Back to Journals » International Journal of Nanomedicine » Volume 14
Antiangiogenic properties of nanoparticles: a systematic review
Authors Saeed BA, Lim V , Yusof NA, Khor KZ , Rahman HS , Abdul Samad N
Received 1 January 2019
Accepted for publication 16 April 2019
Published 11 July 2019 Volume 2019:14 Pages 5135—5146
DOI https://doi.org/10.2147/IJN.S199974
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Brhaish Ali Saeed,1 Vuanghao Lim,1 Nor Adlin Yusof,1 Kang Zi Khor,1 Heshu Sulaiman Rahman2,3, Nozlena Abdul Samad1
1Integrative Medicine Cluster, Advanced Medical and Dental Institute, SAINS@ Bertam, Universiti Sains Malaysia, Kepala Batas 13200, Pulau Pinang, Malaysia; 2Department of Clinic and Internal Medicine, College of Veterinary Medicine, University of Sulaimani, Sulaymaniyah City 46001, Republic of Iraq; 3Department of Medical Laboratory Sciences, College of Health Sciences, Komar University of Science and Technology, Sarchinar District, Republic of Iraq
Abstract: Nanoparticles appear to be one of the most promising agents that offer efficacy in angiogenesis-related disease therapy. The objective of this research is to systematically review studies that have probed into the effect of nanoparticles on angiogenesis. Selected inclusion criteria were used to extract articles, references that were cited in the initial search were sought to identify more potential articles, and articles that did not meet the inclusion criteria and duplicates were discarded. The spherical shape was shown to be the most common shape employed to investigate the role of nanoparticles in angiogenesis therapy. The size of nanoparticles appears to play a crucial role for efficacy on angiogenesis, in which 20 nm emerged as the preferred size. Gold nanoparticles exhibit the most promise as an antiangiogenesis agent, and the toxicity was adjustable based on the dosages applied.
Keywords: nanoparticles, angiogenesis, antiangiogenesis, size, shape, toxicity
Introduction
Angiogenesis refers to the generation of new blood vessels from a preexisting one.1–3 It plays an essential role in various physiological states like embryo growth, ovulation, and wound healing. Moreover, it is important in the progression of many diseases such as diabetic retinopathy (DR), arthritis, and metastasis.4 In the normal physiological state of the body, the censorious balance between the proangiogenic and antiangiogenic keeps the angiogenesis under the critical control.5 The imbalance between these factors may eventually lead to the development of pathological conditions.6
First hypothesis pertaining to angiogenesis was developed almost four decades ago. It indicates that tumor growth depends on the blood supply, and the prevention of this supply will treat the tumor. Hence, the term anti-angiogenesis means any measures that prevent the formation of new blood vessels and reaching of the blood supply into the tumor.7 The major mechanism of action of antiangiogenic drugs is to attach to growth factors such as vascular endothelial growth factor (VEGF), therefore hindering any attachment of growth factors to their respective receptors such asvascular endothelial growth factor receptor (VEGFR). The attachment between the growth factor and its receptor is the main method that induces the generation of new blood vessels.8 Angiogenesis is considered as an intricate process as it involves proliferation and migration of endothelial cell (EC), permeability, and formation of blood vessels.9 There have been many advances in regard to angiogenesis in the past three decades accompanied by the elucidation of numerous antiangiogenic agents that suppress angiogenesis.10
The monoclonal antibody Bevacizumab (Avastin) is the first antiangiogenic therapy that was licensed by the United States (US) Food and Drug Administration (FDA), as a drug to treat colorectal cancer cells by targeting overexpressed VEGF proteins and reducing blood supply of that cell.10 Nevertheless, the therapy seemed insignificant in some cases due to resistance and other limitations such as limiting pharmacokinetics. Therefore, the encapsulation of these drugs into nanocarriers is promising to overcome some of these drawbacks.11 It is anticipated that unique properties of nanoparticles such asantiangiogenic and drug carrier can be applied as an alternative strategy in the treatment of serious angiogenic diseases such as cancer.8 Many studies are emerging to evaluate the influence of nanomaterial in inhibiting angiogenesis.12 Gold, silver, and silica arethe most important inorganic nanoparticles that show antiangiogenic effects.13
Annually, in the USA, the great funds are exploiting in angiogenesis research and development of antiangiogenesis drugs which is more than 4 billion USD.14 On that account, we systematically review the literature focusing on the usage of nanoparticles as antiangiogenic and the impact of nanoparticle’s properties on antiangiogenic activity.
Methodology
Data sources
Several pertinent databases such as Science Direct, PubMed, Google Scholar, ME DLINE via EBSCO, Scopus, and Springer had been combed through to collect relevant data for this review study.
Inclusion and exclusion criteria
All articles published in peer-reviewed journals that looked into the efficacy of nanoparticle as the antiangiogenic agent had been selected, inclusive of in vivo, in vitro, ex vivo, and review-based studies. Nevertheless, the selected studies were limited to articles published in the English language since the past decade (1 January 2008 to 30 January 2018). As such, case reports, editorial/letters, as well as abstract in symposium and Congress were excluded for further analysis. All the articles were independently reviewed by NMY and NAS.
Search strategy
The strategy employed for collecting articles from PubMed and Medline was carried out by using the Medical Subject Heading (MeSH) terms: nanoparticles and antiangiogenesis or angiogenesis, whereas for other databases search terms or keywords from the title of the topic of interest (antiangiogenic properties of the nanoparticle) had been applied. In addition, the following keywords were used in combination with nanoparticles to retrieve all the related studies: antiangiogenic and angiogenesis. Further information pertaining to the study was obtained by using nanotechnology therapy and angiogenesis therapy in the search process. Subsequently, references that were cited in the initial search were sought to identify more potential articles. The titles and abstracts of these articles were assessed to delete any duplicate data. The articles (titles, abstracts, and full texts) were assessed and screened for inclusion. As such, irrelevant or incompatibility papers were excluded.
Results and discussion
A total number of 218 studies were identified through the literature. Of all 218 studies, 34 studies were duplicated studies. After further screening and evaluating the eligibility of titles, abstracts, and full text, only 22 studies were selected for this study. While other 162 failed to meet the inclusion criteria. The study selection stages and results are presented in the flow chart (Figure 1).
 |
Figure 1 Flowchart of the study selection. |
Effect of nanoparticle’s shape on angiogenesis activities
From the 22 selected studies that included 32 experiments, we found that the spherical shape appeared in 16 experiments, while only 8 experiments had used other type of shapes, and 8 experiments did not report on the shape of nanoparticles (Table 1).
 |
Table 1 Characteristics and antiangiogenic effects of nanoparticles |
The inhibitive effect of silver nanoparticles in a spherical shape on angiogenesis has been demonstrated in numerous studies.15–17 Gold and silica nanoparticles in sphere shape had impacted upon angiogenesis by suppressing this process.12,18
Wierzbicki et al (2013) stated that among pristine carbon nanoparticles, spherical diamond nanoparticles (ND) and multiwall nanotubes nanoparticles (MWNTs) exerted the greatest antiangiogenic properties, while spherical graphite nanoparticles (NG) and sheet-like graphene nanoparticles (GNs) had nil effect. Although both diamond nanoparticles and diamond nanoparticles shared the same shape and size, they had a different effect. Meanwhile, spherical fullerenes nanoparticles (C60) exhibited the opposite effect by increasing blood vessel development.31
Shi et al (2017) discovered that spherical hydroxyapatite nanoparticles (HANPs) with the size of NP 20 displayed higher internalization into human umbilical vein endothelial cells (HUVECs) than the rod-shaped NP 80. They observed that the cellular uptake of nanoparticles is strongly affected by particle size and shape. They also claimed that aside from cellular uptake, the shape is also a determinant of biological outcome.21 Thus, in this review, we found that nanoparticles with spherical shape emerge as the most preferable among other shapes in antiangiogenic studies (Figure 2).
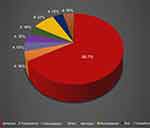 |
Figure 2 Percentage of nanoparticle variety of shapes used in antiangiogenesis studies. The different shapes of nanoparticle used in the 22 studies selected for this review. |
Effect of nanoparticle’s size on angiogenesis activities
The correlation between the size of nanoparticles and their efficacy is elaborately demonstrated in various studies. Arvizo et al (2011) stated that the core size of gold nanoparticles plays an important role in inhibiting the function of vascular endothelial growth factor 165 (VEGF165).20 Nanoparticles of size 20 nm for both gold and silica displayed superiority on VEGF binding in biological media and inhibited angiogenesis when compared to that of size 100 nm.18
Nanoparticles of size 20 nm appeared to be the most effective.19 Gold nanoparticles of size 20 nm showed the greatest effect on the inhibition of angiogenesis when compared to those of sizes 5 nm and 10 nm, especially when they were given at the same concentration. The greatest effect was due to 95% of the protein that was bound to the surface of that 20 nm, as compared to 80% bound to that of the 5 nm.20
Guarneri et al (2014) reported strange results thatdemonstrated that silica as nanoparticles (SiO2NPs) of size 25 nm had no effect on the angiogenic response of ECs even at the highest concentrations (2.5 nM).34 At the same time, other study conducted by Jo et al (2012) found that larger silicate nanoparticles (SiNPs) of size 57 nm effectively inhibited VEGF-induced retinal neovascularization and suppressed ERK 1/2 activation via inhibition of vascular endothelial growth factor receptor 2 (VEGFR-2) phosphorylation.26
HANPs or nano-HAP with 80 nm in size inhibited cell migration, tube formation and nitric oxide (NO) production in HUVECs more than HANPs of size 20 nm.21 Different chemical and physical properties, such as sizes of ultradispersed detonation diamond nanoparticles (UDDs) and microwave-radiofrequency (MW-RF) nanoparticles, showed antiangiogenic effect with different intensities.22
The findings showed that the size of nanoparticles from the selected studies ranges from 4 nm to 100 nm. The size range from 0.0 nm to 20 nm appears more frequent (Figure 3). Nanoparticles sized 20 nm were more common and show to be a potent antiangiogenic agent (Table 1).
 |
Figure 3 Size (nm) of nanoparticles used in antiangiogenesis studies in selected articles. The size distribution of the nanoparticles used in the studies selected for this review. |
The effectiveness of nanoparticles in vitro
The selected studies in this systematic review showed that numerous cell cultures had been applied to investigate the effect of nanoparticles on the formation of new blood vessels, where the cells were cultured in a specific environment so as to better mimic in vivo microenvironment. ECs, namely, HUVECs, human retinal microvascular endothelial cells (HRMECs), bioartificial renal epithelial cells (BRECs), and some cancer cell model cells, were used (Table 1).
In a vitro antiangiogenic study on HUVECs
Song et al (2014) discovered that copper oxide nanoparticles (CO-NPs) displayed the ability to suppress HUVEC proliferation, migration and tube formation in vitro and in dose-dependently upon being treated with varying concentrations (1.25, 2.5, and 5.0 µg/mL) for 24 hrs.9 Silver nanoparticles (AgNPs) also inhibited capillary-like tube formation of HUVEC in a dose-dependent manner, with a 50% inhibitory concentration (IC50) value of 24.97 µg/mL.17 Meanwhile, HANPs treatment especially np 80 decreased HUVEC migration in a dose-dependent manner, in comparison to the control and suppressed tube formation at a concentration of 50 μg/mL.21
Guarnieri et al (2014) demonstrated that silica nanoparticles (SiO2NPs) did not affect the angiogenic response of HUVEC even at the highest concentrations (2,500 pM).34 Whereas in a different study, it was found that the gold nanoparticles (AuNPs) significantly inhibited VEGF-mediated HUVEC proliferation and VEGF induced tube formation in a concentration-dependent manner.23 Additionally, Arvizo et al (2011) revealed that VEGF165-induced proliferation of HUVECs was significantly inhibited by all sizes of graphene nanoplatelets (GNPs) in a concentration-dependent manner.20 Furthermore, nanoceria (NCe) treatment inhibited VEGF-mediated downstream signaling in ECs, which suggested interference with the proliferation and survival of HUVEC.24 In summary, all nanoparticles in selected studies possess antiangiogenic effect in vitro except for SiO2NPs which did not exhibit an antiangiogenic effect on HUVECs although treated in high concentration.
In vitro antiangiogenic study on retinal microvascular ECs
The pathological angiogenesis in the retina is the major cause of vision loss at all ages. This condition leads to the various disorders including retinopathy of prematurity (ROP) in children, DR in young adults, and age-related macular degeneration (AMD) among the elderly.25
Jo et al (2014) demonstrated that titanium dioxide (TiO2) nanoparticles of 130.47 ng/mL had effectively suppressed VEGF-induced tube formation and migration of human retinal microvascular ECs.19 Meanwhile, SiNPs of approximately 5 μg/mL were found to suppress the increment in tube formation and migration induced by VEGF of human retinal microvascular ECs, in comparison to the control values.26
Kim et al (2011) investigated in vitro angiogenesis assays by employing human retinal microvascular ECs, which showed that gold nanoparticles significantly inhibited VEGF-induced proliferation, migration, and capillary-like network formation, when compared to those of the control group.25 Gurunathan et al (2009) revealed that IC50 of Ag-NPs in BRECs was 500 nM that had successfully inhibited VEGF-induced proliferation, migration, and tube formation.16
Overall, several studies have attempted to address the antiangiogenesis properties of nanoparticles in retinal neovascularization. This antiangiogenic effect occurs through the inhibition of proangiogenic factors, namely, VEGF.
In vitro antiangiogenic study in ovarian cancer cells line
NCe-treated human ovarian cancer cell line (A2780), resistant ovarian cancer cell line (C200), and ovarian carcinoma (SKOV3) have possessed the ability to inhibit migration and invasion of ovarian cancer cells without affecting cell proliferation. This type of nanoparticles did not modulate VEGF ovarian cancer cells, but restricted angiogenesis by some other mechanism.24
Antiangiogenic effects of nanoparticle in vivo
The selected studies in systematic review found that nanoparticles suppressed angiogenesis by reducing vascular lumen and inhibiting the formation of new blood vessels, as shown in many in vivo models of these selected studies (Table 1). The literature also portrays several models used to study the effect of antiangiogenic agents; some of the commonly used in vivo models of angiogenesis are chicken embryo chorioallantoic membrane (CAM) assay, rabbit cornea, aortic ring, and the Matrigel implant assay.27
Baharara et al (2014) observed that Ag-NPs exhibited dose-dependent cytotoxic effects on ECs and further inhibited blood vessel formation in the CAM model.15 Meanwhile, the effect of pristine carbon nanoparticles (diamond nanoparticles), multiwalled nanotubes (MWNT), fullerenes nanoparticles (C60), graphite nanoparticles, and graphene nanoparticles on CAM angiogenesis has been studied by Wierzbicki et al (2013) who observed that MWNT and more significant ND displayed antiangiogenic activities. NGs and GNs exerted no activity, whereas C60 exemplified proangiogenic activities.31 The antiangiogenic activity of ND and C60 modified the expression level of kinase insert domain receptor (KDR), but not fibroblast growth factor receptors (FGFRs). Other studies showed that AuNP treatment dramatically decreased the number of branched vessels stimulated by VEGF165, particularly smaller vessels, via chicken embryo CAM assay in a dose-dependent manner.12
Complete suppression of blood vessel formation by zinc oxide nanoparticles coated with biopolymer (Ge-ZnO NPs) (50 μg/mL) was found in chick embryos, whereas gelatin in blood vessels did not show any effect.28 In order to investigate the effect of UDD and MW-RF carbon allotrope nanoparticles on human glioblastoma (U87), cells were cultured on the CAM. As a result, Grodzik et al (2011) observed a decrease in blood vessel area for UDD and MW-RF groups, as compared to the control group.33 The same group also retrieved similar findings upon using diamond nanoparticles at 50 μg/mL, as they reduced the vascular permeability in glioblastoma multiform. By employing the Matrigel model in mice, Song et al (2014) reported that cobalt nanoparticles (CO-NPs) inhibited angiogenesis by reduction of microvessel signals in mice gel plugs, as compared to the control group.9
In the CAM model, gold nanoparticles (Au-DAPHP) or silver nanoparticles (Ag-DAPHP) seemed to exhibit greater antiangiogenesis efficacy at 83% and 76% inhibition, respectively, in comparison to heparin (HP), diaminopyridinyl (DAP)-derivatized heparin (HP) (DAPHP), Ag-glucose, or Au glucose nanoparticles. Nevertheless, as for the mouse Matrigel model, all treatments, including HP, DAPHP, Au/Ag-DAPHP, and Au/Ag-glucose, demonstrated near-maximal and comparable antiangiogenesis activity at 10 μg/Matrigel. This variance in outcome may be due to the varied dose used in CAM and Matrigel models.29
Jo et al (2014) elaborately demonstrated that silicate and titanium dioxide nanoparticles suppressed retinal neovascularization, as demonstrated by the decrease in the number of vascular lumens in the mouse model of C57BL/6 mice, along with being induced by oxygen-induced retinopathy (OIR).19,26 In another study, AuNPs reduced the extent of choroidal neovascularization (CNV) in mice, when compared to the control group.22
Jo et al (2014) also demonstrated that gold and silica at 100 nm nanospheres did not induce any significant change in the area of laser-induced CNV in vivo when compared to the control group, while 20 nm inhibited CNV. They also observed that the higher tendency of large particles to aggregate and agglomerate might attenuate the biological activity in vivo animal experiments.18
The ability of NCe to prevent tumor growth in nude mice when administered even at a very low dose (0.1 mg/kg) appeared to display the novel property of NCe as an antiangiogenic agent.24 The treatment of human hepatocellular carcinoma in a mouse xenograft model by CNP resulted in growth inhibition for both dose- and time-dependent setting.30
The review found that different types of nanoparticles exert a diverse inhibitory effect in vivo. In this respect, spherical shaped carbon nanoparticles showed different effects. For example, C60 with 50 nm size showed a potential angiogenic effect, whereas both NG (4 nm) and GNS (7 nm) did not show a significant effect.. Additionally, spherical silica and gold nanoparticles did not exert an effect at a size of 100 nm in vivo assay.
Mode of action of nanoparticles in inhibiting angiogenesis
The VEGF and FGF are essential promoters of angiogenesis.29 VEGF is a major proangiogenic factor that is vital for the development of the blood vessel network.31 Recently, many types of inorganic nanoparticles have been proven to suppress the formation of new blood vessels by inhibiting VEGF-induced VEGFR2 phosphorylation, and thus deactivation of downstream pathways.9
Jo et al (2014) reported that TiO2 nanoparticles exerted an antiangiogenic effect by suppressing the VEGF/VEGFR2/MAPK pathway without inhibiting the phosphorylation of PI3K/Akt pathway.19 A similar mechanism was portrayed by SiNPs, where Jo et al also observed that SiNPs inhibited VEGF-induced phosphorylation of VEGFR-2 in human retinal microvascular ECs via suppression ERK 1/2 phosphorylation and did not affect AKT phosphorylation.26
Gurunathan et al (2009) demonstrated that the antiangiogenic effect of Ag-NPs was performed by targeting PI3K/Akt the pathway.16 Blocking VEGF-induced Akt phosphorylation with Ag-NPs was also reported by another research group.32 A novel insight highlighted targeting the HIF (hypoxia-inducible factors)-signaling pathway in which HIF-1 induces the gene expression of VEGF-A, while AgNPs inhibit angiogenesisby inhibiting the HIF-1α protein accumulation. , the HIF-1 induces the gene expression of VEGF-A, AgNPs inhibit angiogenesis by inhibiting HIF-1α protein accumulation.17
Kemp et al (2009) stated that Ag or Au nanoparticles conjugated with heparin derivative inhibited FGF-2-induced angiogenesis when compared to the control. Ag or Au can bind to the binding domains of growth factors, such as FGF-2.29 Binding of AuNPs to another growth factor VEGF165 was demonstrated by Pan et al (2014), where the AuNPs inhibited VEGF165-induced HUVEC migration and tube formation by blocking the Akt signaling pathway, as a result of high-affinity binding of AuNPs to the heparin-binding domain of VEGF165, thus preventing the VEGF165–VEGFR-2 interaction.12 Arvizo et al (2011) reported that AuNPs of various sizes suppressed proliferation of HUVECs by binding with VEGF165 and thwarting this growth factor from binding to its extracellular receptor KDR on ECs, thus inhibiting KDR phosphorylation.20 Furthermore, the AuNP exerted its antiangiogenic effect in retinal microvascular ECs by blocking VEGFR-2 autophosphorylation and consequently suppressing of ERK 1/2 activation.25 In addition, the study evaluating the effect of AuNPs on angiogenesis in a specific signaling pathway in HUVECs has been carried out by Kang et al in which they found that AuNPs significantly suppressed VEGF with phosphorylation of ERK1/2, Akt, and FAK, thus inhibited cell proliferation and cell migration .23
Xu et al (2009) suggested that antiangiogenic effect of CNP on HCC leads to a decrease in the levels of VEGFR2 mRNA and protein, but there is a nil effect on VEGF mRNA and protein expression, thus suppressing VEGFR2 expression at both the mRNA and protein levels and leading to blockage of VEGF-induced EC activation.30 Suppressing angiogenesis by inhibiting VEGFR2 expression both at the protein and mRNA level without affecting the expression of VEGF or VEGFR1 also has been the antiangiogenic mechanism of CO-NPs.9
In a study that compared pristine carbon nanoparticles in terms of antiangiogenic properties, Wierzbicki et al (2013) reported that ND may be linked with the inhibition of VEGF receptor KDR expression, but not FGFR protein level, whereas fullerene increased the KDR protein level, thus increasing the occurrence of angiogenesis.31
Grodzik et al (2011) stated that the 2 types of carbon nanoparticles, UDD and MW-RF, exhibited antiangiogenic activities. The UDD nanoparticles performed its activity by reducing FGF-2 and VEGF expression, while MW-RF nanoparticles only reduced VEGF expression, although it exerted a tendency to reduced FGF-2 expression as well.33 The same research group also confirmed that diamond nanoparticles displayed their antiangiogenic effect by decreasing the levels of expressions in VEGF and their receptor, where these diamond nanoparticles can bind with reactive domains of VEGF and VEGF receptor proteins.33 Next, Shi et al (2017) examined the effects of HANPs on ECs, as they discovered that nanoparticles decreased the production of NO in HUVECs as concentration was increased.21 This particular effected can be related to the decrease in p-eNOS expression, while HANPs reduced phosphorylation of Akt. All these verified that HANPs did exert its antiangiogenic effect by inhibiting a PI3K/Akt-dependent eNOS pathway.
A novel antiangiogenic property of NCe that can inhibit VEGF-induced downstream signaling including proliferation, tube formation, and matrix metalloproteinases 2 (MMP2) activation had been investigated. The NCe treatment attenuated VEGF-mediated phosphorylation of VEGFR2, which reduced the phosphorylation of VEGFR2 in HUVECs in response to VEGF165 treatment. Collectively, these findings clearly suggest that NCe does not modulate VEGF in ovarian cancer cells but restricts angiogenesis by some other mechanism.24
Nanoparticles exert its inhibitory effect on angiogenesis by targeting several angiogenic pathways. Excitingly, in this review, we found that different types of carbon nanoparticle-enabled targeting different angiogenesis pathways and exhibited different intensities due to the dissimilarity in physical and chemical properties.
Toxicity effect of nanoparticles and angiogenesis
The studies that had been selected for this systematic review showed that most nanoparticles are safe, although many factors can affect their toxicity.
TiO2 nanoparticles at the concentration of 10 times than presumptive therapeutic concentration (PTC) did not affect the cellular viability of human retinal microvascular ECs, retinoblastoma cell lines, and human brain astrocytes, as well as did not change the histologic integrity and apoptotic activity to the eye of mice when injected locally. 19
Jo et al (2012) observed that SiNPs had no direct cellular toxicity on human retinoblastoma and retinal microvascular ECs, although 10 times the effective therapeutic concentration had been used in vitro, besides, the proliferation of inflammatory cells was not found inthe vitreous and retina. Additionally, a significant difference in the number of apoptotic cell deaths was also not observed in mice that were treated with SiNPs compared to those in the control group.21 At a small size, the SiO2 nanoparticles (25 and 60 nm) did not display any cytotoxic effect after 48 and 96 hrs of incubation. On the other hand, NPs with 100 nm size at lower concentrations (2.5 and 25 PM), did not exert any effect on cell viability, while a slight reduction in cell viability was demonstrated when its concentration was increased (250 and 2,500 PM). 34
GNP and SiO2 NPs did not cause cellular toxicity on HUVECs, human brain astrocytes, or human retinoblastomacells, as well as retinal tissues of mice when an amount 5 times of the therapeutic concentration (83 PM) were injected by those nanoparticles. Therefore, it is safe at the level of cellular viability, apoptotic activity, gene expression (negligible effects) and systemic toxicity.18 When a concentration 5 µM of GNP (5 times of therapeutically effective concentration) was injected into the retina of mice, no retinal toxicity was observed, even at the concentration 10 µM of GNP (10 times of therapeutically effective concentration). Hence, the GNP did not affect the cellular toxicity of human retinal microvascular EC, thus verifying the safe usage of GNP.25
Kang et al (2016) elaborately demonstrated that AuNPs, at a concentration up to 10 µM, did not induce cytotoxic effects on human retinal pigment epithelial (ARPE-19) cells.23 Conjugation of free Au or Ag to glucose or HP improved antiangiogenesis efficacy and increased the safety of these particles when compared to free Au or Ag that exhibited toxic effects alone. The research group found that conjugated Au or Ag at the same dose of lethal free Au or Ag (1–10 μg/CAM) did not induce lethal effects.29 Cytotoxic effect of Ag-NPs on retinal ECs was at concentrations of 500 nM and higher when the viability of BRECs was reduced to 50% of initial through 24 hrs of treatment.16 The biosynthesis of Ag-NPs in using green resources is an approach that is simple, environmentally friendly, cost-effective, and nontoxic.27
The cytotoxicity of AgNPs differed in various cell types as it is influenced by the intracellular physiological conditions, such as pH and redox state, which vary from one type of cell to another, hence giving rise to individual cellular response when exposed to AgNPs. The IC50 value of AgNPs for HUVECs was 24.97 µg/mL, while for human chondrocytes it was 37.35 µg/mL. Moreover, AgNPs have been proven to contribute to cell death through a mechanism of apoptosis.17
As all mice that were treated with CNP did not die and display signs of neurological toxicity or weight loss, thus, oral administration of CNP appears to be safe for the mouse model.30 Astudy conducted by Wierzbicki et al (2013) offers new insights into the bioactive properties of ND and clearly reported that this carbon nanoparticle can be weighed in for use as low-toxicity antiangiogenic therapy.31
No variance was noted for vital physiological functions and tissue cytotoxicity between NCe-treated and untreated mice, which received the dose of 0.1 mg/kg on every third day for 4 weeks, thus emphasizing the safety of NCe.24 CO-NPs could induce apoptosis in HUVECs in a manner that depends on concentration.9
We have found that in almost all included studies, nanoparticles in the mean ranging from 4 nm to 100 nm were safe and their toxicity effects were dose dependent. The synthesis of nanoparticles from biological sources is less toxic than that of the chemical sources. Besides, cytotoxicity of nanoparticles is affected by size, concentration, and surface.
Conclusion
The nanoparticle has been unequivocally shown to be a potent antiangiogenic agent. This systematic review showed that the antiangiogenesis effect of nanoparticles is multifaceted (Figure 4). The type of and physical and chemical properties of nanoparticles such as size and shape have a significant function in antiangiogenesis therapy. The gold nanoparticle is the most promising nanoparticles with antiangiogenesis properties and the biodegradable nanoparticle has portrayed an enhancement in antiangiogenesis activity. Hence, it can be concluded that nanoparticle may ultimately prove useful as a potential preventive and/or therapeutic antiangiogenesis agent.
Future studies recommendations
Future studies should focus on the development of nanoparticles as antiangiogenic agents with more efficacy and less toxicity by exploiting their physical and chemical properties.
Limitation of the studies
Studies are not enough to get a concrete idea of the antiangiogenesis of nanoparticles and also the mechanism behind the antiangiogenesis. But on the basis of the above discussion, it is evident that antiangiogenesis of nanoparticles can be considered as dependent on different kinds of properties such as size, shape, and dose. In addition, different cell lines are used in order to prove the effect of size, shape, and the dose of nanoparticles and all cell lines used do not show the same types of responses.
Acknowledgment
The authors would like to acknowledge Universiti Sains Malaysia (USM) Bridging Grant Number 304/CIPPT/6316487.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gu G, Hu Q, Feng X, et al. PEG-PLA nanoparticles modified with APTEDB peptide for enhanced anti-angiogenic and anti-glioma therapy. Biomaterials. 2014;35(28):8215–8226. doi:10.1016/j.biomaterials.2014.06.022
2. Lu PY, Xie FY, Woodle MC. Modulation of angiogenesis with siRNA inhibitors for novel therapeutics. Trends Mol Med. 2005;11(3):104–113. doi:10.1016/j.molmed.2005.01.005
3. Yu DH, Lu Q, Xie J, Fang C, Chen HZ. Peptide-conjugated biodegradable nanoparticles as a carrier to target paclitaxel to tumor neovasculature. Biomaterials. 2010;31(8):2278–2292. doi:10.1016/j.biomaterials.2009.11.047
4. Kanwar JR, Mahidhara G, Kanwar RK. Antiangiogenic therapy using nanotechnological-based delivery system. Drug Discov Today. 2011;16(5):188–202. doi:10.1016/j.drudis.2011.01.007
5. Behl T, Kotwani A. Possible role of endostatin in the antiangiogenic therapy of diabetic retinopathy. Life Sci. 2015;135:131–137. doi:10.1016/j.lfs.2015.06.017
6. Hsu CC, Chang HM, Lin TC, et al. Corneal neovascularization and contemporary antiangiogenic therapeutics. J Chin Med Assoc. 2015;78(6):323–330. doi:10.1016/j.jcma.2014.10.002
7. Kalishwaralal K, BarathManiKanth S, Pandian SRK, Deepak V, Gurunathan S. Silver nano — A trove for retinal therapies. J Controlled Release. 2010;145(2):76–90. doi:10.1016/j.jconrel.2010.03.022
8. Mukherjee S, Patra C. Therapeutic application of anti-angiogenic nanomaterials in cancers. Nanoscale. 2016;8(25):12444–12470. doi:10.1039/c5nr07887c
9. Song H, Wang W, Zhao P, Qi Z, Zhao S. Cuprous oxide nanoparticles inhibit angiogenesis via down-regulation of VEGFR2 expression. Nanoscale. 2014;6(6):3206–3216. doi:10.1039/c3nr04363k
10. Banerjee D, Harfouche R, Sengupta S. Nanotechnology-mediated targeting of tumor angiogenesis. Vasc Cell. 2011;3:3. doi:10.1186/2045-824X-3-3
11. Sousa F, Cruz A, Fonte P, Pinto IM, Neves-Petersen MT, Sarmento B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci Rep. 2017;7(1):3736. doi:10.1038/s41598-017-03959-4
12. Pan Y, Wu Q, Qin L, Cai J, Du B. Gold nanoparticles inhibit VEGF165-induced migration and tube formation of endothelial cells via the Akt pathway. Biomed Res Int. 2014;2014:1–11.
13. Jo DH, Kim JH, Lee TG, Kim JH. Size, surface charge, and shape determine the therapeutic effects of nanoparticles on the brain and retinal diseases. Nanomedicine. 2015;11(7):1603–1611. doi:10.1016/j.nano.2015.04.015
14. Saghiri MA, Asatourian A, Orangi J, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis—part I: N, Fe, Se, P, Au, and Ca. Crit Rev Oncol Hematol. 2015;96(1):129–142. doi:10.1016/j.critrevonc.2015.05.010
15. Baharara J, Namvar F, Mousavi M, Ramezani T, Mohamad R. Anti-angiogenesis effect of biogenic silver nanoparticles synthesized using saliva officinalis on chick chorioallantoic membrane (CAM). Molecules. 2014;19(9):13498–13508. doi:10.3390/molecules190913498
16. Gurunathan S, Lee K-J, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R, Eom SH. Antiangiogenic properties of silver nanoparticles. Biomaterials. 2009;30(31):6341–6350. doi:10.1016/j.biomaterials.2009.08.008
17. Yang T, Yao Q, Cao F, Liu Q, Liu B, Wang XH. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: insight into the cytotoxicity and antiangiogenesis. Int J Nanomed. 2016;11:6679–6692. doi:10.2147/IJN.S109695
18. Jo DH, Kim JH, Son JG, Piao Y, Lee TG, Kim JH. Inhibitory activity of gold and silica nanospheres to vascular endothelial growth factor (VEGF)-mediated angiogenesis is determined by their sizes. Nano Res. 2014;7(6):844–852. doi:10.1007/s12274-014-0445-8
19. Jo DH, Kim JH, Son JG, et al. Anti-angiogenic effect of bare titanium dioxide nanoparticles on pathologic neovascularization without unbearable toxicity. Nanomed. 2014;10(5):e1109–e1117. doi:10.1016/j.nano.2014.02.007
20. Arvizo RR, Rana S, Miranda OR, Bhattacharya R, Rotello VM, Mukherjee P. Mechanism of the anti-angiogenic property of gold nanoparticles: the role of nanoparticle size and surface charge. Nanomed. 2011;7(5):580–587. doi:10.1016/j.nano.2011.01.011
21. Shi X, Zhou K, Huang F, Wang C. Interaction of hydroxyapatite nanoparticles with endothelial cells: internalization and inhibition of angiogenesis in vitro through the PI3K/Akt pathway. Int J Nanomed. 2017;12:5781–5795. doi:10.2147/IJN.S140179
22. Grodzik M, Sawosz E, Wierzbicki M, et al. Nanoparticles of carbon allotropes inhibit glioblastoma multiforme angiogenesis in ovo. Int J Nanomed. 2011;6:3041–3048.
23. Kang S, Rho C, Cho W, Roh Y. The anti‐angiogenic effects of gold nanoparticles on experimental choroidal neovascularization in mice. Acta Ophthalmol. 2016;94:S256. doi:10.1111/j.1755-3768.2016.0252
24. Giri S, Karakoti A, Graham RP, et al. Nanoceria: a rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancer. PLoS One. 2013;8(1):e54578. doi:10.1371/journal.pone.0054578
25. Kim JH, Kim MH, Jo DH, Yu YS, Lee TG, Kim JH. The inhibition of retinal neovascularization by gold nanoparticles via suppression of VEGFR-2 activation. Biomaterials. 2011;32(7):1865–1871. doi:10.1016/j.biomaterials.2010.11.030
26. Jo DH, Kim JH, Yu YS, Lee TG, Kim JH. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomed. 2012;8(5):784–791. doi:10.1016/j.nano.2011.09.003
27. Baharara J, Namvar F, Ramezani T, Hosseini N, Mohamad R. Green synthesis of silver nanoparticles using Achillea biebersteinii flower extract and its anti-angiogenic properties in the rat aortic ring model. Molecules. 2014;19(4):4624–4634. doi:10.3390/molecules19044624
28. Divya M, Vaseeharan B, Abinaya M, et al. Biopolymer gelatin-coated zinc oxide nanoparticles showed high antibacterial and anti-angiogenic activity. J Photochem Photobiol B. 2018;178:211–218. doi:10.1016/j.jphotobiol.2017.11.008
29. Kemp MM, Kumar A, Mousa S, et al. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology. 2009;20(45):455104. doi:10.1088/0957-4484/20/45/455104
30. Xu Y, Wen Z, Xu Z. Chitosan nanoparticles inhibit the growth of human hepatocellular carcinoma xenografts through an antiangiogenic mechanism. Anticancer Res. 2009;29(12):5103–5109.
31. Wierzbicki M, Sawosz E, Grodzik M, Prasek M, Jaworski S, Chwalibog A. Comparison of anti-angiogenic properties of pristine carbon nanoparticles. Nanoscale Res Lett. 2013;8(1):195. doi:10.1186/1556-276X-8-195
32. Kalishwaralal K, Banumathi E, Pandian SRK, et al. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf B Biointerfaces. 2009;73(1):51–57. doi:10.1016/j.colsurfb.2009.04.025
33. Grodzik M, Sawosz E, Wierzbicki M, et al. VEGF-dependent mechanism of anti-angiogenic action of diamond nanoparticles in glioblastoma multiforme tumor. NSTI-Nanotech. 2012;33:218–221.
34. Guarnieri D, Malvindi MA, Belli V, Pompa PP, Netti P. Effect of silica nanoparticles with variable size and surface functionalization on human endothelial cell viability and angiogenic activity. J Nanopart Res. 2014;16(2):2229. doi:10.1007/s11051-013-2229-6
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.