Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Antibacterial Activity of Surfactin and Synergistic Effect with Conventional Antibiotics Against Methicillin-Resistant Staphylococcus aureus Isolated from Patients with Diabetic Foot Ulcers
Authors Li Z , Li T, Tang J, Huang L, Ding Y, Zeng Z, Liu J
Received 12 September 2023
Accepted for publication 31 October 2023
Published 20 November 2023 Volume 2023:16 Pages 3727—3737
DOI https://doi.org/10.2147/DMSO.S435062
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Zhaoyinqian Li,1– 3,* Tingting Li,1– 4,* Jingyang Tang,1– 3 Li Huang,1– 3 Yinhuan Ding,1– 3 Zhangrui Zeng,1– 3 Jinbo Liu1– 3
1Department of Laboratory Medicine, the Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China; 2Sichuan Province Engineering Technology Research Center of Molecular Diagnosis of Clinical Diseases, Luzhou, People’s Republic of China; 3Molecular Diagnosis of Clinical Diseases Key Laboratory of Luzhou, Luzhou, People’s Republic of China; 4Department of Laboratory Medicine, West China Fourth Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinbo Liu, Department of Laboratory Medicine, the Affiliated Hospital of Southwest Medical University, No. 25, Taiping Street, Jiangyang District, Luzhou, Sichuan, 646000, People’s Republic of China, Tel/Fax +86 08303165730, Email [email protected]
Introduction: The prevalence of diabetic foot ulcers (DFUs) is increasing, leading to a huge financial burden and human suffering. Furthermore, antibiotic resistance is an urgent problem in the realm of clinical practice. Antimicrobial peptides are an effective and feasible strategy for combating infections caused by drug-resistant bacteria. Therefore, we investigated the in vitro antimicrobial ability of the lipopeptide surfactin, either alone or in combination with conventional antibiotics, against the standard and clinical strains of Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA), isolated from patients with DFUs.
Methods: The minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) of surfactin on the selected strains were evaluated by a microbroth dilution technique. The growth curves of the selected strains with and without surfactin were measured, and transmission electron microscopy was used to observe the structure of surfactin-treated bacterial cells. The biofilm inhibitory abilities of surfactin were assessed by crystal violet staining. The antimicrobial interactions between surfactin and conventional antibiotics were established using a checkerboard assay, as well as determining the mutant prevention concentration. The inhibitory effect of surfactin on penicillinase was tested by iodometry.
Results: The MIC and MBC values of surfactin ranged from 512 to 1024 μg/mL and 1024 to 2048 μg/mL, respectively. Moreover, surfactin significantly prevented the S. aureus biofilm formation and displayed limited toxicity on human red blood cells. The synergies between surfactin and ampicillin, oxacillin, and tetracycline against S. aureus were revealed. In vitro resistance was not readily produced by surfactin. The action of surfactin may be by disrupting bacterial cell membranes and inhibiting penicillinase.
Conclusion: Surfactin appears to be a potential option for the treatment of DFUs infected with MRSA, as it is capable of improving antimicrobial activities and can be used alone or in combination with conventional antibiotics to prevent or postpone the emergence of resistance.
Keywords: surfactin, antimicrobial peptides, diabetic foot ulcers, methicillin-resistant Staphylococcus aureus, synergistic effect
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Wardoyo has been published for this article.
Introduction
Diabetic foot infection (DFI) is a frequent and problematic complication of diabetes, and it is becoming an increasingly attractive option as the prevalence of diabetes rises. Previous studies have shown that approximately 15–20% of patients will develop a diabetic foot ulcer (DFU) during their lifetime. The prognosis for those suffering from DFI is generally unfavorable, with a high risk of recurrence, amputation, and even mortality.1 DFIs are usually caused by a variety of microorganisms, but the most common pathogen worldwide is still Staphylococcus aureus.2–4 S. aureus is highly adaptable and can rapidly develop drug resistance by acquiring resistance genes from other bacteria of the same species or even from different genera.5 The prevalence of multidrug-resistant (MDR) strains and Methicillin-resistant S. aureus (MRSA) has complicated the treatment process and decreased the effectiveness of conventional antibiotic therapies. Notably, it has been found that the occurrence of MRSA in DFUs has risen to a range of 15–30%.6 Developing novel antibiotics and reducing existing resistance are thought to be two strategies for managing drug-resistant bacteria.
Antimicrobial peptides (AMPs), small proteins with multiple biological activities, are an important component of the multicellular organisms’ immune defense.7 Over the years, the two characteristics of broad-spectrum antimicrobial activity and less susceptibility to drug resistance make AMPs a promising alternative to antibiotics.8 Surfactin, a macrolide lipopeptide produced by Bacillus subtilis, is one of the most powerful biosurfactants known.9,10 To date, surfactin has proven applications in industry (enhanced oil recovery), agriculture (biological control and promotion of organic pesticide degradation), and animal husbandry.11–13 In recent years, the role of surfactin in the field of medicine has been widely noted. Surfactin has been shown to have potent antimicrobial, anti-inflammatory, antiviral, antimycoplasmal, and anticancer activities, while it also has anti-adhesive properties, inhibiting the production of biofilms and reducing the adhesion of bacteria to the site of infection.14,15 Based on these properties, surfactin has the potential to be loaded into a wound dressing as an antimicrobial substance to control biofilm formation and secondary infection in DFUs.
To our knowledge, there have been no studies to date on the antibacterial effects of surfactin against bacteria infected with DFUs. Therefore, we selected multiple strains of S. aureus (including non-MDR, MDR, and MRSA) isolated from clinical DFUs as well as ATCC 25923 to evaluate the antibacterial activity of surfactin alone or in combination with other conventional antibiotics against S. aureus in vitro and to explore the underlying mechanisms.
Materials and Methods
Bacterial Strains and Reagents
Clinical S. aureus strains were isolated from patients with DFUs admitted to the Affiliated Hospital of Southwest Medical University in Luzhou, China. S. aureus ATCC 25923 was purchased from the American Type Culture Collection. All strains were grown in Mueller-Hinton (MH) Broth Medium (Solarbio, China). Surfactin (99% purity) and clavulanate potassium were purchased from Shanghai yuanye Bio-Technology Co., Ltd. Oxacillin, ampicillin, gentamicin, tetracycline, erythromycin, ciprofloxacin, levofloxacin, and penicillinase were purchased from Solarbio. Surfactin, ampicillin, oxacillin, erythromycin, ciprofloxacin, gentamicin, and tetracycline were prepared in sterilized distilled water and trypticase soy broth (TSB), respectively.
Identification and Antimicrobial Susceptibility Testing
All selected strains were identified by matrix-assisted laser desorption/ionization time-of-flight spectrometry (Bruker, Germany), and antimicrobial susceptibility testing for conventional antibiotics was performed using the MicroScan Walk-Away 96 Plus system (Beckman Coulter, USA). The results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) M100. The minimum inhibitory concentration (MIC) for surfactin was established through the microbroth dilution method, which is the lowest concentration of surfactin that inhibits visible bacterial growth.16 Following MIC determination, 10 µL of bacterial culture was taken from wells with no bacterial growth and smeared on MH agar plates. The lowest concentration at which no bacterial colonies grew on the plate after 24 h of incubation at 37 °C was defined as the minimum bactericidal concentration (MBC).16
Bacterial Growth Curves
ATCC 25923 and a clinical MRSA strain were diluted to 1×106 colony-forming units/mL (CFU/mL) in MH broth containing different concentrations of surfactin (0, 1/2×MIC, MIC, and 2×MIC), and MH broth only was set up as the negative control. All cultures were shaken for 24 h (200 rpm/min, 35 °C). 100 µL of culture was taken from each tube at different time points (0, 4, 8, 12, and 24 h) and the optical density (OD) was measured at 600 nm, respectively.
Biofilm Inhibition Assay
The biofilm-forming ability of ATCC 25923 and a clinical MRSA strain, as well as the biofilm-inhibitory ability of surfactin against them, were assessed by the crystal violet staining method.17 Briefly, the overnight cultures of each strain were diluted with TSB containing serial dilutions of surfactin (1/64×MIC to 2×MIC) to obtain a final concentration of 1×106 CFU/mL, and then 200 µL of each culture were inoculated into a 96-well microtiter plate in triplicates. Then, planktonic cells were removed by washing with 1×phosphate-buffered saline (PBS) three times after incubation at 37°C for 24 h, and each well was stained with 0.5% crystal violet (Solarbio, China) for 20 min. After air-drying, the wells were solubilized with 200 μL of 95% ethanol for 20 min at room temperature. The absorbance of each well was measured at OD570. Moreover, the strength of biofilm formation of the selected strains was classified according to the following criteria: OD ≤ cut-off OD (ODC), no biofilm formation; ODC < OD ≤ 2×ODC, weak biofilm formation; 2×ODC < OD ≤ 4×ODC, moderate biofilm formation; and OD > 4×ODC, strong biofilm formation.18 The ODc was defined as the mean OD of the negative control (TSB only).
Transmission Electron Microscopy (TEM)
ATCC 25923 culture at mid-log growth phase was diluted to 1×108 CFU/mL in MH broth in the presence of 5×MIC of surfactin with 1 h of shaking (180 rpm/min). ATCC 25923 treated with 0.05% DMSO was used as the control. Then, the bacterial culture was centrifuged at 4500×g for 10 min and the pellet was washed with PBS three times. 200 µL of fixative solution containing 2.5% glutaraldehyde and 0.01 M phosphate buffer was added to the washed pellet and left to fix for 2 h at 4 °C. 0.1 M phosphate buffer (pH 7.4) was rinsed three times for 15 min each, and 1% osmium acid/0.1 M phosphate buffer was fixed for 2 h at room temperature. After three washes with 0.1 M phosphate buffer, the fixed cells were dehydrated using ethanol at gradient concentrations (50%, 70%, 80%, and 90%) and were infiltrated overnight with propylene oxide and Epon resin (2:1). Then, the bacterial cells were polymerized at 60 °C for 48 h and were cut using a ultramicrotome (Leica, Germany) followed by staining with lead citrate. Finally, prepared bacterial cells were observed using a TEM (Hitachi, Japan).
Human Red Blood Cells (RBCs) Hemolysis Assay
Whole blood was collected from healthy volunteers at the Hospital of Southwest Medical University. After centrifugation at 1000×g for 5 min, the RBCs were washed with PBS three times and re-suspended in PBS. The RBC suspension (100 µL) and serial dilutions of surfactin (100 µL) were added to a 96-well microtiter plate. Then, 100 µL of the supernatant was taken from the wells and the absorbance at 570 nm was measured after 1 h of incubation at 37 °C. In addition, 0.1% DMSO was used as a negative control, and 0.1% Triton X-100 as a positive control.19 The hemolysis rate was calculated as: Hemolysis rate (%)= (Asample - A0.1% DMSO) / (A0.1% TritonX-100 - A0.1% DMSO) × 100%.
Checkerboard Assay
Antimicrobial interactions between surfactin and conventional antibiotics were determined using a checkerboard assay. 50 µL of a serial dilution of surfactin (64 to 4096 µg/mL) and 50 µL of antibiotics (ampicillin, oxacillin, erythromycin, ciprofloxacin, gentamicin, and tetracycline) (0.125 to 128 µg/mL) were mixed in 96-well microtiter plates in the presence of 1×106 CFU/mL ATCC 25923 and clinical MRSA strain suspensions (100 µL) to establish a two-dimensional tessellation. The OD630 of each well was determined after incubation at 37 °C for 18 h. The fractional inhibitory concentration index (FICI) between surfactin and six conventional antibiotics was calculated as: FICI = MICA(combination) / MICA(alone) + MICB(combination) / MICB(alone). A FICI ≤ 0.5 indicates synergy, 0.5 < FICI < 4 indicates no interaction, and FICI > 4 indicates antagonism.20,21
Penicillinase Inhibition Assay
The inhibitory effect of surfactin on penicillinase was assessed by iodometry. 10,000 U/mL of penicillinase was treated with different concentrations of surfactin (2048, 1024, 512, and 256 μg/mL) or clavulanate potassium (64, 32, 16, and 8 μg/mL) for 18 h at 35 °C. 2mL of penicillin K (10,000 U/mL) was added to 1 mL of the mixture and incubated at 37 °C for 1 h, then 25 mL of 0.01 mol/L iodine standard titrant was added to the solution for 15 min. A 0.01 mol/L solution of sodium thiosulfate (Na2S2O3) was used for the titration. Near the end of the titration, 1–2 drops of starch indicator were added, after which the Na2S2O3 was continued until the color of the solution became colorless. The amount of Na2S2O3 consumed after the action of the corresponding concentration was recorded, and the penicillinase activity was negatively correlated with the amount consumed.
Determination of Mutant Prevention Concentration (MPC)
The procedure of determining the MPC was described previously with slight modifications.22 ATCC 25923 and the clinical MRSA strain were cultured in MH broth and incubated for 24 h. Then, the bacterial suspensions were centrifuged (4000×g for 10 min) and resuspended in MH broth to reach a concentration of 1×1010 CFU/mL. MH plates with oxacillin, surfactin, and oxacillin + surfactin concentrations of MIC, 2×MIC, 4×MIC, 8×MIC, 16×MIC, and 32×MIC were prepared, respectively. 100 μL of 1×1010 CFU/mL bacterial suspensions were inoculated onto each drug-containing MH agar plate and incubated for 72 h at 35 °C. The MPC was defined as the lowest drug concentration that prevented bacterial colony formation from a culture containing over 1×1010 bacteria within 72 h.23 The ratio of MPC to MIC presents the width of the mutant selection window (MSW).24
Statistical Analysis
All experiments were performed in triplicate. Data were analyzed using Prism 9.0 (GraphPad Software, USA) and are presented as the mean ± standard deviation (SD). P-values were calculated using an unpaired Student’s t-test or a one-way ANOVA, and P < 0.05 was considered statistically significant.
Results
Identification of Clinical Strains
Based on our previous study,25 four strains of clinical S. aureus isolated from wound secretions of patients with DFUs, including one non-MDR strain (SA 095), one MDR strain (SA 366), and two MRSA strains (SA 326 and SA 452), were selected for a series of in vitro experiments. All clinical isolates and ATCC 25923 were identified and subjected to antimicrobial susceptibility testing (Table 1).
 |
Table 1 Antimicrobial Susceptibility of Clinical S. aureus Strains Isolated from Patients with DFUs |
Surfactin Inhibits the Growth of MRSA
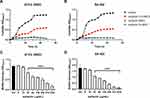
Surfactin showed an antibacterial effect against ATCC 25923 and clinical S. aureus strains isolated from patients with DFUs. The MICs of surfactin against ATCC 25923, SA 095, SA 366, SA 326, and SA 452 were 512, 512, 512, 1024, and 512 μg/mL, respectively. There was no significant difference in MICs between strains with different resistance patterns. The results showed that the MBC values were 1 to 2 times higher than the MIC values. As shown in Figure 1A and B, surfactin inhibited the growth of both ATCC 25923 and SA 452 in a dose-dependent manner during a 24-h exposure period. The growth of two strains was significantly reduced from 4 h when the surfactin concentration was half the MIC. When exposed to MIC and 2×MIC of surfactin, the growth of both strains was almost completely inhibited.
Surfactin Prevents Biofilm Formation of MRSA
Both ATCC 25923 and SA 452 can form strong biofilms, and SA 452 exhibited a higher biofilm-forming ability than ATCC 25923 (OD570 = 1.75 ± 0.24 vs. 1.17 ± 0.05). The concentration of surfactin started to reflect the inhibition of biofilm from 1/16×MIC for both strains, and the inhibition of biofilm was enhanced with an increasing concentration, suggesting that surfactin has a dose-dependent inhibitory effect on S. aureus biofilm formation. Surfactin at a concentration of 2×MIC prevented biofilm formation in more than 95% of SA 452, and 80% inhibition of biofilm formation was achieved for ATCC 25923 (Figure 1C and D).
Surfactin Damages S. aureus Cell Structure without Erythrocytotoxicity
Compared to untreated S. aureus cells, cells treated with 5×MIC surfactin presented a damaged structure, leading to cell lysis and death (Figure 2). However, RBCs hemolysis experiment revealed that hemolytic activity of surfactin (hemolysis > 5%) occurred at 16×MIC, indicating that surfactin had extremely low cytotoxicity to RBCs.
Synergistic Antimicrobial Effects Between Surfactin and Conventional Antibiotics Against MRSA
Checkerboard assays indicated that surfactin showed significantly synergistic effects with ampicillin, oxacillin, gentamicin, and tetracycline against SA 452 with FICIs of 0.133, 0.156, 0.25, and 0.133, respectively. For ATCC 25923, surfactin combined with ampicillin, oxacillin, and tetracycline also exhibited synergistic effects with FICIs of 0.5, 0.25, and 0.188, respectively. Moreover, after combination with surfactin, the MICs of conventional antibiotics against SA 452 and ATCC 25923 decreased 8- to 128-fold and 2- to 16-fold, respectively, except for erythromycin and ciprofloxacin. There was no antagonistic relationship between the tested antibiotics and the surfactin (Table 2). Further penicillinase inhibition experiment showed that surfactin directly inhibited penicillinase activity, but not as much as the clavulanate potassium (Figure 3). When 10,000 U/mL of penicillinase is exposed to 2048, 1024, 512, and 256 μg/mL of surfactin, it requires 7.30, 6.50, 4.00, and 2.25 mL of Na2S2O3 titration, respectively. In contrast, 19.5, 17.0, 14.0, and 11.0 mL of Na2S2O3 were required to titrate to the end point under different concentrations of clavulanate potassium (64, 32, 16, and 8 μg/mL). In addition, the positive control (penicillinase only, no surfactin or clavulanate potassium) required only 0.5 and 0.7 mL of Na2S2O3, respectively.
 |
Table 2 Antimicrobial Activity of the Combination between Surfactin and Conventional Antibiotics Against ATCC 25923 and Clinical MRSA Isolated from Patients with DFUs |
 |
Figure 3 Penicillinase inhibitory activity of surfactin. (A) Inhibition of penicillinase by surfactin and (B) by clavulanate potassium. |
Combination of Surfactin and Oxacillin Narrows the MSW of MRSA
The MPCs of oxacillin alone and in combination with surfactin for ATCC 25923 and SA 452 are listed in Table 3. For ATCC 25923, the MPC of oxacillin used alone was 16 mg/L and the MPC/MIC ratio was 32, while the MPC and MPC/MIC ratio of oxacillin could not be determined for SA 452. However, the MPCs of oxacillin were significantly reduced when in combination with surfactin for both strains, suggesting that the enrichment of S. aureus resistant mutants was limited at a lower concentration. Upon combination with surfactin at 16 (1/32×MIC), 32 (1/16×MIC), and 64 μg/mL (1/8×MIC), the MPCs of oxacillin are reduced to 1, 0.5, and 0.25 μg/mL, respectively. Furthermore, the MPC/MIC ratio of oxacillin is also reduced to 16, 8, and 4 in ATCC25923. Similarly, for SA 452, the MPCs of oxacillin decreased to 16, 8, and 4 μg/mL and the MPC/MIC ratio of oxacillin decreased to 64, 32, and 16 μg/mL, respectively.
 |
Table 3 MPCs of Surfactin and Oxacillin in Combinations with Different Proportions for ATCC 25923 and Clinical MRSA Isolated from Patients with DFUs |
Discussion
DFI indicates an inflammatory process occurring in any tissue below the ankles in patients with diabetes. Almost all DFIs occur in open wounds, and although most are relatively superficial at the presentation, the microorganisms can spread to subcutaneous tissues and even bones, eventually progressing to chronic osteomyelitis and gangrenous lower-extremity amputations.2,26 The etiology of DFI varies by geographic location, disease severity, and patient characteristics (eg, previous antibiotic regimen, recent hospitalization), but more than 65% of DFIs are polymicrobial.27 Appropriate infection control is essential for the treatment of DFUs.26 In this study, we conducted a series of in vitro experiments with the antimicrobial peptide surfactin against S. aureus isolated from clinical patients with DFUs, aiming to evaluate the antibacterial activity of this novel antimicrobial peptide against S. aureus and to explore its potential use for DFI control. Our results suggested that surfactin showed antibacterial and bactericidal activity against S. aureus strains, including non-MDR, MDR, MRSA, and ATCC 25923, in a dose-dependent relationship. This is in agreement with the studies of Liu et al,28 Zhou et al,29 and Gu et al30 MICs varied among studies, probably because surfactin was extracted from different sources and species of Bacillus spp., resulting in slight variations in its biological activities. The efficacy of surfactin in combating Gram-negative bacteria is less than that of Gram-positive bacteria (data not shown), which could be attributed to the distinction in their cellular structures and the action of surfactin.31 It has been found that the special inverted cone molecular structure of surfactin, and the cation it carries, can destabilize the phospholipid structure on the surface of bacterial cell membranes, eventually causing cell membrane disintegration or osmotic pressure imbalance; this is one of the main antibacterial mechanisms of surfactin.32,33 TEM scanning also confirmed that cells treated with 5×MIC surfactin had irregular and collapsed surfaces, content leakage, and cell death when compared to normal S. aureus cells. Moreover, no hemolysis was observed for any of the surfactin concentrations less than 16×MIC, indicating that surfactin has very low cytotoxicity to erythrocytes.
The formation of biofilms on ulcers is a major obstacle in the treatment of DFUs.34 Research has revealed that biofilms are present on more than half of infected DFUs, and that S. aureus is responsible for a quarter of them.35,36 It is widely known that the concentration of antibiotics used to remove biofilms can be 10 to 1,000 times higher than that used to eliminate phytoplankton cells, due to the physical barrier of the biofilm and the interaction between microbial communities.37,38 However, in this study, biofilm formation was significantly inhibited at a concentration of 1/16×MIC, and MRSA biofilm formation was almost completely inhibited at 2×MIC. Liu et al’s study revealed that a one-quarter MIC concentration of surfactin can reduce the biofilm production of S. aureus by 60%, and a concentration of 0.2% surfactin resulted in a biofilm removal rate of 80% after 4 h of treatment.28 One possible reason is that surfactin can inhibit the expression of the S. aureus biofilm-associated gene operon.39 Polysaccharide intercellular adhesin (PIA) is an important component of the S. aureus biofilm structure and is produced by enzymes encoded in the icaADBC locus. Englerová et al revealed that when the surfactin concentration reached 1.5 mg/mL, the expression of the icaA/D/B/C genes was downregulated 2.82-fold, 1.85-fold, 2.25-fold, and 2.37-fold, respectively.39 Meanwhile, Liu et al also demonstrated that a 2×MIC of the surfactin significantly reduced the expression of the icaA and icaD genes.28 Additionally, surfactin was also able to inhibit the expression of sortaseA, the gene encoding the surface adhesion protein anchoring enzyme, and fnbA and fnbB, the genes encoding fibronectin-binding proteins in S. aureus, resulting in impaired biofilm development and reduced biofilm production.40,41
Beta-lactams remain the most essential antibiotic for the treatment of S. aureus infections. Resistance to beta-lactams is primarily due to the bacterially produced beta-lactamases, which hydrolyze the beta-lactam ring, thereby inactivating the drug, and the horizontal acquisition of antimicrobial-resistant genes that weaken their affinity for the antibiotic. In addition, MRSA strains are resistant to methicillin through horizontal acquisition of the mecA gene encoding PBP2a, a transpeptidase with low affinity for most beta-lactam antibiotics and therefore resistant not only to methicillin but to all beta-lactam antibiotics.42 Many AMPs are compatible with being used in a synergistic combination, though the specific mechanism is still not clear. It is widely believed that the enhanced permeability of the bacterial membrane, which is the mechanism of action for most AMPs, facilitates the penetration of antibiotics into the bacterial cells.43,44 Furthermore, AMPs impede the functioning of the antimicrobial-inactivating enzymes, allowing the complementary drug to operate at its full capacity.42 In our study, the combination of surfactin with ampicillin, oxacillin, and tetracycline produced synergistic effects against ATCC 25923 and MRSA, and the addition of at least 64 μg/mL of surfactin converted them to sensitivity when all these antibiotics were resistant alone. A possible explanation is that surfactin can inhibit beta-lactamases and tetracycline destructase enzymes, thus reducing the destruction of the corresponding antibiotics by these enzymes and protecting their antibacterial activity. Notably, although the ability of surfactin to inhibit penicillinase activity was lower than that of clavulanate potassium in this study, it still significantly reduced penicillinase activity, which suggests that the inhibition of penicillinase activity by surfactin may be one of the mechanisms underlying the synergistic antibacterial effects of surfactin with oxacillin and ampicillin against ATCC 25923 and MRSA. Certainly, there are many more possibilities. Some authors have suggested that the synergy must be associated with interaction with specific molecular targets; this requires subsequent experiments to investigate.45,46 In addition, surfactin and oxacillin have distinct molecular structures, so their antibacterial mechanisms and action targets could be completely different. When the two drugs are combined, bacteria must undergo two or more simultaneous resistance mutations, resulting in resistance to both drugs in order to survive. Because of the limitation of their mutation frequency, the emergence of drug-resistant bacteria will be significantly reduced. For ATCC 25923, the MPC/MIC values decreased 8-fold after combination with 64 μg/mL of surfactin compared with oxacillin alone, and for MRSA, there was at least a 2-fold decrease, although exact values were not available. The combination of oxacillin and surfactin narrowed the MSW, suggesting that the combination of them in the clinical setting helps to reduce the production of drug-resistant S. aureus, which is of great importance in controlling the spread of drug resistance.
Conclusion
The natural antimicrobial peptide surfactin effectively inhibited the growth of S. aureus isolated from the wounds of patients with DFUs and ATCC 25923, as well as significantly reducing biofilm production. The combination of surfactin with conventional antibiotics not only presents a synergistic effect, but also converts MRSA to be sensitive to these antibiotics. Moreover, surfactin narrowed the MSW for oxacillin against MRSA, improving the efficiency and safety of antibiotic use. These findings demonstrate that surfactin is likely to be a promising therapeutic option for the control of DFUs caused by S. aureus.
Ethics Approval and Informed Consent
The study protocol was approved by the Institutional Review Board of the Affiliated Hospital of Southwest Medical University. The study was conducted in accordance with the Declaration of Helsinki, and all participants provided written informed consent.
Funding
This study was supported by the Sichuan Province Science and Technology Support Program (2021YFS0329, 2022YFQ0093) and Luxian Government and Southwest Medical University Cooperation Program (2020LXXNYKD-04).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ndosi M, Wright-Hughes A, Brown S, et al. Prognosis of the infected diabetic foot ulcer: a 12-month prospective observational study. Diabet Med. 2018;35(1):78–88.
2. Lipsky BA, Senneville E, Abbas ZG, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3280.
3. Złoch M, Maślak E, Kupczyk W, Jackowski M, Pomastowski P, Buszewski B. Culturomics Approach to Identify Diabetic Foot Infection Bacteria. Int J Mol Sci. 2021;22(17):566.
4. Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis. 2021;21(1):770.
5. Haaber J, Penades JR, Ingmer H. Transfer of Antibiotic Resistance in Staphylococcus aureus. Trends Microbiol. 2017;25(11):893–905.
6. Korting HC, Schollmann C, Stauss-Grabo M, Schafer-Korting M. Antimicrobial peptides and skin: a paradigm of translational medicine. Skin Pharmacol Physiol. 2012;25(6):323–334.
7. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395.
8. Ngambenjawong C, Chan LW, Fleming HE, Bhatia SN. Conditional Antimicrobial Peptide Therapeutics. ACS Nano. 2022;16(10):15779–15791.
9. Bonmatin JM, Laprevote O, Peypoux F. Diversity among microbial cyclic lipopeptides: iturins and surfactins. Activity-structure relationships to design new bioactive agents. Comb Chem High Throughput Screen. 2003;6(6):541–556.
10. Chen X, Lu Y, Shan M, Zhao H, Lu Z, Lu Y. A mini-review: mechanism of antimicrobial action and application of surfactin. World J Microbiol Biotechnol. 2022;38(8):143.
11. Munusamy S, Conde R, Bertrand B, Munoz-Garay C. Biophysical approaches for exploring lipopeptide-lipid interactions. Biochimie. 2020;170:173–202.
12. Penha RO, Vandenberghe LPS, Faulds C, Soccol VT, Soccol CR. Bacillus lipopeptides as powerful pest control agents for a more sustainable and healthy agriculture: recent studies and innovations. Planta. 2020;251(3):70.
13. Zeriouh H, de Vicente A, Perez-Garcia A, Romero D. Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environ Microbiol. 2014;16(7):2196–2211.
14. Abdelli F, Jardak M, Elloumi J, et al. Antibacterial, anti-adherent and cytotoxic activities of surfactin(s) from a lipolytic strain Bacillus safensis F4. Biodegradation. 2019;30(4):287–300.
15. Zouari R, Moalla-Rekik D, Sahnoun Z, Rebai T, Ellouze-Chaabouni S, Ghribi-Aydi D. Evaluation of dermal wound healing and in vitro antioxidant efficiency of Bacillus subtilis SPB1 biosurfactant. Biomed Pharm. 2016;84:878–891.
16. Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175.
17. Li Z, Ding Z, Liu Y, et al. Phenotypic and Genotypic Characteristics of Biofilm Formation in Clinical Isolates of Acinetobacter baumannii. Infect Drug Resist. 2021;14:2613–2624.
18. Stepanović S, Vuković D, Dakić I, Savić B, Švabić-vlahović M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40(2):175–179.
19. Tan P, Lai Z, Jian Q, et al. Design of Heptad Repeat Amphiphiles Based on Database Filtering and Structure-Function Relationships to Combat Drug-Resistant Fungi and Biofilms. ACS Appl Mater Interfaces. 2020;12(2):2129–2144.
20. Doern CD. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol. 2014;52(12):4124–4128.
21. She P, Zhou L, Li S, et al. Synergistic Microbicidal Effect of Auranofin and Antibiotics Against Planktonic and Biofilm-Encased S. aureus. Front Microbiol. 2019;10:2453.
22. Firsov AA, Smirnova MV, Lubenko IY, Vostrov SN, Portnoy YA, Zinner SH. Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to daptomycin and vancomycin in an in vitro dynamic model. J Antimicrob Chemother. 2006;58(6):1185–1192.
23. Zhao X, Drlica K. Restricting the Selection of Antibiotic‐Resistant Mutant Bacteria: measurement and Potential Use of the Mutant Selection Window. J Infect Dis. 2002;185(4):561–565.
24. Jiang L, Xie N, Chen M, et al. Synergistic Combination of Linezolid and Fosfomycin Closing Each Other’s Mutant Selection Window to Prevent Enterococcal Resistance. Front Microbiol. 2020;11:605962.
25. Li T, Li Z, Huang L, et al. Cigarette Smoking and Peripheral Vascular Disease are Associated with Increasing Risk of ESKAPE Pathogen Infection in Diabetic Foot Ulcers. Diabetes Metab Syndr Obes. 2022;15:3271–3283.
26. Pitocco D, Spanu T, Di Leo M, et al. Diabetic foot infections: a comprehensive overview. Eur Rev Med Pharmacol Sci. 2019;23(2 Suppl):26–37.
27. Zubair M, Malik A, Ahmad J. Incidence, risk factors for amputation among patients with diabetic foot ulcer in a North Indian tertiary care hospital. Foot. 2012;22(1):24–30.
28. Liu J, Li W, Zhu X, et al. Surfactin effectively inhibits Staphylococcus aureus adhesion and biofilm formation on surfaces. Appl Microbiol Biotechnol. 2019;103(11):4565–4574.
29. Zhou Z, Liu F, Zhang X, et al. Cellulose-dependent expression and antibacterial characteristics of surfactin from Bacillus subtilis HH2 isolated from the giant panda. PLoS One. 2018;13(1):e0191991.
30. Gu Y, Zheng R, Sun C. Isolation, Identification and Characterization of Two Kinds of Deep-Sea Bacterial Lipopeptides Against Foodborne Pathogens. Front Microbiol. 2022;13:792755.
31. Liston SD, Willis LM. Racing to build a wall: glycoconjugate assembly in Gram-positive and Gram-negative bacteria. Curr Opin Struct Biol. 2021;68:55–65.
32. Mileykovskaya E, Dowhan W. Visualization of Phospholipid Domains in Escherichia coli by Using the Cardiolipin-Specific Fluorescent Dye 10-N-Nonyl Acridine Orange. J Bacteriol. 2000;182(4):1172–1175.
33. Carrillo C, Teruel JA, Aranda FJ, Ortiz A. Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim Biophys Acta. 2003;1611(1–2):91–97.
34. Pouget C, Dunyach-Remy C, Pantel A, Schuldiner S, Sotto A, Lavigne JP. Biofilms in Diabetic Foot Ulcers: significance and Clinical Relevance. Microorganisms. 2020;8(10):1580.
35. Malik A, Mohammad Z, Ahmad J. The diabetic foot infections: biofilms and antimicrobial resistance. Diabetes Metabolic Syndrome. 2013;7(2):101–107.
36. Banu A, Noorul hassan MM, Rajkumar J, Srinivasa S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: a prospective study. Australas Med J. 2015;8(9):280–285.
37. Schilcher K, Horswill AR. Staphylococcal Biofilm Development: structure, Regulation, and Treatment Strategies. Microbiol Mol Biol Rev. 2020;84(3):128.
38. Mottola C, Semedo-Lemsaddek T, Mendes JJ, et al. Molecular typing, virulence traits and antimicrobial resistance of diabetic foot staphylococci. J Biomed Sci. 2016;23:33.
39. Englerova K, Bedlovicova Z, Nemcova R, et al. Bacillus amyloliquefaciens-Derived Lipopeptide Biosurfactants Inhibit Biofilm Formation and Expression of Biofilm-Related Genes of Staphylococcus aureus. Antibiotics. 2021;10(10):1252.
40. Thappeta KRV, Zhao LN, Nge CE, et al. In-Silico Identified New Natural Sortase A Inhibitors Disrupt S. aureus Biofilm Formation. Int J Mol Sci. 2020;21(22):56.
41. Oh KB, Oh MN, Kim JG, Shin DS, Shin J. Inhibition of sortase-mediated Staphylococcus aureus adhesion to fibronectin via fibronectin-binding protein by sortase inhibitors. Appl Microbiol Biotechnol. 2006;70(1):102–106.
42. Vestergaard M, Frees D, Ingmer H. Antibiotic Resistance and the MRSA Problem. Microbiol Spectrum. 2019;7(2):1252.
43. Cassone M, Otvos L Jr. Synergy among antibacterial peptides and between peptides and small-molecule antibiotics. Expert Rev Anti Infect Ther. 2010;8(6):703–716.
44. Ulvatne H, Karoliussen S, Stiberg T, Rekdal O, Svendsen JS. Short antibacterial peptides and erythromycin act synergically against Escherichia coli. J Antimicrob Chemother. 2001;48(2):203–208.
45. Cudic M, Condie BA, Weiner DJ, et al. Development of novel antibacterial peptides that kill resistant isolates. Peptides. 2002;23(12):2071–2083.
46. Cassone M, Vogiatzi P, La Montagna R, et al. Scope and limitations of the designer proline-rich antibacterial peptide dimer, A3-APO, alone or in synergy with conventional antibiotics. Peptides. 2008;29(11):1878–1886.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.