mellitus. Postgrad Med J. 2009;85:129–133. doi:10.1136/pgmj.2008.067900
83. De Feyter HM, Praet SF, van den Broek NM, et al. Exercise training improves glycemic control in long-standing insulin-treated type 2 diabetic patients. Diabetes Care. 2007;30:2511–2513. doi:10.2337/dc07-0183
84. Carlo Negri EB. Influence of acute bouts of exercise on blood glucose in type 2 diabetic patients, as measured by continuous glucose monitoring systems. J Diabetes Metab. 2013;04. doi:10.4172/2155-6156.1000311.
85. Nakhanakhup C, Moungmee P, Appell HJ, Duarte JA. Regular physical exercise in patients with type II diabetes mellitus. Eur Rev Aging Phys Act. 2006;3:10–19. doi:10.1007/s11556-006-0002-x
86. Teo SYM, Kanaley JA, Guelfi KJ, et al. Exercise timing in Type 2 diabetes mellitus: a systematic review. Med Sci Sports Exerc. 2018;50:2387–2397. doi:10.1249/MSS.0000000000001732
87. Adolfsson P, Riddell MC, Taplin CE, et al. ISPAD clinical practice consensus guidelines 2018: exercise in children and adolescents with diabetes. Pediatr Diabetes. 2018;19:205–226. doi:10.1111/pedi.12755
88. Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA. 2002;288:1622. doi:10.1001/jama.288.13.1622
89. Green S, Kiely C, O’Connor E, Gildea N, O’Shea D, Egaña M. Effects of exercise training and sex on dynamic responses of O2 uptake in type 2 diabetes. Appl Physiol Nutr Metab. 2020. doi:10.1139/apnm-2019-0636
90. Yanai H, Adachi H, Masui Y, et al. Exercise therapy for patients with type 2 diabetes: a narrative review. J Clin Med Res. 2018;10:365–369. doi:10.14740/jocmr3382w
91. Asano RY. Acute effects of physical exercise in type 2 diabetes: A review. WJD. 2014;5:659. doi:10.4239/wjd.v5.i5.659
92. Colberg SR, Hernandez MJ, Shahzad F. Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care. 2013;36:e177–e177. doi:10.2337/dc13-0965
93. Yan H, Prista A, Ranadive SM, et al. Effect of aerobic training on glucose control and blood pressure in T2DDM east African males. ISRN Endocrinol. 2014;2014:1–6. doi:10.1155/2014/864897
94. Figueira FR, Umpierre D, Casali KR, et al. Aerobic and combined exercise sessions reduce glucose variability in type 2 diabetes: crossover randomized trial. PLoS One. 2013;8:e57733. doi:10.1371/journal.pone.0057733
95. Kurniawati Y, Baridah HA, Kusumawati MD, Wabula I. Effectiveness of physical exercise on the glycemic control of type 2 diabetes mellitus patients: a systematic review. Jurnal Ners. 2019;14(3si):199–204. doi:10.20473/jn.v14i3(si).17059
96. Embaby H, Elsayed E, Fawzy M. Insulin sensitivity and plasma glucose response to aerobic exercise in pregnant women at risk for gestational diabetes mellitus. Ethiop J Health Sci. 2016;26:409. doi:10.4314/ejhs.v26i5.2
97. Jorge MLMP, de Oliveira VN, Resende NM, et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60:1244–1252. doi:10.1016/j.metabol.2011.01.006
98. Zheng X, Qi Y, Bi L, et al. Effects of exercise on blood glucose and glycemic variability in type 2 diabetic patients with dawn phenomenon. Biomed Res Int. 2020;2020:1–6. doi:10.1155/2020/6408724
99. Jiang Y, Tan S, Wang Z, Guo Z, Li Q, Wang J. Aerobic exercise training at maximal fat oxidation intensity improves body composition, glycemic control, and physical capacity in older people with type 2 diabetes. J Exerc Sci Fit. 2020;18:7–13. doi:10.1016/j.jesf.2019.08.003
100. De Sá CA, Grudka Heizen P, Corralo VS, Gonzaga Dos Santos GA, Moura Soares NM. Chronic effect of aerobic exercise on anthropometric, biochemical and hemodynamic variables in individuals with type 2 diabetes mellitus: A systematic review. Rev Andal Med Deport. 2016;9:173–179. doi:10.1016/j.ramd.2015.09.005
101. Gibala MJ, Little JP. Physiological basis of brief vigorous exercise to improve health. J Physiol. 2020;598:61–69. doi:10.1113/JP276849
102. Manders RJF, Van Dijk J-WM, Van Loon LJC. Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med Sci Sports Exerc. 2010;42:219–225. doi:10.1249/MSS.0b013e3181b3b16d
103. Metcalfe RS, Fitzpatrick B, Fitzpatrick S, et al. Extremely short duration interval exercise improves 24-h glycaemia in men with type 2 diabetes. Eur J Appl Physiol. 2018;118:2551–2562. doi:10.1007/s00421-018-3980-2
104. Coelho LH, Amorim PRDS, Marins JCB, et al. Glycemic response during and after aerobic and resistance exercise training in type 2 diabetics: experimental study. Man Ther Posturol Rehabi. 2018;16:604. doi:10.17784/mtprehabjournal.2018.16.604
105. Bergman BC, Goodpaster BH. Exercise and muscle lipid content, composition, and localization: influence on muscle insulin sensitivity. Diabetes. 2020;69:848–858. doi:10.2337/dbi18-0042
106. Zanuso S, Sacchetti M, Sundberg CJ, Orlando G, Benvenuti P, Balducci S. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. A review of the evidence. Br J Sports Med. 2017;51:1533–1538. doi:10.1136/bjsports-2016-096724
107. Parsian H, Eizadi M, Khorshidi D, et al.; Dept. of Physical Education and Sports Science, Islamic Azad University, Islamshahr Branch, Islamshahr, Iran, Eizadi M, Dept. of Physical Education and Sport Sciences, Islamic Azad University, Saveh Branch, Saveh, Iran, Khorshidi D, Dept. of Physical Education and Sport Sciences, Islamic Azad University, Saveh Branch, Saveh, Iran. The effect of long-term aerobic exercise on serum adiponectin and insulin sensitivity in type 2 diabetic patients. jjums. 2013;11:41–48. doi:10.29252/jmj.11.1.6
108. Cai H, Li G, Zhang P, Xu D, Chen L. Effect of exercise on the quality of life in type 2 diabetes mellitus: a systematic review. Qual Life Res. 2017;26:515–530. doi:10.1007/s11136-016-1481-5
109. Gu Y, Dennis SM, Kiernan MC, Harmer AR. Aerobic exercise training may improve nerve function in type 2 diabetes and pre‐diabetes: A systematic review. Diabetes Metab Res Rev. 2018;e3099. doi:10.1002/dmrr.3099
110. Dos Anjos DMDC, de Moreira BS, Kirkwood RN, Dias RC, Pereira DS, Pereira LSM. Effects of aerobic exercise on functional capacity, anthropometric measurements and inflammatory markers in diabetic elderly women. J Bodyw Mov Ther. 2017;21:509–516. doi:10.1016/j.jbmt.2016.07.012
111. Grace A, Chan E, Giallauria F, Graham PL, Smart NA. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. doi:10.1186/s12933-017-0518-6
112. Alizadeh AA, Rahmani-Nia F, Mohebbi H, Zakerkish M. Effects of eight weeks aerobic exercise on plasma levels of Orexin A, Leptin, glucose, insulin, and insulin resistance in males with type 2 diabetes. Iran J Diabetes Obes. 2015;7:7.
113. Abd El-Kader SM. Aerobic versus resistance exercise training in modulation of insulin resistance, adipocytokines and inflammatory cytokine levels in obese type 2 diabetic patients. J Adv Res. 2011;2:179–183. doi:10.1016/j.jare.2010.09.003
114. Bacchi E, Negri C, Zanolin ME, et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care. 2012;35:676–682. doi:10.2337/dc11-1655
115. Eves ND, Plotnikoff RC. Resistance training and type 2 diabetes: considerations for implementation at the population level. Diabetes Care. 2006;29:1933–1941. doi:10.2337/dc05-1981
116. Pesta DH, Goncalves RLS, Madiraju AK, Strasser B, Sparks LM. Resistance training to improve type 2 diabetes: working toward a prescription for the future. Nutr Metab (Lond). 2017;14:24. doi:10.1186/s12986-017-0173-7
117. Strasser B, Pesta D. Resistance training for diabetes prevention and therapy: experimental findings and molecular mechanisms. Biomed Res Int. 2013;2013:1–8. doi:10.1155/2013/805217
118. Brown EC, Franklin BA, Regensteiner JG, Stewart KJ. Effects of single bout resistance exercise on glucose levels, insulin action, and cardiovascular risk in type 2 diabetes: A narrative review. J Diabetes Complications. 2020;34:107610. doi:10.1016/j.jdiacomp.2020.107610
119. Irvine C, Taylor NF. Progressive resistance exercise improves glycaemic control in people with type 2 diabetes mellitus: a systematic review. Aust J Physiother. 2009;55:237–246. doi:10.1016/S0004-9514(09)70003-0
120. Santos GMD, Montrezol FT, Pauli LSS, et al. Undulatory physical resistance training program increases maximal strength in elderly type 2 diabetics. Einstein (São Paulo). 2014;12:425–432. doi:10.1590/S1679-45082014AO3162
121. Fenicchia LM, Kanaley JA, Azevedo JL, et al. Influence of resistance exercise training on glucose control in women with type 2 diabetes. Metabolism. 2004;53:284–289. doi:10.1016/j.metabol.2003.10.007
122. Azari N, Rahmati M, Fathi M. The effect of resistance exercise on blood glucose, insulin and insulin resistance in iranian patients with type II diabetes: a systematic review and meta-analysis. Iran J Diabetes Obes. 2018;10:11.
123. Mendes R, Sousa N, Themudo-Barata JL, Reis VM. High-intensity interval training versus moderate-intensity continuous training in middle-aged and older patients with type 2 diabetes: a randomized controlled crossover trial of the acute effects of treadmill walking on glycemic control. IJERPH. 2019;16:4163. doi:10.3390/ijerph16214163
124. Cassidy S, Vaidya V, Houghton D, et al. Unsupervised high-intensity interval training improves glycaemic control but not cardiovascular autonomic function in type 2 diabetes patients: A randomised controlled trial. Diabetes Vasc Dis. 2019;16:69–76. doi:10.1177/1479164118816223
125. Savikj M, Gabriel BM, Alm PS, et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia. 2019;62:233–237. doi:10.1007/s00125-018-4767-z
126. Terada T, Wilson BJ, Myette-Côté E, et al. Targeting specific interstitial glycemic parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolism. 2016;65:599–608. doi:10.1016/j.metabol.2016.01.003
127. Gillen JB, Little JP, Punthakee Z, Tarnopolsky MA, Riddell MC, Gibala MJ. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes Metab. 2012;14:575–577. doi:10.1111/j.1463-1326.2012.01564.x
128. Takenami E, Iwamoto S, Shiraishi N, et al. Effects of low-intensity resistance training on muscular function and glycemic control in older adults with type 2 diabetes. J Diabetes Investig. 2019;10:331–338. doi:10.1111/jdi.12926
129. Gordon BA, Benson AC, Bird SR, Fraser SF. Resistance training improves metabolic health in type 2 diabetes: A systematic review. Diabetes Res Clin Pract. 2009;83:157–175. doi:10.1016/j.diabres.2008.11.024
130. Heden TD, Liu Y, Kanaley JA. A comparison of adipose tissue interstitial glucose and venous blood glucose during postprandial resistance exercise in patients with type 2 diabetes. J Appl Physiol. 2018;124:1054–1061. doi:10.1152/japplphysiol.00475.2017
131. Chen S-M, Shen F-C, Chen J-F, Chang W-D, Chang N-J. Effects of resistance exercise on glycated hemoglobin and functional performance in older patients with comorbid diabetes mellitus and knee osteoarthritis: a randomized trial. IJERPH. 2019;17:224. doi:10.3390/ijerph17010224
132. Liu J, Zhu L, Li P, Li N, Xu Y. Effectiveness of high-intensity interval training on glycemic control and cardiorespiratory fitness in patients with type 2 diabetes: a systematic review and meta-analysis. Aging Clin Exp Res. 2019;31:575–593. doi:10.1007/s40520-018-1012-z
133. Liu Y, Ye W, Chen Q, Zhang Y, Kuo C-H, Korivi M. Resistance exercise intensity is correlated with attenuation of HbA1c and insulin in patients with type 2 diabetes: a systematic review and meta-analysis. IJERPH. 2019;16:140. doi:10.3390/ijerph16010140
134. Schwingshackl L, Missbach B, Dias S, König J, Hoffmann G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia. 2014;57:1789–1797. doi:10.1007/s00125-014-3303-z
135. Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357. doi:10.7326/0003-4819-147-6-200709180-00005
136. Sigal RJ, Kenny GP. Combined aerobic and resistance exercise for patients with type 2 diabetes. JAMA. 2010;304:2298. doi:10.1001/jama.2010.1719
137. Tan S, Li W, Wang J. Effects of six months of combined aerobic and resistance training for elderly patients with a long history of type 2 diabetes. J Sports Sci Med. 2012;11:495–501.
138. Maiorana A, O’Driscoll G, Goodman C, Taylor R, Green D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract. 2002;56:115–123. doi:10.1016/S0168-8227(01)00368-0
139. Johannsen NM, Swift DL, Lavie CJ, Earnest CP, Blair SN, Church TS. Combined aerobic and resistance training effects on glucose homeostasis, fitness, and other major health indices: a review of current guidelines. Sports Med. 2016;46:1809–1818. doi:10.1007/s40279-016-0548-3
140. Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–2982. doi:10.2337/diacare.26.11.2977
141. Maiorana A, O’Driscoll G, Cheetham C, et al. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001;38:860–866. doi:10.1016/S0735-1097(01)01439-5
142. Zanuso S, Jimenez A, Pugliese G, Corigliano G, Balducci S. Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol. 2010;47:15–22. doi:10.1007/s00592-009-0126-3
143. Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A 1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253. doi:10.1001/jama.2010.1710
144. AminiLari Z, Fararouei M, Amanat S, et al. The effect of 12 weeks aerobic, resistance, and combined exercises on omentin-1 levels and insulin resistance among type 2 diabetic middle-aged women. Diabetes Metab J. 2017;41:205. doi:10.4093/dmj.2017.41.3.205
145. Bgeginski R, Ribeiro PAB, Mottola MF, Ramos JGL. Effects of weekly supervised exercise or physical activity counseling on fasting blood glucose in women diagnosed with gestational diabetes mellitus: A systematic review and meta-analysis of randomized trials. J Diabetes. 2017;9:1023–1032. doi:10.1111/1753-0407.12519
146. Pan B, Ge L, Xun Y, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. 2018;15:72. doi:10.1186/s12966-018-0703-3
147. Sampath Kumar A, Maiya AG, Shastry BA, et al. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62:98–103. doi:10.1016/j.rehab.2018.11.001
148. Thomas D, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006. doi:10.1002/14651858.CD002968.pub2
149. Umpierre D. Physical activity advice only or structured exercise training and association with HbA 1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790. doi:10.1001/jama.2011.576
150. Yuing T, Lizana PA, Berral FJ. Hemoglobina glicada y ejercicio: una revisión sistemática. Rev Med Chile. 2019;147:480–489. doi:10.4067/S0034-98872019000400480
151. Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218. doi:10.1001/jama.286.10.1218
152. Way KL, Hackett DA, Baker MK, Johnson NA. The effect of regular exercise on insulin sensitivity in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab J. 2016;40:253. doi:10.4093/dmj.2016.40.4.253
153. Blood Sugar and Exercise | ADA. Available from: https://www.diabetes.org/fitness/get-and-stay-fit/getting-started-safely/blood-glucose-and-exercise.
154. Peirce NS. Diabetes and exercise. Br J Sports Med. 1999;33:161–172. doi:10.1136/bjsm.33.3.161
155. Yamanouchi K, Shinozaki T, Chikada K, et al. Daily walking combined with diet therapy is a useful means for obese NIDDM patients not only to reduce body weight but also to improve insulin sensitivity. Diabetes Care. 1995;18:775–778. doi:10.2337/diacare.18.6.775
156. Medscape: diabetes mellitus and exercise;2010. Available from: https://www.medscape.com/viewarticle/717051.
157. Dubé JJ, Allison KF, Rousson V, Goodpaster BH, Amati F. Exercise dose and insulin sensitivity: relevance for diabetes prevention. Med Sci Sports Exerc. 2012;44:793–799. doi:10.1249/MSS.0b013e31823f679f
158. Newsom SA, Everett AC, Hinko A, Horowitz JF. A single session of low-intensity exercise is sufficient to enhance insulin sensitivity into the next day in obese adults. Diabetes Care. 2013;36:2516–2522. doi:10.2337/dc12-2606
159. Mann S, Beedie C, Balducci S, et al. Changes in insulin sensitivity in response to different modalities of exercise: a review of the evidence: insulin sensitivity and exercise modality. Diabetes Metab Res Rev. 2014;30:257–268. doi:10.1002/dmrr.2488
160. Wang X, Patterson BW, Smith GI, et al. A ~60-min brisk walk increases insulin-stimulated glucose disposal but has no effect on hepatic and adipose tissue insulin sensitivity in older women. J Appl Physiol. 2013;114:1563–1568. doi:10.1152/japplphysiol.01364.2012
161. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2017;2:e000143. doi:10.1136/bmjsem-2016-000143
162. Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action: exercise and insulin action. Acta Physiol. 2007;192:127–135. doi:10.1111/j.1748-1716.2007.01783.x
163. Kirwan JP, Solomon TPJ, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151–6. doi:10.1152/ajpendo.00210.2009
164. Wojtaszewski JFP, Nielsen JN, Richter EA. Invited review: effect of acute exercise on insulin signaling and action in humans. J Appl Physiol. 2002;93:384–392. doi:10.1152/japplphysiol.00043.2002
165. Hayashi T, Wojtaszewski JFP, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol Endocrinol Metab. 1997;273:E1039–51. doi:10.1152/ajpendo.1997.273.6.E1039
166. Liu S-X, Zheng F, Xie K-L, Xie M-R, Jiang L-J CY. Exercise reduces insulin resistance in Type 2 diabetes mellitus via mediating the lncRNA MALAT1/MicroRNA-382-3p/Resistin Axis. Mol Ther Nucleic Acids. 2019;18:34–44. doi:10.1016/j.omtn.2019.08.002
167. Dubé JJ, Amati F, Toledo FGS, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54:1147–1156. doi:10.1007/s00125-011-2065-0
168. Yokoyama H, Emoto M, Fujiwara S, et al. Short-term aerobic exercise improves arterial stiffness in type 2 diabetes. Diabetes Res Clin Pract. 2004;65:85–93. doi:10.1016/j.diabres.2003.12.005
169. Ross R. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann Intern Med. 2000;133:92. doi:10.7326/0003-4819-133-2-200007180-00008
170. Mourier A, Gautier J-F, Kerviler ED, et al. Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM: effects of branched-chain amino acid supplements. Diabetes Care. 1997;20:385–391. doi:10.2337/diacare.20.3.385
171. Campos RMDS, Masquio DCL, Corgosinho FC, et al. Effects of magnitude of visceral adipose tissue reduction: impact on insulin resistance, hyperleptinemia and cardiometabolic risk in adolescents with obesity after long-term weight-loss therapy. Diabetes Vasc Dis. 2019;16:196–206. doi:10.1177/1479164118825343
172. Vissers D, Hens W, Taeymans J, Baeyens J-P, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One. 2013;8:e56415. doi:10.1371/journal.pone.0056415
173. Sabag A, Way KL, Keating SE, et al. Exercise and ectopic fat in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2017;43:195–210. doi:10.1016/j.diabet.2016.12.006
174. Bacchi E, Negri C, Targher G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial): hepatology. Hepatology. 2013;58:1287–1295. doi:10.1002/hep.26393
175. Teich T, Zaharieva DP, Riddell MC. Advances in exercise, physical activity, and diabetes mellitus. Diabetes Technol Ther. 2019;21:
176. Heiskanen MA, Motiani KK, Mari A, et al. Exercise training decreases pancreatic fat content and improves beta cell function regardless of baseline glucose tolerance: a randomised controlled trial. Diabetologia. 2018;61:1817–1828. doi:10.1007/s00125-018-4627-x
177. Nieuwoudt S, Fealy CE, Foucher JA, et al. Functional high-intensity training improves pancreatic β-cell function in adults with type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313:E314–20. doi:10.1152/ajpendo.00407.2016
178. Solomon TPJ, Malin SK, Karstoft K, Kashyap SR, Haus JM, Kirwan JP. Pancreatic β-cell function is a stronger predictor of changes in glycemic control after an aerobic exercise intervention than insulin sensitivity. J Clin Endocrinol Metab. 2013;98:4176–4186. doi:10.1210/jc.2013-2232
179. Brankston GN, Mitchell BF, Ryan EA, Okun NB. Resistance exercise decreases the need for insulin in overweight women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;190:188–193. doi:10.1016/S0002-9378(03)00951-7
180. Olver TD, Laughlin MH. Endurance, interval sprint, and resistance exercise training: impact on microvascular dysfunction in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2016;310:H337–50. doi:10.1152/ajpheart.00440.2015
181. Qiu S, Cai X, Yin H, et al. Exercise training and endothelial function in patients with type 2 diabetes: a meta-analysis. Cardiovasc Diabetol. 2018;17:64. doi:10.1186/s12933-018-0711-2
182. Plotnikoff RC, Costigan SA, Karunamuni ND, Lubans DR. Community-based physical activity interventions for treatment of Type 2 diabetes: a systematic review with meta-analysis. Front Endocrinol. 2013;4. doi:10.3389/fendo.2013.00003.
183. Byrne H, Caulfield B, De Vito G. Effects of self-directed exercise programmes on individuals with Type 2 diabetes mellitus: a systematic review evaluating their effect on HbA1c and other metabolic outcomes, physical characteristics, cardiorespiratory fitness and functional outcomes. Sports Med. 2017;47:717–733. doi:10.1007/s40279-016-0593-y
184. Umpierre D, Ribeiro PAB, Schaan BD, Ribeiro JP. Volume of supervised exercise training impacts glycaemic control in patients with type 2 diabetes: a systematic review with meta-regression analysis. Diabetologia. 2013;56:242–251. doi:10.1007/s00125-012-2774-z
185. Eriksson JG. Exercise and the treatment of Type 2 diabetes mellitus: an update. Sports Med. 1999;27:381–391. doi:10.2165/00007256-199927060-00003
186. Eckstein ML, Williams DM, O’Neil LK, Hayes J, Stephens JW, Bracken RM. Physical exercise and non‐insulin glucose‐lowering therapies in the management of Type 2 diabetes mellitus: a clinical review. Diabet Med. 2018;
187. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. CCJM. 2017;84 7 suppl 1:S15–21. doi:10.3949/ccjm.84.s1.03
188. Ishiguro H, Kodama S, Horikawa C, et al. In search of the ideal resistance training program to improve glycemic control and its indication for patients with Type 2 diabetes mellitus: a systematic review and meta-analysis. Sports Med. 2016;46:67–77. doi:10.1007/s40279-015-0379-7
189. Wang T, Liu Y, Zhong R, Xu D, Wang H, Fu BS-C. Benefit effects of aerobic exercise and resistance training on the management of type 2 diabetes. Int J Clin Exp Med. 2018;11:10433–10445.
190. Rijal A, Nielsen EE, Hemmingsen B, et al. Adding exercise to usual care in patients with hypertension, type 2 diabetes mellitus and/or cardiovascular disease: a protocol for a systematic review with meta-analysis and trial sequential analysis. Syst Rev. 2019;8:330. doi:10.1186/s13643-019-1233-z
191. Francois ME, Baldi JC, Manning PJ, et al. ‘Exercise snacks’ before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia. 2014;57:1437–1445. doi:10.1007/s00125-014-3244-6
192. Marliss EB, Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes. 2002;51 Supplement 1:S271–83. doi:10.2337/diabetes.51.2007.S271
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
