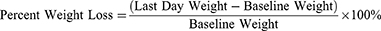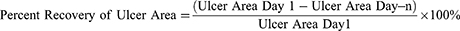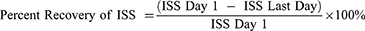Back to Journals » Journal of Inflammation Research » Volume 15
Anti-Inflammatory Activity and Wound Healing Effect of Kaempferia galanga L. Rhizome on the Chemical-Induced Oral Mucosal Ulcer in Wistar Rats
Authors Wahyuni IS , Sufiawati I , Nittayananta W, Levita J
Received 26 January 2022
Accepted for publication 17 March 2022
Published 8 April 2022 Volume 2022:15 Pages 2281—2294
DOI https://doi.org/10.2147/JIR.S359042
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Adam D Bachstetter
Indah Suasani Wahyuni,1,2 Irna Sufiawati,2 Wipawee Nittayananta,3 Jutti Levita1,4
1Doctoral Program in Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 2Department of Oral Medicine, Faculty of Dentistry, Universitas Padjadjaran, Bandung, Indonesia; 3Faculty of Dentistry, Thammasat University, Pathum Thani, Thailand; 4Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, West Java, Indonesia
Correspondence: Indah Suasani Wahyuni, Faculty of Pharmacy, Universitas Padjadjaran, Jl. Raya Bandung-Sumedang km 21, Jatinangor-Sumedang, West Java, 45363, Indonesia, Tel +62-842-888888 Ext: 3510, Email [email protected]
Introduction: Kaempferia galanga L. (K. galanga; local name kencur, Zingiberaceae) is a plant commonly used as a kitchen spice, and empirically it is often used for medicinal purposes. This plant has been shown to have an anti-inflammatory role, but no research has been found on its effect on oral mucosal ulcer. This study aimed to investigate anti-inflammatory activity and wound healing effect of the ethanol extract of K. galanga L. rhizome (EEKG) on the chemical-induced oral mucosal ulcer in Wistar rats.
Methods: In this study, 35 rats were divided into 7 groups (normal, negative, triamcinolone acetonide, and 4 EEKG groups). Acetic acid 70% was used as the oral mucosal ulcer inducer. Parameters observed were macroscopic and microscopic histopathological examinations.
Results: The results revealed that dose of 0.5% of the EEKG was effective in increasing the percent recovery of ulcer area and inflammation sign scores. Meanwhile, doses of 0.5– 2% of EEKG were effective in reducing the histopathological score. Interestingly, topical EEKG in our study was more effective compared with triamcinolone acetonide (the conventional therapy for oral mucosal ulceration).
Discussion: The EEKG has been confirmed its anti-inflammatory activity by accelerating the healing process on the chemical-induced oral mucosal ulcer in Wistar rats, based on the percent recovery of the ulcer area, the percent recovery of the inflammation sign score, and the histopathology score.
Conclusion: Taken together, K. galanga L. is very potential to be developed as a prospective phytopharmaceutical for the treatment of oral mucosal ulceration in human after clinical trials.
Keywords: anti-oral mucosal ulcer, anti-inflammation, Kaempferia galanga L, wound healing
Introduction
The mouth is the gateway for the entry of food that can sustain human life, although not directly, this organ plays an important role in maintaining general health. Thus, oral mucosal health problems will affect the function of the mouth.1,2 The most common oral mucosal health problem is ulceration, which can be self-healing, recurrent, or persistent, and the ulcerative lesion is also the most common sign of inflammation of the oral mucosa. Oral mucosal ulceration is an inflammatory condition that begins with the disintegration of the oral mucosal epithelium by various causes.3 This condition eventually affects the quality of life of the patients.
Four phases of wound healing which include: hemostasis, inflammation, proliferation, and remodeling, partially overlapped. Impaired immunity locally and or systemically will prolong the inflammatory phase and delay wound healing.4 In vivo studies on wound healing of the induced-oral mucosa required 10 days to approach the completely cicatrized condition without any therapy.5 To suppress excessive inflammatory reactions and speed up the healing process, we need drugs that work as an anti-inflammatory which will stimulate the wound healing process.
The treatment of oral mucosal ulceration mostly requires steroid anti-inflammatory drugs (SAIDs). These drugs can be administered orally or topically.1,6 SAIDs, eg, glucocorticoids, are recognized to initial steroid binding to intracellular NF-kappaB, which inhibits its translocation to the nucleus and DNA-binding, and also IKK activity.7,8 However, topical SAIDs, eg, triamcinolone acetonide topical gel preparations, or oral prednisolone tablets, are not recommended for long-term use due to their effect on the suppression of the immune system, both locally and systemically.3,6 Topical administration of triamcinolone acetonide gel has also been reported, showing unsatisfactory results for the treatment of oral mucosal ulceration of rats, both in normal rats and diabetic rats.9
Besides, there are non-steroids anti-inflammatory drugs (NSAIDs) that also can be used for the treatment of oral mucosal ulcerations, eg, hyaluronic acid (gel preparations, topical use), benzydamine hydrochloride (liquid preparations/lozenges, topical use), or diclofenac sodium (tablet preparations, oral use). These NSAIDs could alter prostaglandin biosynthesis by inhibiting the cyclooxygenase enzyme (COX). NSAIDs mostly work by blocking both isomers, causing gastric irritation,10 as well as other side effects, such as numbness/discomfort of the tongue, or hypersensitivity.6 Therefore, it is necessary to develop the alternative for high quality and efficacy anti-inflammatory drugs, with safer and fewer side effects. Recent research has begun to lead to the development of potential medicinal plants because they are usually edible, then believed to be safer, and cause fewer side effects.11
K. galanga L. rhizome, local name kencur, is one of the Indonesian-originated plants, that have been utilized empirically as pain-reducers. It has shown various activities including anti-inflammatory, analgesic, and antioxidant effects, due to their secondary metabolites content.12,13 K. galanga L. is easy to grow in Indonesia and other countries on the Asian continent.14 The results of our preliminary study have identified the content of ethyl-p-methoxycinnamate (EPMC) and flavonoids in the ethanolic extract of K. galanga L. rhizome.15 We also have reported that the best percentage of yield and water, as well as the secondary metabolites content, was obtained from plants harvested in the rainy season.16 The phytochemical screening of K. galanga L. rhizome indicated the presence of: kaempferol;12,13 EPMC and ethyl cinnamate, that have showed anti-inflammatory and antinociceptive activity better than that of indomethacin as the COX inhibitor17 and aspirin;18 quinones and terpenes;19 polyphenols and flavonoids.15 It has been reported in a study that diarylheptanoids can be isolated from K. galanga and stated to have a potential effect for the treatment of diseases associated with inflammation.20
K. galanga L. exhibited inhibitory activity against several inflammatory parameter proteins, namely: IL-6 and PGE2.21 Moreover, this plant also showed inhibition of PGH2 formation via the COX signaling pathway.19 The acute and sub-chronic toxicity study showed that K. galanga L. rhizome extract on Wistar rats did not indicate toxicity and mortality.22 Moreover, the purified EPMC was confirmed its safety at 2 g/kg BW (the LD50 value >2 g/kg BW).17 In vivo studies of K. galanga L extract had been carried out and showed an effect on relieving carrageenan-induced paw edema of rats better than controls.23–25 Based on this, K. galanga L., is very possible to be developed as a prospective phytopharmaceutical. Several in vivo studies of other medicinal plants have been conducted to determine their therapeutic effect for oral mucosal ulceration.9,26–29
A clinical trial study of K. galanga L. extract has been carried out and concluded that the extract in oral administration (dose 160 mg/day, for 10 days) has the same effectiveness as meloxicam for the treatment of knee osteoarthritis.30 Meanwhile, another clinical trial of topical K. galanga extract on 16 patients with recurrent aphthous stomatitis has showed good results in reducing the ulcer size and pain.31
Nevertheless, there is a lack of information about the effect of the ethanol extract of K. galanga L. rhizome (EEKG) on the chemical-induced oral mucosal ulcer in Wistar rats. This particular plant still needs further exploration to be developed as an anti-oral mucosal ulceration phytopharmaceutical, therefore this research aimed to investigate the effect of the EEKG on the chemical-induced oral mucosal ulcer in Wistar rats, via macroscopic and microscopic histopathological examination.
Materials and Methods
Plant Material and Extract Preparation
K. galanga herbs and fresh rhizomes were purchased from Subang, West Java, Indonesia. The herbs were identified and the specimens were stored at the Bandung Institute of Technology (ITB) Bandung, Indonesia. The herbs were extracted using cold-maceration method (for 3×24 hours at room temperature). Ethanol 70% was employed as the solvent. The excess solvent was removed using a rotary evaporator (Buchi®, R-220, at 40–45°C) to a thick extract.15,16 CMC (dissolved in ddH2O) was prepared as an extract solvent and as a material applied to the negative controls. EEKG was dissolved in 2% (w/w) of carboxymethylcellulose sodium salt (CMC, Merck®, CAS Number: 9004-32-4, C4888). Concentration of the extract in 2% of CMC were: 0.5%, 1%, 2%, and 4% (w/w). The extracts were provided in semi solid gel formula.
Animals
Male Wistar albino rats were obtained from the Iratco® Laboratory (animal experiment laboratory), Bogor, Indonesia. The animals were strain identified at the Iratco® Laboratory, Bogor, Indonesia, by a certified veterinarian. Calculation of the number of the rats was based on the Federer formula. Based on these calculations, the minimum total sample required is 28 rats. In order to avoid a shortage of sample size in the event of a drop-out, in this study we used 35 rats divided into 7 groups. The inclusion criteria for the animals were: healthy male Wistar albino rats, aged 10–11 weeks, weighing 150–200 gram. Exclusion criteria were: the rat looked sick or died, or decreased in weight loss during the acclimatization period more than 10%.
The ethical recommendation for health research using animals already approved to fulfil the proper health research requirements, from the Ethical Committee of Health Research of Universitas Padjadjaran (http://kepk.fk.unpad.ac.id/, recognized by the FERCAP-Forum of Ethics Review Committee in Asia & Western Pacific Region since 2014). The document number was 523/UN6.KEP/EC/2020 and the amendment of the ethical approval was document no. 031/UN6.KEP/EC/2021. Ethical considerations on the use of experimental animals paid attention to the 3R principle, namely: reduction, refinement, and replacement.32 This 3R principle combined with the 5F principle for animal welfare, namely: freedom from hunger and thirst; freedom from feeling discomfort; freedom from pain, injury, and disease; freedom from fear and distress; and freedom to express their normal behavior.33
The rats were fed and drank ad libitum. Feed and water consumption was measured every day. The feed provided was 75 grams per day per group/cage for 5 rats and 350 mL of water/day/cage for 5 rats. The standard pellet feed used in this study contained a low fiber (5%), protein (20%), and fat (5–10%). Rats were placed in cages with a length of 55 cm x width 45 cm x height 45 cm, a maximum capacity of 5 rats with the same treatment group. The cages were facilitated with a husk that will be replaced every day and ventilation was facilitated by a special air conditioning machine. The environmental humidity was also considered around 30–70%, normal room temperature (18–26°C), and the environment was kept clean. The dark-light cycle of 12 hours using a lamp was facilitated to provide the natural life and behavior of the rats.
Experimental Study Design and Treatment
A total of 35 male Wistar rats were used in this in vivo study. Rats were grouped randomly into 7 groups (n = 5), namely: normal control group (Normal), negative control group (NegCon), Triamcinolone acetonide (TRA) group, test 1 group (T1), test 2 group (T2), test 3 group (T3), and test 4 group (T4). Each group consisted of 5 rats. Rats in the normal control group were not given induction of oral mucosal ulcer and were not given any treatment/administration. Rats in the NegCon, TRA, T1, T2, T3, and T4 groups were given oral mucosal ulcer induction. Rats in the NegCon group were treated with 2% CMC while rats in the TRA group were treated with Triamcinolone acetonide 0.1% in oral-base (Kenalog in oral-base, Taisho®). Rats in groups T1, T2, T3, and T4, were treated with 0.5% EEKG, 1% EEKG, 2% EEKG, and 4% EEKG, respectively.
All rats in TRA and EEKG groups (T1 - T4) were anesthetized with intraperitoneal ketamine (80 mg/kg) and xylazine (10 mg/kg) to facilitate the procedure of oral mucosal ulcer induction. Antisepsis was performed with a mouthwash solution of 0.2% chlorhexidine gluconate in sterile and disposable cotton balls before the induction process. Induction of oral mucosal ulcer was using a cotton ball 10 mm in diameter, in a syringe without needle moistened with 70% acetic acid as much as 100 microliters, applied to the ventral of rat’s tongue for 2 minutes (modification from Kang and Kim, 2018; Miao et al, 2019; Idrus et al, 2019).26,27,34 The induction technique was standardized for all animals and performed by the same operator.
Triamcinolone acetonide (TRA) in oral-base (Kenalog® in oral-base, Taisho) was used as a comparison material, because it is commonly used in the conventional therapy of stomatitis. TRA was applied topically to oral mucosal ulcers of rats once a day, as well as EEKG in several concentrations, according to the group of rats.
After the experiment finished, the rats being euthanized using overdosed sodium pentobarbital. The rat’s tongue was taken as a sample and placed in a closed container (5 mL tube), then stored in the freezer −20°C until the next experimental procedure. A small portion of the tongue tissue was taken and then stored in a vial container containing 10% formaldehyde (Indokimia®), for tissue staining and histopathology examination. Furthermore, the dead rats were put in a freezing box and then sent to a special incinerator to destroy the research animals.
Daily Visual Observation
The weight of rats, feed, and water consumption were evaluated every day. The base-line weight of the rats was measured on the day before induction. The first day was the day when ulcers formed on the oral mucosa of rats after induction. On the 1st day of the ulcer formation, the maximum ulcer area (consider to be 100% of ulcer area) and the maximum inflammation signs score/ISS (consider to be 100% of ISS) were also measured. Then from the first day until the 6th day, treatments were given in the form of topical drug administration, 1 time/day. Ulcer photography documentation was carried out every day to obtain data on ulcer area (in mm), with the help of a dental probe or scaled dental pinset, and ISS in all groups. Final body weight was measured before euthanasia was performed on the 7th day. The percent weight loss was measured in all rats. The percent weight loss formula is:
The area of ulcer/lesion with a tendency for linear regression with time then observed and analyzed using digital photography and ImageJ® computer software (https://imagej.nih.gov/ij/) (modification from Cavalcante et al, 2011; Oliveira et al, 2016; Kang and Kim, 2018; Miao et al, 2019).5,9,26,27 The first thing to do was to calibrate the scale (Analyze>Calibrate>select “Straight freehand tools”). Measure the known length of the scale on the dental pinset or dental probe and make adjustments (Analyze>set scale). The known distance was 10 and unit of length in mm. Then click OK, select the “Freehand selection tool”, and start measuring the ulcer area by circling the edges of the ulcer. Furthermore, the results were obtained in the form of ulcer area in millimeters (Analyze>Measure). The percent recovery of ulcer area was measured in all rats.
The percent recovery of ulcer area formula is:
The visual inflammation sign score was measured by observing the presence of the signs of inflammation, namely: edema, tissue necrosis, erythema, and lesions. The four signs were scored as +/presence or -/absent, then the results were summed for each rat in all groups (minimum score = 0, maximum score = 4) (modification from Kang and Kim, 2018; Miao et al, 2019).26,27 All measurements were performed by the same operator. The ISS day 1 was considered to become 100% when ulcers were formed after induction using acetic acid. The percent recovery of the ISS was compared the last day to the first-day measurement. The percent recovery of inflammation sign score formula is:
Histopathological Analysis
The collected mucosa fragments that will be identified, immersed in 10% formalin for 24 hours. After fixation, the specimens were subjected to dehydration in crescent alcoholic series, diaphanized in xylol, impregnated in paraffin, and melted at 60°C. Then, the fragments were placed into paraffin-forming blocks at room temperature, and were sectioned to 5 μm in thickness using a microtome. Finally the slides were stained with hematoxylin-eosin.5,9
The analysis was performed with an optical microscope and the histological characteristics of the ulcer along with their corresponding wound healing phases will be described. The histopathological score was measured by two examiners who had received training and were calibrated, with a good agreement level of kappa score = 0.85. The histopathological parameters were determined and scored from 0 to 4 according to the previously published criteria.5,9,28
0 = No ulcer, remodelled epitel.
1 = No ulcer, fibrosis, and slight inflammation.
2 = With ulcer, fibrosis, and moderate inflammation.
3 = With ulcer and inflammation, with granulation tissue.
4 = With ulcer and acute inflammation process (dilated vessels, mixed inflammatory infiltrate with neutrophils).
Statistical Analysis
The feed and water consumption, percent weight loss, percent recovery of ulcer area, and percent recovery of inflammation sign score were analyzed by the one-way and two-way ANOVA test (Excel 2020 software). The Mann–Whitney test was used to analyzed the histopathology scores (using the online software at https://www.statskingdom.com/170median_mann_whitney.html). A 95% confidence level (p<0.05) was used to compare the differences and significance between variables.
Results
Feed and Water Consumptions
Table 1 shows the average and standard deviation of daily feed and water consumption of rats. The average daily feed consumption of the rat groups that were given oral mucosal ulcer induction in our study, tended to show an increase compared to the normal group. However, this difference was not significant when compared to the NegCon (the highest average of daily feed consumption) and the normal group. Meanwhile, on the contrary, the average daily water consumption in the groups that received oral mucosal ulcer induction tended to decrease in water consumption when compared to normal. A significant decrease in water consumption compared to normal only occurred in the test 3 group. When compared with the NegCon, the group of rats treated with TRA and EEKG, except for group 3, tended to consume more water on average. However, water consumption in all induced rat groups did not show a significant difference compared to the NegCon.
 |
Table 1 Daily Feed Consumption (Gram) and Water Consumption (mL) |
Percent Weight Loss
The weight of the rats on the first day was considered as 100% body weight or baseline weight. Baseline weight of the rats were not significantly different. Table 2 shows the average and standard deviation of percent weight loss of rats. The weight loss of rats in the normal group and the EEKG treatment groups compared to the NegCon was not significantly different. Only the TRA group showed a significant difference in percent weight loss compared to the NegCon.
 |
Table 2 Percent Weight Loss (%) |
Ulcer Area Measurement
Figure 1 shows the daily dynamics of the percent recovery of the ulcer area. Day first was one day after the induction of ulcers in the oral mucosa of rats, so the percent recovery of ulcer areas in all groups was 0%. On day 3 only the NegCon group did not show an increase in the percentage of recovery of ulcer area compared to day 2, while the other groups showed an increase.
 |
Figure 1 Daily dynamics of the percent recovery of ulcer area. |
Table 3 shows the average and standard deviation of the percent recovery of ulcer area measurement of rats. The ulcer area at day 1 is considered to become 100% when ulcers are formed after induction using acetic acid. The percent recovery of the ulcer area was compared the day-n (3rd day and last day) to the first-day measurement. On day-3 all groups, except rats of Test 2, showed a greater percentage in the recovery of the ulcer area compared to the NegCon, but on day-6, only rats of Test 1 showed a greater percentage. Statistical analysis was carried out using one-way and two-way ANOVA.
 |
Table 3 Percent Recovery of Ulcer Area (%) |
Inflammation Sign Score Measurement
Figure 2 shows the example of inflammation sign score (ISS) measurement of rats and Figure 3 shows the comparison of ulcer healing between groups on day 6 post-induction.
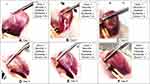 |
Figure 2 The example of inflammation sign score measurement (test 1, rat no 5). |
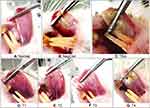 |
Figure 3 The comparison of ulcer healing between groups on day-6 post-induction. Abbreviations: NegCon, negative control; TRA, triamcinolone acetonide; T1, test 1; T2, test 2; T3, test 3; T4, test 4. |
Table 4 shows the average and standard deviation of percent recovery of ISS of rats on day 6 (the last day). It appears that only the Test 1 showed a percent recovery of ISS greater than the NegCon group although not significantly different (statistical analysis was carried out using one-way ANOVA).
 |
Table 4 Percent Recovery of Inflammation Sign Score (%) |
Table 5 shows the frequency distribution of ISS based on the test group on the last day. The maximum total score is 20 and the minimum is 0, so if the total score is between 0 and 10 then the intervention is effective, whereas if the total score is more than 10, then the drug is not effective, based on the visual observation of signs of inflammation. Only Test 1 group shows a better score than the NegCon group.
 |
Table 5 Frequency Distribution of Macroscopic Signs of Inflammation |
Histopathology Score Measurement
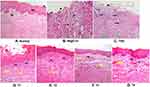
Figure 4 shows the differentiation in histopathological features (magnification 100x) between groups. The distribution of inflammatory cells (purple spots of leukocyte/black arrows) increased in the inflammatory phase and lower when approaching wound healing, on the other hand, fibrosis (yellow arrows) shows an increase when approaching the wound healing stage. Green arrows show the dilated vessels which increased in the acute phase of inflammation and decreased when entering the chronic phase or wound healing.
Table 6 shows the average, standard deviation, and p-value compared to the NegCon, of the histopathology score measurement of rats. The difference test between groups was calculated using Mann–Whitney statistical analysis. The level of significance is p<0.05. The T2 and T3 groups showed better effectiveness and significantly different than NegCon in treating the oral mucosal ulceration of Wistar rats.
 |
Table 6 Histopathology Score |
Table 7 shows the frequency distribution of histopathology scores based on the test group. The maximum total score is 20 and the minimum is 0, so if the total score is between 0 and 10 then the intervention is effective, whereas if the total score is more than 10, then the drug is not effective, based on the histopathological picture. The T1, T2, and T3 groups showed better scores than the NegCon, which means effective in reducing the histopathology score.
 |
Table 7 The Frequency Distribution of Histopathology Scores |
Discussion
This study revealed that EEKG exerts anti-inflammatory activity and promotes wound healing. The administration of topical EEKG affected the ulcer area, reduced signs of inflammation, and improved histopathological feature of the chemical-induced oral mucosal ulcer in Wistar rats. We proved that there is a positive correlation between the development of the oral mucosal ulcer with their daily feed consumption. However, a negative correlation is observed between the development of the oral mucosal ulcer with water consumption and bodyweight.
The study showed that the daily consumption of feed and water for all groups of rats was still within normal limits (Table 1). Normally rats need feed as much as 5 g/100 g of BW per day.35 This research used rats with body weight ranging from 150–200 g so that in one cage/per 5 rats need between 37.5–50 g of feed. The average consumption of rat feed per cage in this study was following this theory, which ranged from 46.88 to 52.73 g. The slight increase in feed consumption in the ulcer-induced group suggests the possibility that the pain experienced by the rat triggered stress and increased the instinct to eat more.36
Meanwhile, the average water consumption requirement of normal rats is 10–25 mL/100 g of BW per day,35 so that in one cage/per 5 rats need between 75–250 mL of water. The water consumption per cage in this study was following with this theory, which ranged from 202.38 to 290 mL per day. Water consumption is also related to the health condition of the rats, in this case the ulcers were made on the ventral part of the tongue, so that it is possible to make difficulties or disturbances for rats in consuming water. Rats are rodents that can gnaw/bite on feed using their teeth. However, the pain caused by the ulcer lesions limited the movement of the rat’s tongue needed when drinking, so that water consumption was decreased compared to normal. The results of this study are in line with research conducted by Kang and Kim, which stated that there was no significant difference in feed and water consumption between the experimental groups.26
The percent of weight loss measured by comparing the initial body weight (day-1) with the final body weight of the rats after therapy (day-6), as shown in Table 2. When compared with normal (−4.30%), the percent weight loss in ulcer-induced rats tended to be greater (−5.24% to −11.88%), except for the NegCon (−0.82%) and the Test 2 group (+1.19%), even though not significantly different. The weight loss of ulcer-induced rats and treated with TRA appeared to be the largest and most significant (−11.88%), compared to the negative control. This is in line with the study by Oliveira et al, that the weight loss of rats treated with TRA was greater than rats treated with chamomile, on study of ulcer healing on normoglycemic and diabetic rats.9 However, this contradicts with the results of the study by Sari et al, which were showed a significant increase in body weight of rats treated with TRA and areca nut-chrysanthemum extract for oral mucosal ulcers in rats.28
Weight loss was observed in normal rat group which is predicted due to the possibility of experiencing stress, even though the experimental environment has been well conditioned. Nevertheless, the negative control group showed a lower percentage of weight loss compared to that of the normal group (provided in Table 2).
Figure 1 shows the dynamics of the percent recovery of ulcer area. It appears that on day 3 can be said to be the peak of ulcer development in our study, this is indicated by the absence of an increase in the percent recovery of ulcer area in the negative control group. This is in line with previous studies which stated that days 3 to 5 were the peak of oral mucosal ulcer development in rats, if no intervention was given.4,5 Meanwhile, the group of rats that were given topical drug intervention, both TRA and EEKG, showed an increase in percent recovery of ulcer area day by day. However, after day 3, rats in the negative control group began to show an increase in the percent recovery of ulcer area. This is thought to be because 2% of the CMC given acted as a coating agent which also had the effect of protecting ulcer from external disturbances that could inhibit wound healing. It can be said that 0.5% of EEKG (T1) showed the best effectiveness in increasing the percent recovery of ulcer area during the experiment.
The administration of 0.5–4% of EEKG (Test 1, Test 2, and Test 3) were better at reducing the ulcer area macroscopically compared to that of TRA, on day-3 or day-6 (Table 3). Furthermore, 0.5% EEKG (Test 1) indicated a better percent recovery of ulcer area (85.24%) compared to the NegCon (79.82%). The treatment with 0.5%, 1%, and 4% of EEKG (Test 1, Test 2, and Test 4) was better than TRA in reducing the inflammation sign score (ISS) by visual observation (Tables 4 and 5). However, only 0.5% of EEKG (Test 1) indicated a better recovery (76.67%) compared to the NegCon (71.67%). As a consequence, it is confirmed that the low dose of EEKG (0.5%) possesses the best anti-inflammatory activity by healing the chemical-induced oral mucosal ulcer Wistar rats, both in terms of the percent recovery on ulcer area and inflammation signs scores. Statistical analysis using one-way ANOVA showed that there was no significant difference in percent recovery of ulcer area between the test group and NegCon (p-value >0.05). Meanwhile, using two-way ANOVA showed that there was a significant difference in percent recovery of ulcer area between day 3 and day 6 (p = 0.0012), but there was no significant difference in percent recovery of ulcer area with increasing extract dose (p = 0.4412).
The results of this study are not in accordance with the previous study on oral ulcers in rats, which reported that low, middle, and high dose of Shuangjinlian mixture have anti-inflammatory effect and was effective for the prevention and treatment of oral ulcers in rats, in dose-dependent manner.27 Likewise with the results of the study on the effect of acai berry water extract (ABWE) on oral mucosal ulcers, confirmed that medium (3%) and high (5%) doses of ABWE were better in the wound healing process, than the lower dose.26
In our study, signs of inflammation in the form of ulceration, pseudomembrane/necrotic tissue, and edema were more commonly found in the early phase or day 1 to day 3 after induction of oral mucosal ulcers, while the appearance of erythema lesions persist to the wound healing phase. The lesion in the form of pseudomembrane/necrotic tissue showed thickening in the mid-inflammatory phase, around day 3 to day 4, then began to thin out as it entered the remodeling phase and approached the wound healing phase (depicted in Figure 2).
The administration of lower doses EEKG on the chemical-induced oral mucosal ulcers in Wistar rats, conveyed a better histopathology score than the NegCon. The 0.5%, 1%, and 2% of EEKG exhibited a better effect than TRA, as shown in Tables 6 and 7. Even the 1% and 2% of EEKG showed statistically significant differences (p<0.05), which was better than the NegCon. Only the application of 4% EEKG which was not showing better effect than TRA application and NegCon.
The results of measuring the ulcer area and inflammation sign score through the visual observation method (macroscopic) in this study were not in line with the results of the histopathology score measurement (microscopic). This is presumably because physiologically the oral mucosal epitel has a very good healing speed, supported by various components in saliva,4 hence the lesions appear to heal with marked closure of the mucosal epithelial discontinuities. Meanwhile, the microscopic observation revealed that the inflammatory process still occurs in the connective tissue/lamina propria, even though the mucosal epithelial surface has been covered.
In general, the topical application of 0.5–2% of EEKG on the chemical-induced oral mucosal ulcer of Wistar rats, could accelerate wound healing and manifested a better anti-inflammatory activity compared to TRA. This is in line with the results of research by Oliveira et al.9 In our study the effect of EEKG did not show a dose-dependent pattern, in line with the results of research conducted by Sari et al.28
Several studies have proven that infusion, methanol extract, ethanol extract, petroleum extract, and EPMC isolated from K. galanga rhizome, possess an anti-inflammatory effect.17–19,23,24,37,38 The EEKG used in this in vivo study has been proven to contain various secondary metabolites such as polyphenols, flavonoids, alkaloids, triterpenoids, tannins, and saponins,15,16 which is in line with the research results of Elshami et al.39 The identification of Ethyl-p-methoxy cinnamate (EPMC) as the most abundant active substance in the EEKG has also been proven using the thin layer chromatography (TLC) method, as well as spectrophotometric analysis and chromatogram profiles using High-performance liquid chromatography (HPLC).15 These secondary metabolites in the EEKG are thought to work synergistically as anti-inflammatory, thus facilitating the healing process of oral mucosal ulcers.
Triamcinolone acetonide is a steroid anti-inflammatory drug that is commonly used to treat various types of stomatitis, but it is also able to inhibit wound healing in ulcerated oral mucosa of rats. In addition, because the oral mucosal lesions of rats may be easily invaded by various microorganisms, the administration of steroids will suppress local immunity which exacerbates inflammatory conditions, and delays wound healing.9,40 Meanwhile, EEKG was thought to have an antimicrobial effect, so it could show superiority in accelerating wound healing.14 This could be the answer to the phenomenon that EEKG is superior to TRA in treating oral mucosal ulceration in rats. Thus, the development of this EEKG will be able to help solve the problem of discovering alternative drugs for oral mucosal ulceration therapy.
Conclusion
The ethanol extract of K. galanga L. rhizome has confirmed its anti-inflammatory activity by accelerating the healing process on the chemical-induced oral mucosal ulcer in Wistar rats, based on the percent recovery of the ulcer area, the percent recovery of the inflammation sign score, and the histopathology score. However, clinical trials need to be conducted for drug development and its application in humans. Taken together, K. galanga L. is potential to be developed as a prospective phytopharmaceutical for the treatment of oral mucosal ulceration.
Acknowledgment
The authors thank the Rector of UNIVERSITAS PADJADJARAN via the Directorate of Research and Community Engagement for funding the publication fee of this article and the whole research, by the research of dissertation grant number 1595/UN6.3.1/PT.00/2021. This study is in the framework of the first author’s dissertation project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chiang CP, Chang JYF, Wang YP, Wu YH, Wu YC, Sun A. Recurrent aphthous stomatitis etiology, serum autoantibodies, anemia, hematinic deficiencies, and management. J Formosan Med Assoc. 2019;118:1279–1289. doi:10.1016/j.jfma.2018.10.023
2. Svalastog AL, Donev D, Kristoffersen NJ, Gajović S. Concepts and definitions of health and health-related values in the knowledge landscapes of the digital society. Croat Med J. 2017;58:431–435. doi:10.3325/cmj.2017.58.431
3. Fitzpatrick SG, Cohen DM, Clark AN. Ulcerated lesions of the oral mucosa: clinical and histologic review. Head Neck Pathol. 2019;13(1):91–102. doi:10.1007/s12105-018-0981-8
4. Waasdorp M, Krom BP, Bikker FJ, van Zuijlen PPM, Niessen FB, Gibbs S. The bigger picture: why oral mucosa heals better than skin. Biomolecules. 2021;11(1165):22. doi:10.3390/biom11081165
5. Cavalcante GM, Paula RJS, Souza LP, Sousa FB, Mota MRL, Alves APNN. Experimental model of traumatic ulcer in the cheek mucosa of rats. Acta Cirúrgica Brasileira. 2011;26(3):227–234. doi:10.1590/s0102-86502011000300012
6. Woo SB, Setterfield JF, Greenberg MS. Ulcerative, vesicular, and bullous lesions. In: Glick M, Greenberg MS, Lockhart PB, Challacombe SJ, editors. Burket’s Oral Medicine.
7. De Bosscher K, Berghe WV, Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene. 2006;25(51):6868–6886. doi:10.1038/sj.onc.1209935
8. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi:10.1038/sigtrans.2017.23
9. Oliveira BV, de Barros-silva PG, Nojosa JDS, et al. TNF-alpha expression, evaluation of collagen, and TUNEL of Matricaria recutita L. extract and triamcinolone on oral ulcer in diabetic rats. J Appl Oral Sci. 2016;24(3):278–290. doi:10.1590/1678-775720150481
10. Ferrer MD, Busquets-Cortés C, Capó X, et al. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr Med Chem. 2019;26(18):3225–3241. doi:10.2174/0929867325666180514112124
11. Wahyuni IS, Sufiawati I, Nittayananta W, Puspitasari IM, Levita J. Efficacy and safety of plant-based therapy on re-current aphthous stomatitis and oral mucositis in the past decade: a systematic review. J Herbmed Pharmacol. 2021;10(2):179–187. doi:10.34172/jhp.2021.19
12. Umar MI, Asmawi MZ, Sadikun A, Altaf R, Iqbal MA. Review: phytochemistry and medicinal properties of Kaempferia galanga L. (Zingiberaceae) extracts. Afr J Pharm Pharmacol. 2011;5(14):1638–1647. doi:10.5897/AJPP11.388
13. Wang SY, Zhao H, Xu HT, et al. Kaempferia galanga L.: progresses in phytochemistry, pharmacology, toxicology and ethnomedicinal uses. Front Pharmacol. 2021;12:675350. doi:10.3389/fphar.2021.675350
14. Kumar A. Phytochemistry, pharmacological activities and uses of traditional medicinal plant Kaempferia galanga L. - an overview. J Ethnopharmacol. 2020;10(253):112667. doi:10.1016/j.jep.2020.112667
15. Wahyuni IS, Sufiawati I, Nittayananta W, Levita J. Identification of ethyl para-methoxycinnamate and kaempferol in the ethanol extract of Kaempferia galanga L. rhizome as biomaterial for drug candidate using spectrophotometric and chromatographic analysis. Mater Sci Forum. 2021;1028:371–376. doi:10.4028/www.scientific.net/MSF.1028.371
16. Wahyuni IS, Sufiawati I, Nittayananta W, Levita J. The determination of ethyl p-methoxycinnamate in Kaempferia galanga L. rhizome extract harvested in rainy and dry seasons. Int J App Pharm. 2021;13(4):132–135. doi:10.22159/ijap.2021.v13s4.43841
17. Umar MI, Asmawi MZ, Sadikun A, et al. Bioactivity-guided isolation of Ethyl-p-methoxycinnamate, an anti-inflammatory constituent from Kaempferia galanga L. extracts. Molecules. 2012;17:8720–8734. doi:10.3390/molecules17078720
18. Umar MI, Asmawi MZ, Sadikun A, et al. Ethyl-p-methoxy cinnamate isolated from Kaempferia galanga inhibits inflammation by suppressing interleukin-1, tumor necrosis factor-alpha, and angiogenesis by blocking endothelial functions. CLINICS. 2014;69(2):134–144. doi:10.6061/clinics/2014(02)10
19. Levita J, Wijaya LK, Celcilia S, Mutakin M. Inhibitory activity of Kaempferia galanga and Hibiscus sabdariffa on the rate of PGH2 formation. J Appl Sci. 2015;15(7):1032–1036. doi:10.3923/jas.2015.1032.1036
20. Yao F, Huang Y, Wang Y, He X. Anti-inflammatory diarylheptanoids and phenolics from the rhizomes of kencur (Kaempferia galanga L.). Ind Crops Prod. 2018;125:454–461. doi:10.1016/j.indcrop.2018.09.026
21. Aroonrerk N, Kamkaen N. Anti-inflammatory activity of Quercus infectoria, Glycyrrhiza uralensis, Kaempferia galanga, and Coptis chinensis, the main components of Thai herbal remedies for aphthous ulcer. J Health Res. 2009;23(1):17–22.
22. Kanjanapothi D, Panthong A, Lertprasertsuke N, et al. Toxicity of crude rhizome extract of Kaempferia galanga L. (Proh Hom). J Ethnopharmacol. 2004;90:359–365. doi:10.1016/j.jep.2003.10.020
23. Vittalrao AM, Shanbhag T, Kumari M, Bairy KL, Shenoy S. Evaluation of antiinflammatory and analgesic activities of alcoholic extract of Kaempferia galanga in rats. Indian J Physiol Pharmacol. 2011;55(1):13–24. PMID: 22315806.
24. Riasari H, Rachmaniar R, Febriani Y. Effectiveness of anti-inflammatory plaster from Kencur (Kaempferia galanga L.) rhizome ethanol extract. Int J Pharm Sci Res. 2016;7(4):1746–1749. doi:10.13040/IJPSR.0975-8232
25. Samodra G, Febrina D. Anti-inflammatory effects of Kaempferia galanga L. rhizome extract in carrageenan-induced female rats. Adv Health Sci Res. 2019;20(1):13–17. doi:10.2991/ahsr.k.200204.004
26. Kang MH, Kim BH. Oral wound healing effects of acai berry water extracts in rat oral mucosa. Toxicol Res. 2018;34(2):97–102. doi:10.5487/TR.2018.34.2.097
27. Miao M, Peng M, Xing Z, Liu D. Effect of Shuangjinlian mixture on oral ulcer model in rat. Saudi J Biol Sci. 2019;26:790–794. doi:10.1016/j.sjbs.2019.02.005
28. Sari LM, Mubarak Z, Sari DK. Evaluation of clinical, histology, TNF-α, and collagen expressions on oral ulcer in rats after treatment with areca nut and chrysanthemum oral gel. F1000 Res. 2021;10:623. doi:10.12688/f1000research.54887.1
29. Surboyo MDC, Mahdani FY, Ayuningtas NF, Santosh ABR, Ernawati DS, Mansur D. The cytotoxicity, anti-inflammation, anti-nociceptive and oral ulcer healing properties of coconut shell liquid smoke. J Herbmed Pharmacol. 2021;10(4):459–467. doi:10.34172/jhp.2021.53
30. Syahruddin AN, Dahlan CK, Taslim NA. The effects of Kaempferia galanga L. extract on pain, stiffness and functional physic in patient with knee osteoarthritis: double blind randomized clinical trial. Int J Sci Healthcare Res. 2017;2(4):37–43.
31. Tambunan LV, Lubis W. Effectiveness of Kaempferia galanga Linn Rhizome’s extract towards the healing of minor re-current aphthous stomatitis in RSGMP USU’s patients. Uip Heath Med. 2017;1(1):100–103. doi:10.7454/uiphm.v1i0.35
32. Kischkel S, Brietzke A, Matschegewski C, Schmidt W, Eickner T, Grabow N. Application of 3R principles in small animal GLP testing of biomaterials. Curr Direct Biomed Eng. 2019;5(1):335–338. doi:10.1515/cdbme-2019-0084
33. Mellor DJ. Updating animal welfare thinking: moving beyond the “five freedoms” towards “a life worth living”. Animals. 2016;6(21):1–20. doi:10.3390/ani6030021
34. Idrus E, Hartanti PD, Suniarti DF, Prasetyo SR, Wimardhani YS, Subarnbhesaj A. An experimental model of chemically-induced ulceration of the buccal mucosa of Mus musculus. Makara J Health Res. 2019;23(3):181–187. doi:10.7454/msk.v23i3.1158
35. Lesmana R, Goenawan H, Dewi FNA. Pedoman Penggunaan Tikus Sebagai Hewan Uji Laboratorium (Guidelines for the Use of Rats as Laboratory Test Animals).
36. Yau YH, Potenza MN. Stress and eating behaviors. Minerva Endocrinol. 2013;38(3):255–267. PMID: 24126546; PMCID: PMC4214609.
37. Ridtitid W, Sae-wong C, Reanmongkol W, Wongnawa M. Antinociceptive activity of the methanolic extract of Kaempferia galanga Linn. in experimental animals. J Ethnopharmacol. 2008;118(2):225–230. doi:10.1016/j.jep.2008.04.002
38. Jagadish PC, Latha KP, Mudgal J, Nampurath GK. Extraction, characterization and evaluation of Kaempferia galanga L. (Zingiberaceae) rhizome extracts against acute and chronic inflammation in rats. J Ethnopharmacol. 2016;194:434–439. doi:10.1016/j.jep.2016.10.010
39. Elshamy AI, Mohamed TA, Essa AF, et al. Recent advances in Kaempferia phytochemistry and biological activity: a comprehensive review. Nutrients. 2019;11(10):2396. doi:10.3390/nu11102396
40. Sidhu G, Preuss CV. Triamcinolone. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544309/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.