Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Anti-Lipolysis Induced by Insulin in Diverse Pathophysiologic Conditions of Adipose Tissue
Authors Zhao J, Wu Y, Rong X, Zheng C, Guo J
Received 21 February 2020
Accepted for publication 19 April 2020
Published 11 May 2020 Volume 2020:13 Pages 1575—1585
DOI https://doi.org/10.2147/DMSO.S250699
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Jia Zhao,1 YaYun Wu,1 XiangLu Rong,1– 3 CuiWen Zheng,1 Jiao Guo1– 3
1Guangdong Metabolic Diseases Research Center of Integrated Chinese and Western Medicine, Guangdong Pharmaceutical University, Guangdong, People’s Republic of China; 2Guangdong TCM Key Laboratory for the Prevention and Treatment of Metabolic Diseases, Guangdong, People’s Republic of China; 3Joint Laboratory of Guangdong Province and Hong Kong and Macao Regions on Metabolic Diseases, Guangdong, People’s Republic of China
Correspondence: Jiao Guo
Guangdong Metabolic Disease Research Center of Integrated Chinese and Western Medicine, Guangdong Pharmaceutical University, 280 Waihuan East Road, Guangzhou University City, Panyu District, Guangzhou, Guangdong Province 510006, People’s Republic of China
Tel +86 20 39352818
Email [email protected]
Abstract: As an important energy reservoir, adipose tissue maintains lipid balance and regulates energy metabolism. When the body requires energy, adipocytes provide fatty acids to peripheral tissues through lipolysis. Insulin plays an important role in regulating normal fatty acid levels by inhibiting lipolysis. When the morphology of adipose tissue is abnormal, its microenvironment changes and the lipid metabolic balance is disrupted, which seriously impairs insulin sensitivity. As the most sensitive organ to respond to insulin, lipolysis levels in adipose tissue are affected by impaired insulin function, which results in serious metabolic diseases. However, the specific underlying mechanisms of this process have not yet been fully elucidated, and further study is required. The purpose of this review is to discuss the effects of adipose tissue on the anti-lipolysis process triggered by insulin under different conditions. In particular, the functional changes of this process respond to inconsonantly morphological changes of adipose tissue.
Keywords: adipose tissue, insulin, anti-lipolysis
As an important endocrine organ and energy reservoir, adipose tissue is regulated by various esterases and hormones. Lipolysis, as the primary energy supply pathway, is involved in various metabolic processes, and the fatty acids released by lipolysis are important energy substrates and signal molecules.1 Insulin plays an important role in regulating lipolysis and controlling the lipolysis of adipose tissues. When insulin binds to insulin receptors on the cytomembranes of adipocytes, it reduces the levels of cyclic adenosine phosphate (cAMP) through the phosphatidylinositol kinase-3/protein kinase B (PI3K/AKT) pathway, thereby inhibiting lipolysis.2 At present, studies regarding insulin regulation of lipolysis have not yet established the anti-lipolysis process induced by insulin under distinct metabolic conditions, especially in terms of the functional differences due to morphological changes in adipose tissues. Therefore, it is necessary to further explore the underlying mechanism through which insulin regulates lipolysis when adipose tissues initiate functional changes to seek effective targets for the treatment of metabolic diseases.
Regulation of Lipolysis
Lipolysis is a biochemical pathway for the catabolism and metabolism of triglycerides (TAG) stored in lipid droplets in cells, and primarily occurs in adipocytes. In lipolysis, TAG is hydrolyzed into glycerol and free fatty acids (FFA) via lipases to mobilize stored energy during fasting or exercise. FFAs from the hydrolysis and cleavage of TAG are then used as energy substrates, being essential precursors for lipid and membrane synthesis, or as media in the cell signal transduction process. Therefore, lipolysis plays an important role in maintaining the function of adipose tissue and the energy balance of the body.3
The Basic Process of Lipolysis
During fasting or starvation, lipolysis is activated to increase the concentration of fatty acids and glycerol in serum and meet the energy requirements of other metabolic tissues. Catecholamines trigger lipolysis during fasting. Catecholamine norepinephrine binds to the β-adrenergic receptors of adipocytes. These receptors bind to adenylate cyclase G protein and transmit the signal to adenylate cyclase to produce cAMP. cAMP binds to PKA and stimulates the activation of lipase.4 In addition, when fasting, a lower plasma glucose level stimulates the secretion of glucagon. Glucagon elevates intracellular cAMP levels by increasing the activity of adenylate cyclase, thus, promoting lipolysis.5 The lipolysis process requires the participation of a variety of lipases. When lipases are phosphorylated, they contact the lipid droplets and hydrolyze TAG into diacylglycerol (DAG), monoacylglycerol (MAG), glycerol, and FFAs.
Perilipin1A can be phosphorylated by PKA, which is a key protein regulator of adipose tissue lipolysis, releasing comparative gene Identification-58 (CGI-58) to promote phosphorylated lipase entry into lipid droplets.6–8 CGI-58 can be further phosphorylated by PKA and diffused to the cytoplasm, activating adipose triglyceride lipase (ATGL) to initiate lipolysis.9–12
The first step in lipolysis is ATGL.13–15 ATGL initiates lipolysis by specifically hydrolyzing the ester bond of TAG, but it has little effect on the hydrolysis of other lipids, which is, thus, considered the rate-limiting enzyme in the TAG hydrolysis process.16,17 The reduced expression of ATGL leads to the increased accumulation of TAG in adipose cells and other tissues, which leads to obesity and other metabolic complications.18 However, ATGL overexpression leads to increased lipolysis, fatty acid oxidation, decreased TAG deposition, and decreased adipocyte size.19 In addition, the lipolysis involved by ATGL can promote the production of lipid signaling molecules to positively regulate glucose-stimulated insulin secretion.20
Subsequently, hormone-sensitive lipase (HSL), an intracellular neutral lipase, catalyzes the hydrolysis of DAG to MAG. Haemmerle21 observed that HSL-deficient mice accumulated large amounts of DAG instead of TAG in adipose and other tissues. HSL knockout mice were found to have a lower lipolysis rate and TAG level in vivo as well as reduced FFA release and increased DAG accumulation. In vitro, HSL-deficient fat pads showed that isoproterenol-stimulated FFA release decreased and DAG accumulation and glycerol production in adipocytes were absent, indicating that HSL is a rate-limiting enzyme for DAG catabolism in adipose tissue.22 HSL is strongly regulated by hormones, including catecholamine, ANP, and growth hormones, in which insulin is an important inhibitor of HSL.23 HSL-deficient mice showed low hormone-stimulated lipolysis levels.21 However, HSL-overexpressing mice showed normal basal lipolysis activity but increased excitatory lipolysis.24
Finally, monoglyceride lipase (MGL), which is located in the cytoplasm, plasma membranes, and lipid droplets, catalyzes the hydrolysis of MAG to glycerol and FFAs. MGL belongs to the serine hydrolytic enzyme superfamily25 and is considered the rate-limiting enzyme for MAG hydrolysis.26 Taschler27 found that MGL-deficient mice exhibited reduced diet-induced insulin resistance despite altering the lipolysis levels. MGL is also an important component of the endocannabinoid system, which regulates peripheral lipogenesis.28 After treatment with rosiglitazone, white adipose tissue (WAT) lipolysis in rats increased, as did the mRNA transcriptional level of MGL.29 However, no relevant studies have shown that MGL activity is affected by hormones.
Regulation of Lipolysis
Lipolysis is regulated by various hormones and cytokines. The activity of lipases is strictly regulated by hormones. During fasting, elevated levels of glucocorticoids upregulate the ATGL transcription level.15 Catecholamine promotes the phosphorylation of HSL by binding to the β-adrenoreceptor, increasing cAMP levels, and activating protein kinase A(PKA).30 When refeeding, insulin binds to insulin receptors in adipocytes and reduces cAMP and ultimately inhibits lipolysis by phosphorylation and the activation of PDE3B.31 Additionally, insulin may inhibit the expression of ATGL through FoxO1.32
Local autocrine/paracrine cytokines secreted from adipocytes also regulate lipolysis. For example, tumor necrosis factor-α (TNF-α) induces lipolysis via p44/42 and Jun kinases, while endogenous adenosine produces an antilipolytic effect through the adenosine A1 receptor.33,34 Certain prostaglandin types affect lipolysis to a greater or lesser degree depending on concentration.33 Jaworski35 found that aliphatic specific phospholipase A2 (AdPLA) can regulate the level of prostaglandin PGE2, and the functional loss of AdPLA leads to a decrease in PGE2 levels, thereby increasing the levels of cAMP through PKA-mediated HSL phosphorylation and the activation of lipolysis. In addition, other factors from the peripheral organs or central nervous system regulate lipolysis. NPY is involved in visceral adipose obesity induced by stress: knockdown of the NPY receptor reduces visceral adipose mass. Nicotinic acid inhibits lipolysis through PUMA-G, a Gi/o-coupled seven-transmembrane domain receptor expressed in mouse adipocytes.36
Anti-Lipolysis Effects of Insulin
Insulin plays a crucial role in regulating glucose and lipid metabolisms. Insulin promotes lipid synthesis and storage, reduces plasma FFAs, and inhibits the catabolism of lipids and FFA oxidation. Insulin is the most important hormone that inhibits lipolysis. As early as 1960, in vitro experiments showed that adding glucose and insulin to a culture medium inhibited the release of FFAs in adipocytes, while adrenalin stimulated the release of FFAs, suggesting that the lipolysis of adipocytes negatively responds to insulin.37
Insulin Mechanism of Action Against Lipolysis
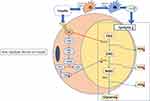
The anti-lipolysis mechanism induced by insulin is relatively well understood. Insulin regulates the glucose uptake of adipocytes and triggers the transport of fatty acid transporters and the FFA uptake of adipocytes.38 Insulin binds to specific membrane insulin receptors to initiate and activate tyrosine phosphorylation, then receptors interact with insulin receptor substrates (IRS-1 and IRS-2) to activate the phosphatidylinositol 3-kinase (PI3K) complex.39 Subsequently, phosphodiesterase 3B (PDE3B) is activated through the PKB/Akt pathway to inhibit basal and catecholamine-induced lipolysis.40,41 Phosphodiesterase catalyzes the decomposition of cAMP, then inactive 5′ -amp, thereby reducing the activation level of PKA, thus, reducing the activity of HSL and inhibiting lipolysis42 (Figure 1).
Scherer43 found that injecting insulin into the middle brain hypothalamus (MBH) of SD rats increased the expression of adipogenic gene-related proteins in WAT, reduced the activity of HSL, and thus inhibited lipolysis. In contrast, mice lacking insulin receptors in neurons showed no significant inhibition of lipolysis and a decrease in adipogenesis. Therefore, it is believed that in the brain, especially in the hypothalamus, insulin maintains the function of WAT.43 In addition, the insulin signaling pathway in POMC neurons controls the lipolysis of adipose tissues.44 In addition, previous studies have shown that cerebral insulin acts primarily on non-subcutaneous adipose tissue to control systemic lipolysis in healthy individuals.45
Recent studies have shown that the α/β-hydrolase domain containing protein 15 (ABHD15) is necessary for anti-lipolysis via insulin in WAT. It has also been found that neither insulin nor glucose treatment can inhibit fatty acid mobilization in ABHD15 knockout mice. Insulin signaling was impaired in ABHD15 knockout adipocytes along with reduced AKT phosphorylation, decreased glucose uptake, and lower adipogenesis. In vitro experiments showed that ABHD15 can bind and stabilize phosphodiesterase 3B (PDE3B); accordingly, PDE3B expression was reduced in the WAT of ABHD15 knockout mice. This explained the lack of decrease in FFA efflux despite increasing protein kinase A(PKA) activity and phosphorylation levels of hormone-sensitive lipase (HSL).46 Insulin may also inhibit lipolysis through the regulatory subunit of phosphorylated protein phosphatase-1 (PP-1), which, once activated, rapidly dephosphorylates and deactivates HSL, thereby reducing the lipolysis rate.47,48
In addition, insulin appears to inhibit lipolysis independently of Akt under certain conditions. Akt2-deficient mice developed glucose intolerance and hyperinsulinemia, but still showed normal serum NEFA and glycerol levels. During the insulin tolerance test (ITT) and hyperinsulin-hyperglycemia clamp test, insulin partially inhibited lipolysis in akt2-deficient mice. Consistent with the in vivo results, insulin antagonized the lipolysis of primary brown adipocytes from Akt2-deficient mice induced by catecholamines. These data suggest that insulin inhibits lipolysis in the absence of Akt2 in hyperinsulinemia conditions.49 Studies have shown that zinc finger protein transcription factor (Snail1) in adipocytes inhibit ATGL expression and lipolysis, while insulin increases the levels of Snail1 in mouse and human adipocytes, thereby regulating lipolysis.50 A recent study found that physiologically low levels of insulin converted CD11c- adipose tissue macrophages (ATMs) into CD11c+ATMS that produce GDF3 and increase lipid accumulation dependent on ALK7 in vivo. In ALK7-intact obese mice, depletion of ATMs by clodronate upregulated lipase activity and reduced fat mass, but in ALK7 deficient mice, the opposite was observed. Meanwhile, ALK7 intact mice showed attenuated effects of insulin on lipolysis and lipid accumulation in vivo after ATMS removal or bone marrow transplantation from mice lacking GDF3, which represents a new mechanism of insulin regulating lipid metabolism.51
In addition, genes encoding various enzymes involved in adipogenesis, such as fatty acid synthetase (FASN), are transcribed and activated by insulin to stimulate adipogenesis.52 Chakrabarti53,54 found that insulin can reduce ATGL transcription through the mTORc1-mediated pathway, thereby inhibiting lipolysis and promoting triglyceride storage. High plasma insulin levels lead to the dephosphorylation of acetyl-CoA carboxylase, thereby promoting acetyl-CoA converting to malonyl-CoA and the conversion of carbohydrates into fatty acids.55–57 Campbell58 found that the re-esterification process of FFAs had marked insulin sensitivity. When increasing insulin concentration, although the absolute rate of FFA re-esterification in adipocytes remains unchanged, the amount of FFAs involved in re-esterification is twice that in the basal state, indicating that insulin can promote FFA re-esterification and indirectly inhibit lipolysis.
Insulin Resistance Results in Abnormal Lipolysis
The abnormal function of insulin leads to unstable systemic lipolysis. In obese individuals, the increase in basal lipolysis is closely related to insulin resistance, but not to BMI or age.48,59 Studies have shown that insulin reduces the outflow of signals from the sympathetic nervous system to WAT, while excessive diets may impair insulin function in the hypothalamus, which may lead to uncontrolled lipolysis in patients with obesity and type 2 diabetes.60 To study the relationship between lipolysis and insulin sensitivity in humans, Langin61 studied 367 subjects and measured spontaneous glycerol release in vitro after overnight fasting. The results showed a positive correlation between glycerol release and the homeostasis model assessment of insulin resistance (HOMA-IR). When adjusting for age, sex, and body mass index, 8% of HOMA-IR variation was still explained by lipolysis, suggesting a relationship between the high lipolysis of adipose tissues and insulin resistance. Meanwhile, insulin tolerance was assessed in 126 subjects after intravenous insulin administration. A negative association was identified between insulin tolerance and lipolysis. Next, lipolysis and HOMA-IR were measured in 25 patients with morbid obesity who had previously undergone bariatric surgery. The results showed that the lower the lipolysis rate, the more significant the improvement in insulin resistance. Previous studies have shown that the production of endogenous glucose and the lipolysis rate of adipose tissue are very sensitive to circulating insulin. For individuals with obesity but normal glucose tolerance, hyperinsulinemia within the normal physiological range can compensate for the insulin resistance of the liver and adipose tissue.62 This indicates that there may be a mutual regulatory relationship between adipose lipolysis and insulin.
Anti-Lipolysis Regulation by Insulin for Morphological Differences in Adipose Tissue
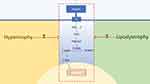
The abnormal morphology of adipose tissue leads to changes in the microenvironment of the tissue, which impairs the function of insulin. The morphology of adipose tissue is positively correlated with lipolysis and inversely correlated with insulin-stimulated adipogenesis.63 At present, studies have found that insulin resistance is not dependent on insulin, but is instead primarily driven by the morphological changes of adipose tissue.64 However, the mechanism through which adipose tissues act on the anti-lipolysis process in insulin has not yet been clarified. The deletion of FSP27, which is related to the formation of lipid droplets in human adipocytes, increases lipolysis and inhibits insulin signal transduction by suppressing the phosphorylation of AKT.65,66 Hypertrophy and lipodystrophy represent two abnormal morphologies of adipose tissue, both resulting in the dysfunction of lipolysis and lipid metabolism. The functional alterations induced by morphological changes in adipose tissue might impair the anti-lipolytic function of insulin directly or indirectly (Figure 2).
Anti-Lipolysis Induced by Insulin in Adipose Hypertrophy
In individuals with obesity, basal lipolysis increases but lipolysis stimulated by catecholamine decreases. Impaired sensitivity to insulin signals in adipocytes may be a cause of the increased basal lipolysis.67 Clinical studies have shown that in adults with obesity, insulin poorly inhibits lipolysis, which may indicate that the hypertrophy of adipose tissue is resistant to insulin anti-lipolysis.68 Clinically, it showed less insulin sensitivity in young adults with obesity accompanied by impaired glucose tolerance, including insufficient insulin action inhibiting lipolysis and lipid oxidation, as well as β-cell dysfunction in lipid and glucose metabolism.69
Wueest70 showed that adipose tissue lipolysis mediated by glycoprotein 130 (gp130) promotes liver steatosis and insulin resistance. Kuang71 found that adipose-specific SIRT6 knockout (FKO) was sensitive to diet-induced obesity, in which adipocyte hypertrophy exists, rather than adipocyte hyperplasia. Specific knockout of SIRT6 in adipose tissues increased the phosphorylation and acetylation of FoxO1, thereby reducing the transcriptional activity of ATGL, leading to reduced lipolysis levels in vivo. The increased inflammation of adipose tissue in FKO mice may also lead to insulin resistance in a high-fat diet. A recent study72 showed that, compared to the adipocytes of lean individuals, anti-lipolysis and adipogenesis activities sensitive to insulin in adipocytes from individuals who are overweight or obese significantly receded. In particular, anti-lipolysis sensitivity correlated with systemic insulin sensitivity. These differences were already evident in the overweight state, were only slightly worse in the unhealthy obese state, and were not related to adipocyte size. The following analysis showed that the epigenetic dysregulation of AKT2 is involved in disturbed adipocyte insulin signaling.
Insulin inhibits lipolysis in adipose tissue through the PI3K/Akt pathway. However, in patients with obesity, the PI3K signaling pathway is inhibited. When insulin/PI3K signaling is blocked, β-cells produce more insulin to maintain normal glucose and lipid levels, but the adipose tissues fail to efficiently respond to insulin, leading to increased circulation levels of FFAs and subsequent obesity-related metabolic diseases.73,74 This indicates that the unhealthy expansion of adipose impairs insulin sensitivity, in which the anti-lipolysis effect of insulin is altered by adipose tissue hypertrophy.
Previous studies have found that TGF-β is related to obesity in humans and mice.75–79 The TGF-β/Smad3 pathway is upregulated in obese adipose tissue, and TGF-β signaling factor Smad3 occupies the promoter of the insulin gene and inhibits insulin gene transcription. In Smad3 knockout mice fed a high-fat diet, it was found that mice showed increased insulin sensitivity without the obesity phenotype, suggesting that the absence of Smad3 enhances insulin sensitivity and prevents diet-induced obesity and insulin resistance.80 These results suggest that, via the inhibition of the TGF-β pathway, upregulating the PI3K/Akt signal is a potential strategy to improve insulin sensitivity in adipose tissue hypertrophy.
Anti-Lipolysis Induced by Insulin in Lipodystrophy
According to previous studies, compared to the adipocytes found in patients with obesity, smaller adipocytes have higher sensitivity to insulin-stimulated glucose uptake, higher glucose oxidation levels, and lower sensitivity to anti-lipolysis.81–83 However, when adipocytes are deficient in lipid storage and catabolism, they are insufficient to maintain homeostasis. Adipocyte-specific insulin receptor gene knockout mice display lipodystrophy with severe insulin resistance, hyperglycemia, organ enlargement, and adipokine secretion disorders, suggesting the indispensable role of insulin signaling in adipose tissue development,84 and that the dysfunction of insulin might also give rise to lipodystrophy.
Lipodystrophy is a rare disease characterized by the selective loss of body fat, although the extent of fat loss varies and can be caused by genetic defects or acquired diseases. Adipocytes of lipodystrophy individuals are generally smaller, and fat loss is proportional to the total adipocyte amount. Insulin resistance, dyslipidemia, hypertension, and diabetes mellitus are often associated with lipodystrophy, and the extent of fat loss determines the extent of the metabolic disease.85
Studies have shown that the abnormal mitochondrial function of white adipose tissue may be related to the secretion of lipid metabolites and lactic acid, which leads to insulin resistance in peripheral tissues.86 However, the correlation between the energy metabolism of adipose tissue and insulin sensitivity remains to be elucidated. It is notable that the double deficiency of Cidea and Cidec activates both WAT and BAT to consume more energy and increase insulin sensitivity.87 Wei88 found that Plin1 knockout mice had reduced lipid accumulation and increased adipose lipid catabolism and fatty acid efflux, thus, impairing insulin sensitivity in adipose tissue, suggesting that Plin1 may be a potential target for regulating insulin function in adipose tissue. In clinical practice, Brown89 regarded lipodystrophy as a human model of leptin deficiency and found that recombinant leptin (Metrelptin) can improve insulin sensitivity, reduce liver steatosis, and lower circulating triglycerides independently of food intake restriction induced by leptin, suggesting that leptin may be a regulatory site of insulin resistance in lipodystrophy. In individuals with obesity, treatment with partial leptin reduction restores hypothalamic leptin sensitivity and leads to reduced food intake, increased energy expenditure, and improved insulin sensitivity.90 However, how leptin affects insulin resistance in the case of lipodystrophy remains unclear.
Lipolysis activity in patients with lipodystrophy is currently ambiguous. The A-ZIP/F-1 “leanness” mouse is a model for studying lipodystrophy that has notable dyslipidemia and insulin resistance and lower levels of lipolysis.91 AP2-SREBP1c overexpressing mice are another model of lipodystrophy that show significant insulin resistance. Overexpression of nSREBP1c in adipose tissue leads to systemic fat loss and severe metabolic syndrome. In a previous study, the mRNA levels of PPARγ, C/EBPα, and some lipoproteins related to adipocyte differentiation were significantly decreased. The mRNA levels of insulin receptor, insulin receptor substrate, and GLUT4, which are related to insulin function, were also significantly decreased.92–94
As an insulin-sensitive organ, the atrophy of WAT impacts the function of insulin, which is largely reflected in the inhibition of insulin’s anti-lipolytic action. In theory, imperfect insulin function leads to increased levels of WAT lipolysis, but in the case of lipodystrophy, lipolysis is more complicated. On the one hand, due to the loss of WAT, fewer adipocytes respond to insulin. On the other hand, as a result of the metabolic disorder caused by lipodystrophy, insulin loses its function in anti-lipolysis, leading to an imbalance in lipolysis that impairs metabolic stability. Therefore, it is necessary to understand the mechanism by which immature adipose tissue impacts insulin function, especially the lipolysis levels, in insulin-resistant adipose tissue in the lipodystrophic state.
Discussion
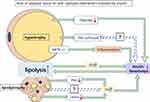
Obesity and lipodystrophy seem to be opposing diseases, yet both share similar pathological features, including insulin resistance, hepatic steatosis, and unstable adipokine levels.95 These pathological differences might in turn alter the morphology and function of adipose tissue, leading to abnormal FFA levels, impairing normal lipid metabolism. As mentioned above, excess circulating FFA may accumulate in insulin-sensitive tissues, impairing insulin sensitivity. Increased basal lipolysis may also alter the microenvironment of adipose tissue and worsen systemic insulin sensitivity. Meanwhile, excessive release of FFAs may increase inflammation in adipose tissue, leading to insulin resistance (Figure 3).
As previously discussed, lipolysis levels in adipose tissue are theoretically elevated in insulin resistance due to the inability of insulin to function properly against lipolysis, but this does not appear to be the case. Relevant in vitro studies have shown that insulin has a biphasic effect on lipolysis,96,97 indicating that the effect of insulin on lipolysis is equivalent to a superposition of synergistic and inhibitory effects, all of which are related to the synthesis of new proteins and the activation of cAMP.98 In a recent study, mature adipocytes collected from adult women with and without diabetes reached the same conclusion.99 However, these conclusions are based on in vitro studies and lack corresponding in vivo studies. This suggests that insulin regulates anti-lipolysis depending on adipose tissue state through a specific pathway that is not yet fully understood.
Metabolic diseases, including obesity and diabetes, seriously affect human health. Insulin resistance, as the most important pathological process of metabolic diseases, is needed for effective control. In general, insulin resistance leads to increased levels of lipolysis, and improvement in insulin sensitivity inhibits excessive lipolysis in adipose tissue. Currently, insulin sensitizers, such as TZDs and metformin, are widely used in the treatment of insulin resistance. Studies have shown that biguanides and thiazolidinediones inhibit stimulated lipolysis in human adipocytes through the activation of AMP-activated protein kinase.100 TZDs may attenuate lipolysis and FFA efflux by activating Akt signaling to decrease cAMP levels, thereby reducing lipase activity in adipocytes.101 The PPARγ receptor agonist troglitazone increases insulin sensitivity in visceral adipocytes, increases fat mass, and interferes with Beta-3-triggered lipolysis.102 Pioglitazone can mitigate glucocorticoid receptor-induced excessive lipolysis in adipocytes.103 Clinically, in well-controlled T2DM patients, whole body lipolysis is insulin resistant, pioglitazone improves the insulin sensitivity of lipolysis,104 and thiazolidinediones can modify GH-induced insulin resistance by suppressing lipolysis.105 Metformin inhibits lipolysis by preventing PKA/HSL activation by decreasing the accumulation of cAMP by preserving PDE3B.106 It has also been found that metformin decreases cellular cAMP production, reduces the activities of PKA and MAPK1/3, and attenuates the phosphorylation of perilipin during isoproterenol-stimulated lipolysis.107 These studies have shown that insulin sensitizers effectively inhibit excessive lipolysis by stimulating the insulin signaling pathway.
Obesity and lipodystrophy are two different abnormal morphologies of adipose tissue that both affect insulin function. Recent studies have confirmed that improving lipolysis in adipose tissue can enhance insulin sensitivity, but the mechanism is not presently clear, especially in the two extreme states. Preclinical studies have shown that inhibiting lipolysis may be an effective treatment strategy for patients with obesity or early stages of diabetes.108,109 However, at present, there is no effective inhibitor applicable to the human body. Therefore, identifying targets acting on the insulin anti-lipolysis pathway (to improve insulin sensitivity by regulating lipolysis levels) to effectively rescue morphological and functional conversion will provide a new direction for our future research regarding the treatment of insulin resistance.
Acknowledgments
This study was sponsored by the National Natural Science Foundation of China (No: 81274156), the Natural Science Foundation of Guangdong Province (No: 2015A030311033), and the Science and Technology Planning Project of Guangdong Province (No: 2017B050504005).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Kalderon B, Mayorek N, Berry E, Zevit N, Bar-Tana J. Fatty acid cycling in the fasting rat. Am J Physiol Endocrinol Metab. 2000;279(1):E221–E227. doi:10.1152/ajpendo.2000.279.1.E221
2. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–2223. doi:10.1152/physrev.00063.2017
3. Coleman RA, Mashek DG. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem Rev. 2011;111(10):6359–6386. doi:10.1021/cr100404w
4. Kim C, Xuong NH, Taylor SS. Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science. 2005;307(5710):690–696. doi:10.1126/science.1104607
5. Perea A, Clemente F, Martinell J, Villanueva-Penacarrillo ML, Valverde I. Physiological effect of glucagon in human isolated adipocytes. Horm Metab Res. 1995;27(8):372–375. doi:10.1055/s-2007-979981
6. Miyoshi H, Souza SC, Zhang HH, et al. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem. 2006;281(23):15837–15844. doi:10.1074/jbc.M601097200
7. Martinez-Botas J, Anderson JB, Tessier D, et al. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet. 2000;26(4):474–479. doi:10.1038/82630
8. Miyoshi H, Souza SC, Endo M, et al. Perilipin overexpression in mice protects against diet-induced obesity. J Lipid Res. 2010;51(5):975–982. doi:10.1194/jlr.M002352
9. Itabe H, Yamaguchi T, Nimura S, Sasabe N. Perilipins: a diversity of intracellular lipid droplet proteins. Lipids Health Dis. 2017;16(1):83. doi:10.1186/s12944-017-0473-y
10. Sahu-Osen A, Montero-Moran G, Schittmayer M, et al. CGI-58/ABHD5 is phosphorylated on Ser239 by protein kinase A: control of subcellular localization. J Lipid Res. 2015;56(1):109–121. doi:10.1194/jlr.M055004
11. Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab. 2009;297(2):E289–E296. doi:10.1152/ajpendo.00099.2009
12. Lass A, Zimmermann R, Haemmerle G, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in chanarin-dorfman syndrome. Cell Metab. 2006;3(5):309–319. doi:10.1016/j.cmet.2006.03.005
13. Zimmermann R, Strauss JG, Haemmerle G, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386. doi:10.1126/science.1100747
14. Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A 2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279(47):48968–48975. doi:10.1074/jbc.M407841200
15. Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279(45):47066–47075. doi:10.1074/jbc.M403855200
16. Wilson PA, Gardner SD, Lambie NM, Commans SA, Crowther DJ. Characterization of the human patatin-like phospholipase family. J Lipid Res. 2006;47(9):1940–1949. doi:10.1194/jlr.M600185-JLR200
17. Girousse A, Langin D. Adipocyte lipases and lipid droplet-associated proteins: insight from transgenic mouse models. Int J Obes (Lond). 2012;36(4):581–594. doi:10.1038/ijo.2011.113
18. Bezaire V, Langin D. Regulation of adipose tissue lipolysis revisited. Proc Nutr Soc. 2009;68(4):350–360. doi:10.1017/S0029665109990279
19. Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006;7(1):106–113. doi:10.1038/sj.embor.7400559
20. Tang T, Abbott MJ, Ahmadian M, Lopes AB, Wang Y, Sul HS. Desnutrin/ATGL activates PPARdelta to promote mitochondrial function for insulin secretion in islet beta cells. Cell Metab. 2013;18(6):883–895. doi:10.1016/j.cmet.2013.10.012
21. Haemmerle G, Zimmermann R, Hayn M, et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277(7):4806–4815. doi:10.1074/jbc.M110355200
22. Wang SP, Laurin N, Himms-Hagen J, et al. The adipose tissue phenotype of hormone-sensitive lipase deficiency in mice. Obes Res. 2001;9(2):119–128. doi:10.1038/oby.2001.15
23. Zechner R, Zimmermann R, Eichmann TO, et al. FAT SIGNALS–lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15(3):279–291. doi:10.1016/j.cmet.2011.12.018
24. Lucas S, Tavernier G, Tiraby C, Mairal A, Langin D. Expression of human hormone-sensitive lipase in white adipose tissue of transgenic mice increases lipase activity but does not enhance in vitro lipolysis. J Lipid Res. 2003;44(1):154–163. doi:10.1194/jlr.M200250-JLR200
25. Vaughan M. The production and release of glycerol by adipose tissue incubated in vitro. J Biol Chem. 1962;237:3354–3358.
26. Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272(43):27218–27223. doi:10.1074/jbc.272.43.27218
27. Taschler U, Radner FP, Heier C, et al. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem. 2011;286(20):17467–17477. doi:10.1074/jbc.M110.215434
28. Cota D, Marsicano G, Tschop M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112(3):423–431. doi:10.1172/JCI17725
29. Festuccia WT, Laplante M, Berthiaume M, Gelinas Y, Deshaies Y. PPARγ agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia. 2006;49(10):2427–2436. doi:10.1007/s00125-006-0336-y
30. Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48(12):2547–2559. doi:10.1194/jlr.R700014-JLR200
31. Choi YH, Park S, Hockman S, et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest. 2006;116(12):3240–3251. doi:10.1172/JCI24867
32. Chakrabarti P, Kandror KV. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J Biol Chem. 2009;284(20):13296–13300. doi:10.1074/jbc.C800241200
33. Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G1–G4. doi:10.1152/ajpgi.00554.2006
34. Dhalla AK, Chisholm JW, Reaven GM, Belardinelli L. A1 adenosine receptor: role in diabetes and obesity. Handb Exp Pharmacol. 2009;193:271–295.
35. Jaworski K, Ahmadian M, Duncan RE, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15(2):159–168. doi:10.1038/nm.1904
36. Lafontan M. Advances in adipose tissue metabolism. Int J Obes (Lond). 2008;32(Suppl 7):S39–S51. doi:10.1038/ijo.2008.237
37. Engel F, WhIte J. Some hormonal influences on fat mobilization from adipose tissue. Am J Clin Nutr. 1960;8(5):691–704. doi:10.1093/ajcn/8.5.691
38. Lafontan M. Fat cells: afferent and efferent messages define new approaches to treat obesity. Annu Rev Pharmacol Toxicol. 2005;45:119–146. doi:10.1146/annurev.pharmtox.45.120403.095843
39. Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19(4):471–482. doi:10.1016/j.beem.2005.07.004
40. Choi SM, Tucker DF, Gross DN, et al. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol. 2010;30(21):5009–5020. doi:10.1128/MCB.00797-10
41. DiPilato LM, Ahmad F, Harms M, Seale P, Manganiello V, Birnbaum MJ. The role of PDE3B phosphorylation in the inhibition of lipolysis by insulin. Mol Cell Biol. 2015;35(16):2752–2760. doi:10.1128/MCB.00422-15
42. Mei J, Holst LS, Landstrom TR, et al. C(2)-ceramide influences the expression and insulin-mediated regulation of cyclic nucleotide phosphodiesterase 3B and lipolysis in 3T3-L1 adipocytes. Diabetes. 2002;51(3):631–637. doi:10.2337/diabetes.51.3.631
43. Scherer T, O’Hare J, Diggs-Andrews K, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13(2):183–194. doi:10.1016/j.cmet.2011.01.008
44. Shin AC, Filatova N, Lindtner C, et al. Insulin receptor signaling in POMC, but not AgRP, neurons controls adipose tissue insulin action. Diabetes. 2017;66(6):1560–1571. doi:10.2337/db16-1238
45. Iwen KA, Scherer T, Heni M, et al. Intranasal insulin suppresses systemic but not subcutaneous lipolysis in healthy humans. J Clin Endocrinol Metab. 2014;99(2):E246–E251. doi:10.1210/jc.2013-3169
46. Xia W, Pessentheiner AR, Hofer DC, et al. Loss of ABHD15 impairs the anti-lipolytic action of insulin by altering PDE3B stability and contributes to insulin resistance. Cell Rep. 2018;23(7):1948–1961. doi:10.1016/j.celrep.2018.04.055
47. Ragolia L, Begum N. Protein phosphatase-1 and insulin action. Mol Cell Biochem. 1998;182(1–2):49–58. doi:10.1023/A:1006827227162
48. Geetha T, Langlais P, Caruso M, Yi Z. Protein phosphatase 1 regulatory subunit 12A and catalytic subunit delta, new members in the phosphatidylinositide 3 kinase insulin-signaling pathway. J Endocrinol. 2012;214(3):437–443. doi:10.1530/JOE-12-0145
49. Koren S, DiPilato LM, Emmett MJ, et al. The role of mouse Akt2 in insulin-dependent suppression of adipocyte lipolysis in vivo. Diabetologia. 2015;58(5):1063–1070. doi:10.1007/s00125-015-3532-9
50. Sun C, Jiang L, Liu Y, et al. Adipose snail1 regulates lipolysis and lipid partitioning by suppressing adipose triacylglycerol lipase expression. Cell Rep. 2016;17(8):2015–2027. doi:10.1016/j.celrep.2016.10.070
51. Bu Y, Okunishi K, Yogosawa S, et al. Insulin regulates lipolysis and fat mass by upregulating growth/differentiation factor 3 in adipose tissue macrophages. Diabetes. 2018;67(9):1761–1772.
52. Viscarra JA, Wang Y, Hong IH, Sul HS. Transcriptional activation of lipogenesis by insulin requires phosphorylation of MED17 by CK2. Sci Signaling. 2017;10(467):eaai8596.
53. Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 2010;59(4):775–781. doi:10.2337/db09-1602
54. Chakrabarti P, Kim JY, Singh M, et al. Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1-Egr1-ATGL-mediated pathway. Mol Cell Biol. 2013;33(18):3659–3666. doi:10.1128/MCB.01584-12
55. Bhathena SJ, Avigan J, Schreiner ME. Effect of insulin on sterol and fatty acid synthesis and hydroxymethylglutaryl CoA reductase activity in mammalian cells grown in culture. Proc Natl Acad Sci U S A. 1974;71(6):2174–2178. doi:10.1073/pnas.71.6.2174
56. Stansbie D, Brownsey RW, Crettaz M, Denton RM. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976;160(2):413–416. doi:10.1042/bj1600413
57. Holland R, Hardie DG. Both insulin and epidermal growth factor stimulate fatty acid synthesis and increase phosphorylation of acetyl-CoA carboxylase and ATP-citrate lyase in isolated hepatocytes. FEBS Lett. 1985;181(2):308–312. doi:10.1016/0014-5793(85)80282-9
58. Campbell PJ, Carlson MG, Hill JO, Nurjhan N. Regulation of free fatty acid metabolism by insulin in humans: role of lipolysis and reesterification. Am J Physiol. 1992;263(6):E1063–E1069. doi:10.1152/ajpendo.2006.263.6.E1063
59. Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48(5):275–297. doi:10.1016/j.plipres.2009.05.001
60. Scherer T, Lindtner C, Zielinski E, O’Hare J, Filatova N, Buettner C. Short term voluntary overfeeding disrupts brain insulin control of adipose tissue lipolysis. J Biol Chem. 2012;287(39):33061–33069. doi:10.1074/jbc.M111.307348
61. Girousse A, Tavernier G, Valle C, et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol. 2013;11(2):e1001485. doi:10.1371/journal.pbio.1001485
62. Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care. 2012;35(6):1316–1321. doi:10.2337/dc11-1951
63. Dahlman I, Ryden M, Arner P. Family history of diabetes is associated with enhanced adipose lipolysis: evidence for the implication of epigenetic factors. Diabetes Metab. 2018;44(2):155–159. doi:10.1016/j.diabet.2017.10.010
64. Ryden M, Hrydziuszko O, Mileti E, et al. The adipose transcriptional response to insulin is determined by obesity, not insulin sensitivity. Cell Rep. 2016;16(9):2317–2326. doi:10.1016/j.celrep.2016.07.070
65. Wu L, Xu D, Zhou L, et al. Rab8a-AS160-MSS4 regulatory circuit controls lipid droplet fusion and growth. Dev Cell. 2014;30(4):378–393. doi:10.1016/j.devcel.2014.07.005
66. Grahn TH, Kaur R, Yin J, et al. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J Biol Chem. 2014;289(17):12029–12039. doi:10.1074/jbc.M113.539890
67. Langin D, Dicker A, Tavernier G, et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes. 2005;54(11):3190–3197. doi:10.2337/diabetes.54.11.3190
68. Yan H, Pierce JR, Myers KB, et al. Exercise effects on adipose tissue postprandial lipolysis and blood flow in children. Med Sci Sports Exerc. 2018;50(6):1249–1257. doi:10.1249/MSS.0000000000001566
69. Kim JY, Nasr A, Tfayli H, Bacha F, Michaliszyn SF, Arslanian S. Increased lipolysis, diminished adipose tissue insulin sensitivity, and impaired beta-cell function relative to adipose tissue insulin sensitivity in obese youth with impaired glucose tolerance. Diabetes. 2017;66(12):3085–3090. doi:10.2337/db17-0551
70. Wueest S, Item F, Lucchini FC, et al. Mesenteric fat lipolysis mediates obesity-associated hepatic steatosis and insulin resistance. Diabetes. 2016;65(1):140–148. doi:10.2337/db15-0941
71. Kuang J, Zhang Y, Liu Q, et al. Fat-specific Sirt6 ablation sensitizes mice to high-fat diet-induced obesity and insulin resistance by inhibiting lipolysis. Diabetes. 2017;66(5):1159–1171. doi:10.2337/db16-1225
72. Ryden M, Petrus P, Andersson DP, et al. Insulin action is severely impaired in adipocytes of apparently healthy overweight and obese subjects. J Intern Med. 2019;285(5):578–588.
73. Zick Y. Insulin resistance: a phosphorylation-based uncoupling of insulin signaling. Trends Cell Biol. 2001;11(11):437–441. doi:10.1016/S0962-8924(01)02129-8
74. Zick Y. Role of Ser/Thr kinases in the uncoupling of insulin signaling. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S56–S60. doi:10.1038/sj.ijo.0802503
75. Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3(1):37–48. doi:10.1007/BF03401666
76. Samad F, Uysal KT, Wiesbrock SM, Pandey M, Hotamisligil GS, Loskutoff DJ. Tumor necrosis factor alpha is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc Natl Acad Sci U S A. 1999;96(12):6902–6907. doi:10.1073/pnas.96.12.6902
77. Alessi MC, Bastelica D, Morange P, et al. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes. 2000;49(8):1374–1380. doi:10.2337/diabetes.49.8.1374
78. Fain JN, Tichansky DS, Madan AK. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism. 2005;54(11):1546–1551. doi:10.1016/j.metabol.2005.05.024
79. Lin Y, Nakachi K, Ito Y, et al. Variations in serum transforming growth factor-beta1 levels with gender, age and lifestyle factors of healthy Japanese adults. Dis Markers. 2009;27(1):23–28. doi:10.1155/2009/529253
80. Yadav H, Quijano C, Kamaraju AK, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14(1):67–79. doi:10.1016/j.cmet.2011.04.013
81. Jacobsson B, Smith U. Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells. J Lipid Res. 1972;13(5):651–656.
82. Olefsky JM. Effects of fasting on insulin binding, glucose transport, and glucose oxidation in isolated rat adipocytes: relationships between insulin receptors and insulin action. J Clin Invest. 1976;58(6):1450–1460. doi:10.1172/JCI108601
83. Smith U. Effect of cell size on lipid synthesis by human adipose tissue in vitro. J Lipid Res. 1971;12(1):65–70.
84. Qiang G, Whang Kong H, Xu S, et al. Lipodystrophy and severe metabolic dysfunction in mice with adipose tissue-specific insulin receptor ablation. Mol Metab. 2016;5(7):480–490. doi:10.1016/j.molmet.2016.05.005
85. Garg A, Agarwal AK. Lipodystrophies: disorders of adipose tissue biology. Biochim Biophys Acta. 2009;1791(6):507–513. doi:10.1016/j.bbalip.2008.12.014
86. Bodis K, Roden M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur J Clin Invest. 2018;48(11):e13017.
87. Zhou L, Yu M, Arshad M, et al. Coordination among lipid droplets, peroxisomes, and mitochondria regulates energy expenditure through the CIDE-ATGL-PPARalpha pathway in adipocytes. Diabetes. 2018;67(10):1935–1948. doi:10.2337/db17-1452
88. Wei S, Liu S, Su X, et al. Spontaneous development of hepatosteatosis in perilipin-1 null mice with adipose tissue dysfunction. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(2):212–218. doi:10.1016/j.bbalip.2017.11.007
89. Brown RJ, Valencia A, Startzell M, et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504–3516. doi:10.1172/JCI95476
90. Zhao S, Zhu Y, Schultz RD, et al. Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab. 2019;30(4):706–719 e706. doi:10.1016/j.cmet.2019.08.005
91. Gavrilova O, Marcus-Samuels B, Reitman ML. Lack of responses to a beta3-adrenergic agonist in lipoatrophic A-ZIP/F-1 mice. Diabetes. 2000;49(11):1910–1916. doi:10.2337/diabetes.49.11.1910
92. Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10(9):1096–1107. doi:10.1101/gad.10.9.1096
93. Shimomura I, Hammer RE, Richardson JA, et al. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12(20):3182–3194. doi:10.1101/gad.12.20.3182
94. Li FX, Li JL, Rong XL, et al. Study on mouse model characterized by insulin resistance- fatty liver and hypercholesteremia induced by lipodystrophy. Tradit Chin Drug Res Clin Pharmacol. 2014;25(1):96–101.
95. Vegiopoulos A, Rohm M, Herzig S. Adipose tissue: between the extremes. EMBO J. 2017;36(14):1999–2017. doi:10.15252/embj.201696206
96. Desai KS, Li KC, Angel A. Bimodal effect of insulin on hormone-stimulated lipolysis: relation to intracellular 3ʹ,5ʹ-cyclic adenylic acid and free fatty acid levels. J Lipid Res. 1973;14(6):647–655.
97. Mooney RA, Ebersohl RD, McDonald JM. Insulin-mediated antilipolysis in permeabilized rat adipocytes. J Biol Chem. 1984;259(12):7701–7704.
98. Lavis VR, Williams RH. Lipolytic effects of high concentrations of insulin on isolated fat cells. Enhancement of the response to lipolytic hormones. Diabetes. 1973;22(8):629–636. doi:10.2337/diab.22.8.629
99. Strålfors P, Jönsson C, Batista A, Kjölhede P. Insulin and β-adrenergic receptors mediate lipolytic and anti-lipolytic signalling that is not altered by type 2 diabetes in human adipocytes. Biochem J. 2019;476(19):2883–2908. doi:10.1042/BCJ20190594
100. Bourron O, Daval M, Hainault I, et al. Biguanides and thiazolidinediones inhibit stimulated lipolysis in human adipocytes through activation of AMP-activated protein kinase. Diabetologia. 2010;53(4):768–778. doi:10.1007/s00125-009-1639-6
101. He J, Xu C, Kuang J, et al. Thiazolidinediones attenuate lipolysis and ameliorate dexamethasone-induced insulin resistance. Metabolism. 2015;64(7):826–836. doi:10.1016/j.metabol.2015.02.005
102. Hodis J, Vaclavíková R, Farghali H. Beta-3 agonist-induced lipolysis and nitric oxide production: relationship to PPARgamma agonist/antagonist and AMP kinase modulation. Gen Physiol Biophys. 2011;30(1):90–99. doi:10.4149/gpb_2011_01_90
103. Hasan AU, Ohmori K, Hashimoto T, et al. PPARγ activation mitigates glucocorticoid receptor-induced excessive lipolysis in adipocytes via homeostatic crosstalk. J Cell Biochem. 2018;119(6):4627–4635. doi:10.1002/jcb.26631
104. Gastaldelli A, Casolaro A, Ciociaro D, et al. Decreased whole body lipolysis as a mechanism of the lipid-lowering effect of pioglitazone in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2009;297(1):E225–E230. doi:10.1152/ajpendo.90960.2008
105. Krag MB, Nielsen S, Guo Z, et al. Peroxisome proliferator-activated receptor gamma agonism modifies the effects of growth hormone on lipolysis and insulin sensitivity. Clin Endocrinol (Oxf). 2008;69(3):452–461. doi:10.1111/j.1365-2265.2008.03231.x
106. Zhao W, Li A, Feng X, et al. Metformin and resveratrol ameliorate muscle insulin resistance through preventing lipolysis and inflammation in hypoxic adipose tissue. Cell Signal. 2016;28(9):1401–1411. doi:10.1016/j.cellsig.2016.06.018
107. Zhang T, He J, Xu C, et al. Mechanisms of metformin inhibiting lipolytic response to isoproterenol in primary rat adipocytes. J Mol Endocrinol. 2009;42(1):57–66. doi:10.1677/JME-08-0130
108. Arner P, Andersson DP, Backdahl J, Dahlman I, Ryden M. Weight gain and impaired glucose metabolism in women are predicted by inefficient subcutaneous fat cell lipolysis. Cell Metab. 2018;28(1):45–54.e43. doi:10.1016/j.cmet.2018.05.004
109. Mayer N, Schweiger M, Romauch M, et al. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat Chem Biol. 2013;9(12):785–787. doi:10.1038/nchembio.1359
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.