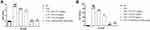Back to Journals » Drug Design, Development and Therapy » Volume 17
Anti-Arthritic Effect of Edaravone Against Complete Freund Adjuvant Induced Arthritis via Osteoclast Differentiation and HIF-1α–VEGF–ANG-1 Axis
Authors Liu J, Zhao N, Su SH, Gao Y, Qi B
Received 28 September 2022
Accepted for publication 15 December 2022
Published 18 February 2023 Volume 2023:17 Pages 519—534
DOI https://doi.org/10.2147/DDDT.S391606
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Manfred Ogris
Jichao Liu,1 Nan Zhao,2 Shi-Han Su,3 Yun Gao,2 Bo Qi4
1Department of Hand and Foot Micro Burn Plastic Surgery, 3201 Hospital, Hanzhong, People’s Republic of China; 2Department of Neurosurgery, The First Hospital of Kunming, Kunming, People’s Republic of China; 3Department of Internal Medicine-Neurology, 920th Hospital of Joint Logistics Support Force, Kunming, People’s Republic of China; 4Department of Orthopaedics, 920th Hospital of Joint Logistics Support Force, Kunming, People’s Republic of China
Correspondence: Bo Qi, Department of Orthopaedics, 920th Hospital of Joint Logistics Support Force, Kunming, 650000, People’s Republic of China, Email [email protected]
Background: Bone dysfunction is a crucial problem that occurs during rheumatoid arthritis (RA) disease. Osteoclast plays a significant role in bone resorption and osteoclast differentiation and its enhancement of bone destruction. Edaravone remarkably exhibited free radical scavenging and anti-inflammatory effects. The objective of the current investigation is to comfort the inhibitory effect of Edaravone (ED) against complete Freund adjuvant (CFA) rat model via inhibition of angiogenesis and inflammation.
Methods: Subcutaneous injection of CFA (1%) was used to induce arthritis; the rats were divided into different groups and received the oral administration of ED. Paw edema, body weight, and arthritis score were regularly estimated. Biochemical parameters were estimated, respectively. We also estimate the level of hypoxia-inducible factor-1α (HIF-1α), angiopoietin 1 (ANG-1), and vascular endothelial growth factor (VEGF). We also checked into how ED affected the differentiation of osteoclasts utilising a co-culture system with monocytes and synovial fibroblasts in arthritis rats.
Results: ED treatment significantly (P< 0.001) suppressed the arthritis score and paw edema and improved the body weight. ED treatment significantly (P< 0.001) altered the antioxidant parameters and pro-inflammatory cytokines: inflammatory mediator nuclear kappa B factor (NF-κB), cyclooxygenase-2 (COX-2), and prostaglandin E2 (PGE2), respectively. Furthermore, ED treatment significantly (P< 0.001) suppressed the level of ANG-1, HIF-1α, and VEGF, respectively. The results suggest that ED suppressed osteoclast differentiation and also decreased the level of cytokines and osteopontin (OPN), receptor activator for nuclear factor-κ B Ligand (RANKL) and macrophage colony stimulating factor (M-CSF) in the co-culture supernatant of monocytes and synovial fibroblasts.
Conclusion: Edaravone could mitigate CFA via inhibiting angiogenesis and inflammatory reactions, which may be linked with the HIF-1α–VEGF–ANG-1 axis and also enhance the bone destruction of murine arthritis via suppression of osteoclast differentiation and inflammatory reaction.
Keywords: Edaravone, oxidative stress, arthritis, inflammation, angiogenesis, osteoclast differentiation
Introduction
Persistent autoimmune joint disorder known as RA disease is associated with a remarkable increase in inflammatory cells in the synovial tissue of joints, chronic proliferative synovitis, obesity, osteoporosis, cardiovascular risk and causes high death rate.1–4 RA affects 0.5% to 1% of the global population.5,6 RA having unidentified etiology by the presence of hyperplastic synovial membrane, which is known as pannus. The pannus is capable of destroying the bone and adjacent articular cartilage RA characterized via synovial membrane, bone destruction, inflammation, autoantibody production, and swelling cartilage destruction.5,7 Systemic complications, injury, early death, and social cost are also associated with RA. According to previous studies, RA primarily affects middle-aged women (50–60 years).5,7,8 According to studies, females are more affected by RA in comparison to males. Clinical studies have shown that RA affects the foot and hand joints, inducing constant painful swelling, atypical synovium widening, pannus formation, exaggeration, and changes in joint morphology.9,10 While the exact cause of arthritis is unknown, previous literature indicates that inflammatory reaction and immune cell play a critical part in the spread of RA.5,7
Symptoms of early stages of arthritis such as heat, swelling, and diminished joint discomfort and function; later stages include varied degrees of joint stiffness and deformity that go hand-to-hand with bone loss and enhance the chance of disability.6,11,12 Inflammation is natural reaction to damage the stimuli like infections.13,14 During the RA state, inflammation has a positive effect that deactivates a number of dangerous microorganisms, starting with the release of signalling molecules.8,15 Macrophages play a crucial role in the defense against the attack of invasive species like bacteria, viruses, and fungus.16 The synthesis of numerous signalling molecules and inflammatory-related cytokines such as interferon-γ (INF-γ), IL-6, Il-1β, TNF-α, and inflammatory mediators include COX-2, PGE2, and NF-κB are initiated in response to an invading agent’s attack.17 The alteration in the production of cytokines and inflammatory mediators induces various diseases like cancer, arthritis, diabetes, and cardiovascular and obesity diseases. Few clinical reports suggest that inflammatory cells are commonly observed in the infected area of RA patients.18 IL-6, promote the conversion of native CD4+ cells into T-helper lymphocytes by upregulating the intracellular expression of the transcription activator STAT-3 and the signal transducer. By stimulating immune cells and synovial fibroblasts, they further increasing the NF-κB expression, IL-17 plays a crucial role in the progression of RA disease.19,20 IL-17 induces the oxidative stress via synthesis of reactive oxygen species (ROS). IL-17 and VEGF, work together to trigger the expression of VEGF. The importance of VEGF in extending the inflammatory response and promoting the onset of chronic arthritis is well documented.21
The expansion of new capillaries from pre-existing ones is known as angiogenesis and it is essential in the development of RA. The majority of angiogenesis research has focused on VEGF, ANG-1, and HIF-1α, they regulate hypoxia-driven angiogenesis.21,22 During RA condition, start the deposition of HIF-1 into the inflamed synovium region and start the production of VEGF, which with the aid of ANG (ANG-1 and 2) enhances the migration and proliferation of endothelial cells (ECs). ANG-1 is responsible for vessel maturation during angiogenesis and ANG-2 (a natural antagonist of ANG-1) enhances the activity of ANG-1, which may be referred to as the HIF-1-VEGF-ANG-1 axis.21–23 Furthermore, the degree of the inflammatory reaction, as well as the expansion of joint damage in rheumatoid synovium, depend on the growth of new vasculature. As a result, the role of ANG-1, HIF-1, and VEGF is more important, and angiogenesis suppression may be a useful therapy for treating RA disease.21
Osteoblasts and osteocytes regulate bone formation.24 The multinucleated osteoclast acts on somatic cells to demonstrate the potential effect to resorb the bone.24,25 Previous reports suggest that osteoclastic bone resorption and bone formation regulate bone remodeling.26,27 M-CSF and RANKL are both significant cytokines of osteoclast differentiation. Inflammatory markers like lymphocytes and fibroblast start the secretion of RANKL from the joint and boost the osteoclast differentiation.28–30 Bone resorption factors such as interleukin and prostaglandin act on the osteoblasts and play a crucial role in altering the RANKL level. While RA disease increases the osteoclasts, it further leads to dysfunction of bone and ultimately induces bone loss and erosion. Some researchers target osteoclast differentiation for the treatment of RA disease.31
Edaravone (3-methyl-1-phenyl-pyrazoline-5-one) is a potent free radical scavenger and it also interacts with both hydroxyl and peroxyl radicals to generate oxidized compounds.32–35 ED showed immunomodulation effects to avoid negative effects on autoimmune diseases.33,34 Moreover, the protective effect and the underlying mechanism of ED against arthritis are still unknown. This is due to its antioxidant and anti-inflammatory effects against various animal disease models. In this investigation, we evaluated whether ED protected against CFA-induced arthritis in rats and investigated the potential mechanisms.
Materials and Methods
Rodent
Swiss Albino Wistar rats (6–8 weeks old; 100–125 g) were obtained from the Institute’s animal house and kept under standard laboratory conditions (22±5℃; 60–70% relative humidity; 12/12 h dark and light cycles). Access to food and water was allowed for the rats. Institute guidelines were followed throughout the entire experimental process. The 3201 Hospital animal ethical committee authorised this study (Approved No.3201–2021-0120).
CFA Induced Arthritis Model
Previously reported method was used for induction arthritis in rats with minor modifications.16 CII solution was emulsified in equal quantities of complete Freund’s adjuvant (CFA) by dissolving bovine natural collagen II (2 mg/mL) in acetic acid (50 mM) via continuous stirring at 4℃. The rats were given a 0.1 mL intradermal emulsion of CFA through loose skin at the tail’s base (day 0). Following successful primary vaccination, 0.1 mL CII in incomplete Freund’s adjuvant was injected intradermally.16 The rats were then divided into the following classes, each of which contains 10 rats. The following are the groups:-
Group I: normal control received the vehicle only (1% CMC solution).
Group II: CFA control received vehicle only (1% CMC solution).
Group III–V: CFA + ED (12.5, 25, and 50 mg/kg body weight) and:
Group VI: CFA + indomethacin (400 µg/kg), respectively.
The rats were given the appropriate doses once a day, and the normal and CFA groups were given the same amount of CMC (carboxy methyl cellulose). After initial immunisation, the procedure begins 22 days later. After the last intragastric injection, the rats were CO2 anaesthetized, and blood was taken from the cardiac apex.
Paw Swelling
The paw swelling of the experimental rats were estimated at regular time intervals (0, 7, 14, 21, and 28 days) using the plethysmometer via following the previously reported method.36
Thymus and Spleen Index
After scarifying the rats, the spleens and thymus tissues were successfully removed and weighted. A ratio of spleen and thymus wet weight to body weight was used to calculate the spleen and thymus index.37
Arthritis Score
For the estimation of arthritis score previous reported method of Kumar et al was used with minor modification.38,39 Two independent arthritis scores were carried out by blinded observation. During the observation, the severity and frequency of arthritis was scrutinized. From day 0 (primary immunization) to day 32 (last oral administration) arthritis scores were regularly recorded. During the arthritis score, the inflammation, its peaks, extends from the ankle via digits and it is characterized through erythema and extreme swelling were recorded. Arthritis score was estimated using a visual score from 0 to 4, where score 1 shows minimal inflammation and score 4 indicates the extensive erythema and swelling of all paws. Scores acquired for the four limbs were added to calculate the arthritis index for all animals, with a maximum score of 16 per rat.
Biochemical and Immune Parameters
After collecting the blood into the tube (without containing anticoagulant) was centrifuged at 5000 rpm for 10 min at 4℃. The serum was successfully separated and divided into the aliquots at 4℃. The levels of alkaline phosphatase (ALP), C-reactive protein (CRP), alanine amino transaminase (ALT), rheumatoid factor (RF), amino transaminase (AST), total protein, and serum nitrate were estimated using commercially available colorimetric assay kits following the manufacturing method (Jian Cheng Bioengineering Institute, China).
Antioxidant Parameters
The antioxidant parameters GSH, MDA, SOD, and CAT were calculated with minor modifications using the previous study.38,40
Hematological Parameters
Hematological parameters viz., haemoglobin (Hb), leukocytes (WBC), mean cell volume (MCV), erythrocytes (RBC), HCT (hematocrit), lymphocytes (LYM), MCHC (mean corpuscular hemoglobin concentration), and platelet (PLT) were assessed using readymade diagnostic reagent kits (Sunlong Biotech Co., Ltd. Zhejiang, China).
Elisa
Pro-inflammatory cytokines such as IL-2, IL-17, TNF-α, IL-10, IL-1β, IL-16, IL-6 (Bender MedSystem, Vienna, Austria), and TGF-β; angiogenesis markers such as VEGF, ANG-1, and HIF-1α were estimated using ELISA kits (Elabscience, China) following the manufacture protocol.
Cell Culture
The isolation of osteoclast-like cells was accomplished using a previously published method.41,42 Fresh blood from CFA induced rats was collected into an anticoagulant containing a test tube for the isolation of lymphocytes and transferred the sample into the cell bottle and cultured for the next 12 h. After culturing the sample, discard the upper layer (containing lymphocytes) and collect the lower adherent cells, which contain the mononuclear cells. The synovial tissue of CFA rats were collected and washed with the phosphate buffer saline (PBS) 3 times and superficially cut the synovium about 1–2 mm3 and kept into the Dulbecco’s modification Eagle’s medium and incubated for 3–4 h at 37℃. The tissues were entirely removed once the synovial fibroblasts were successfully grown into sheets. 1×106 mL−1 of monocytes were added to the 6 well plates. The synovial fibroblasts were then processed into a cell suspension 1×105 mL−1 and mixed into a hanging transwell chamber on a 6 well plate. The cells were then co-cultured in a CO2 incubator (5%) at 37℃ to produce osteoclast-like cells. Every day, while the cells are being cultured, a fresh culture medium is used.
Cell Viability Assay
The ED was sterilised and used for an in-vitro experimental study to determine the cell viability. To assess cell viability, a 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was used. In the 96 well plates, the cells (8×103 cells/well) were added together with M-CSF (30 ng/mL). Following a 12-hour culture, the cells were treated with various concentrations of ED (10–250 μg/mL), next 72 h, and estimated the optical density at 570 nm.43
TRAP Capacity
The estimation of TRAP levels in osteoclast-like cells was done using a previously described approach.25,44 The co-culture cells were exposed to ED at various concentrations (10, 20, 50, 100, and 250 μg/mL) for 48 hours, and the amount of TRAP in the supernatant was calculated.43
Elisa
The synovial fibroblasts and monocytes were co-cultured for 48 hours and the different dosages of ED (10, 20, 50, 100, and 250 μg/mL) were used. The supernatant of the culture medium was collected for the estimation of the level of TNF-α, IL-1, IL-6, IL-37 (Nanjing Jiancheng Bioengineering Institute, China) and RANKL, OPN, and M-CSF (Shanghai Yuanye Bio-Technology Co., Ltd., China) via manufacturing instructions.
Statistical Analysis
The results, which are presented as mean standard error mean (SEM), were produced using GraphPad Prism 7 software (St. Louis, USA). Dunnett’s t-test comparison and one-way ANOVA were used to assess the groups’ significance. Statistical significance was defined as a P value of 0.05.
Result
Body Weight
Reduction in body weight is a common feature of arthritis. In this experimental protocol, we selected almost similar body weight rats. Comparison with the normal and tested drug-treated rats, CFA rats exhibited a reduction in body weight. The ED and indomethacin group rats confirmed significantly (P<0.001) augmented the body weight (Figure 1A). The body weight of the ED (50 mg/kg) treated group of rats was remarkably similar to that of normal control group.
Arthritic Score
Arthritic score is an important feature of arthritis. During arthritis, it increases the swelling and redness in the rat paw. Normal rats (without treatment) did not show any signs and symptoms of arthritis and this group score 0. CFA-induced rats showed amplified arthritis scores as they increased the experimental study time. The maximum arthritis score was observed in this group at the end of the study. ED (12.5 mg/kg and 25 mg/kg) treated rats significantly (P<0.001) decreased arthritis score. ED (and 50 mg/kg) treated group rats established maximum down-regulation in the arthritic score (Figure 1B). Indomethacin treated group rats confirmed a reduction in the arthritic score.
Thymus and Spleen Index
The spleen and thymus indexes of the various groups of rats are shown in Figure 2. CFA-induced rats presented a significantly (P<0.001) amplified index level of spleen (Figure 2A) and thymus (Figure 2B). ED and indomethacin-treated group rats significantly (P<0.001) down-regulated the spleen and thymus index.
Paw Swelling
Table 1 shows an enhancement of paw swelling in CFA induced arthritis rats. ED treatment and indomethacin treated rats showed significant (P<0.001) reduction in paw swelling.
 |
Table 1 Effect of Edaravone on Paw Swelling in CFA Induced Arthritis |
CRP Level
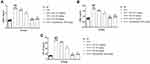
During injury, infection, or inflammatory reaction, the level of CRP boosted due to the expansion of disease. Normal levels of CRP were detected in the untreated rats. CFA-induced rats demonstrated 3–5 times higher CRP level as compared to normal rats, and ED treated rats significantly (P<0.001) diminution the level. Indomethacin treated group exhibited similar results (Figure 3A).
Rheumatoid Factor
RF is a significant indicator of arthritis. During arthritis conditions, RF levels are boosted due to increased inflammation reactions. The normal group did not show RF level. Alternatively, CFA induce rats revealed that boosted levels of RF and ED treatment significantly (P<0.001) suppressed the level of RF at dose dependent levels, suggesting the anti-arthritis effect. A similar momentum was observed after indomethacin treatment (Figure 3B).
Hematological Parameters
During arthritis, the alteration of hematological parameters observation is a common feature. Hematological parameters are commonly used to identify arthritis. Normal levels of hematological parameters were observed in the untreated rats. CFA treated rats presented a reduction in the level of HCT, RBC, Hb and boosted the level of LYM, WBC, PLT, MCV, MCHV. ED treated rats significantly (P<0.001) altered the level of HCT, RBC, Hb, PLT, LYM, WBC, MCV, MCHV (Figure 4).
Antioxidant Parameters
In the progression of arthritis, oxidative stress plays a significant role. CFA-treated rats displayed boosted MDA levels and reduced levels of SOD, GSH, and CAT compared with those without treated and tested drug-treated rats. ED and indomethacin received rats significantly (P<0.001) demonstrated the suppression in the level of MDA and improvement in the levels of SOD, GSH, CAT (Figure 5).
Nitrate and Total Protein Level
CFA treated rats significantly (P<0.001) exhibited reduced levels of total protein and ED treated rats significantly (P<0.001) increased the level of total protein (Figure 6A). CFA-treated rats had higher nitrate levels, but ED-treated rats had considerably lower nitrate level (Figure 6B).
Hepatic Parameters
The effect of ED on hepatic parameters is shown in Figure 7. An increased level of hepatic markers including ALP, ASL, and ALT was observed in CFA-induced arthritic rats. Rats given ED or indomethacin treatment significantly (P<0.001) had lower levels of hepatic markers.
Pro-Inflammatory Cytokines and Inflammatory Mediators
Figure 8 confirms the effect of ED and indomethacin on the level of pro-inflammatory cytokines. The levels of pro-inflammatory cytokines were altered in CFA-induced arthritis rats. ED and indomethacin-treated group rats significantly (P<0.001) modulated the level of pro-inflammatory cytokines.
The levels of COX-2, PGE2, and NF-κB in the untreated and treated groups of rats are shown in Figure 9. CFA-induced rats exhibited an enhanced level of COX-2 (Figure 9A), PGE2 (Figure 9B), and NF-κB (Figure 9C). ED and indomethacin-treated rats significantly (P<0.001) demonstrated reduced levels of COX-2, PGE2, and NF-κB.
VEGF, ANG-1, and HIF-1α Level
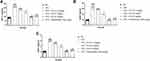
Figure 10 shows the effect of VEGF, ANG-1and HIF-1α in untreated and treated group rats. CFA-induced rats exhibited augmented levels of HIF-1α (Figure 10A), VEGF (Figure 10B) and ANG-1 (Figure 10C). ED and indomethacin-treated rats significantly (P<0.001) down-regulated the level of HIF-1α (Figure 10A), VEGF (Figure 10B) and ANG-1 (Figure 10C), respectively.
Cell Viability
The co-culture cells were treated with ED (10, 20, 50, 100, and 250 μg/mL), respectively. The results demonstrated the suppression in the viability of osteoclast cells (Figure 11A). ED treatment also showed a reduction in the level of TRAP (Figure 11B).
Cytokines
Figure 12 exhibits the effect of ED on cytokine level of co-culture cells. ED treatment significantly (P<0.001) showed the suppressed level of TNF-α (Figure 12A), IL-1 (Figure 12B), IL-6 (Figure 12C), and IL-37 (Figure 12D).
M-CSF, OPN, and RANKL
Figure 13 displays the effect of ED on the level of M-CSF, OPN, and RANKL of the co-culture cells. ED treated cells exhibited significant (P<0.001) reduction in the level of M-CSF (Figure 13A), OPN (Figure 13B), and RANKL (Figure 13C).
Discussion
RA disease treatment not only require inflammatory reaction control but also require suppression of bone erosion and joint destruction.43 It is well known that bone erosion is a hallmark of RA.45 Concurrently with the expansion of bone loss, anti-inflammatory drugs exhibited poor protection against bone loss. Therefore, urgently needed discovery of novel treatment for the treatment of bone erosion during RA disease. Chinese herbal drugs, their phytoconstituents and various chemical constituents showed protective effects against arthritis disease. Using osteoclast differentiation and HIF-1-VEGF-ANG-1 axis, we examined the anti-arthritis impact of edaravone against Complete Freund Adjuvant Induced Arthritis in this experimental investigation.
It is well known that RA disease is linked with bone destruction.43 Bone destruction occurs due to an imbalance between bone formation and bone resorption. Osteoclasts are significant cells that induce bone destruction during RA disease.46,47 Therefore, researchers are targeting osteoclast proliferation, differentiation, and formation for the treatment of RA disease.47 Under diverse circumstances, synovial macrophages and circulating monocytes can be stimulated to differentiate into functional osteoclasts, and they are also considered as the source of osteoclasts in RA joints.48 During RA conditions, synovial fibroblasts boost the monocyte to differentiate into the osteoclasts via secreting the cytokines like TNF-α, IL-1, IL-6, and IL-34.49–51
Inflammatory cytokines and related signaling pathways play a crucial role in osteoclast differentiation.52 Inflammatory cytokines secreted from the RA synovial fibroblasts boost the OPN level in the monocytes and also take part in the osteoclast maturation and differentiation regulation.51 Cytokine like IL-1 involved in the regulation of OPN, RANKL, and M-CSF. M-CSF and RANKL are significant cytokines for osteoclast differentiation. M-CSF and RANKL, two crucial receptors, demonstrated various stages of the osteoclastogenesis process, including the proliferation stage through M-CSF binding to c-FMS and the differentiation stage through RANKL binding to RANK.49,51,52 A further distinct cytokine, IL-34, can be triggered by M-CSF and functions as a ligand for the colony stimulating factor 1 receptor. Previous reports suggest that the IL-34 can boost the RANKL mediated osteoclast differentiation via enhancing the proliferation and adhesion of osteoclast precursors.51
Previous reports suggest that increased paw edema and altered organ weight during RA disease. According to Ahmad et al, paw edema enhanced during arthritis disease1 and CFA-induced group rats exhibited the same result. ED treatment remarkably suppressed the paw edema along with arthritis score, suggesting the anti-arthritic effect.
In this investigation, we found that CFA-induced arthritic rats had a lower body weight. Previous research has linked with low body weight observed during the arthritic condition due to the intestine’s inability to absorb glucose and leucine.40,54 We observed that ED therapy considerably raised body weight, implying that glucose and leucine absorption by the gut has improved.
The increase in inflammation during RA disease is well documented to be influenced by hepatic factors. The hepatic parameters are considered as an important marker of lysosomal membrane enzyme integrity, during the injury start the secretion of these enzymes into the serum and induce the hepatic tissue injury and finally cause the tissue damage.40,54 A cytoplasmic enzyme, such as ALP, is the α-glucuronidase lysosomal enzyme (phagocytic indicator) indicator, which is considered as the sensitive parameter of cellular injury caused via pathological conditions. The boosted level of ALP observed in this experimental group in the CFA group suggest the induction of inflammation.38,55 A similar result was seen in the levels of AST and ALT, with both enzymes rising in CFA-induced arthritis group rats and a decline level of hepatic parameters indicating a reduction in lysosomal stability.
It is well proven that alterations in the hematological parameters are commonly observed during arthritic conditions. The deformability of erythrocytes during the arthritic condition was generated due to reduction of RBC level, which was frequently seen and led to the animatic condition (shortened life of erythrocytes).5–7 The destruction of premature RBCs and the reduction of bone marrow erythropoietin cause reduction in the level of haemoglobin (Hb). WBC is a crucial immune system component linked to the production of inflammatory responses and disorders that are related to it.7,8 Kumar et al suggest that the boosted level of IL-1β and WBC starts the generation of inflammatory parameters such as macrophages and granulocytes and causes the expansion of colony stimulating factors.39,54 ED treatment remarkably altered the haematological parameters, suggesting an anti-arthritis effect.
Reactive oxygen and nitrogen species are generated during the body's cellular metabolism and play a crucial role in the development of disease. Free radicals (FR), reactive oxygen species (ROS), and reactive nitrogen species (RNS), all contribute to cellular instability and the emergence of unexpected inflammatory diseases like arthritis.5,6 ROS can damage tissue and various endogenous antioxidant mechanisms to protect the tissue from ROS-induced injury. Arthritis condition boosted synthesis of ROS via activation of phagocyte and neutrophils and finally causes oxidative stress in the tissue. The constant formation of oxygen species in the synovial membrane damages proteins, lipids, collagen, and nucleic acids, which also serves as a warning to inflammatory cells, aggravating arthritic disorder.8,9 Previous research indicates that the NF-κB pathway can be activated by ROS as a signal, which changes pro-inflammatory cytokines and other transcription mediators.5,11 In this experimental work, we saw a drop in the level of endogenous antioxidant enzymes, and ED therapy altered the level of endogenous antioxidants.
The autoimmune disorder RF (autoantibody) systemic changes and deformities are described by the induction of chronic inflammation in the synovial joint. During RA, the synovium swells as a result of the multiplication of synovial cells, which significantly contributes to the degeneration of cartilage.5,6 Bone erosion is the most common symptom of RA, which is related to prolonged and increased inflammation in the joints and affects 80% of the patients.5,53 Furthermore, the deficit in immune function is primarily due to a mismatch between the humoral and cellular immunity. Although cellular immunity increases and Th1 cells become activated and start the secretion of pro-inflammatory cytokines, while humoral immunity decreases, they suppress Th2 inflammatory cytokine secretion. According to previous research, cytokine development in the synovium begins with lymphocytes and mononuclear macrophages; it plays a central role in RA pathogenesis.5,7,8 Furthermore, the dynamic role of pro-inflammatory cytokines in the survival of RA lesions and the progression of RA disease, which is considered as a significant factor during RA disease. Inflammatory related cytokines are divided into two categories: one category of cytokines secreted by lymphocytes and the second category of cytokines secreted from the monocytes/macrophages.6,36 Previous research suggests that numerous markers directly or indirectly interact with inflammatory-related cytokines and play an important role in the expansion or incidence of arthritis disease.10,36 The well-known cytokine, TNF-α, altered the numerous functions in the pathogenesis of RA including accumulation of chemokines, leukocytes, activated endothelial cells, activation of osteoclasts and chondrocytes.11,56 During the arthritis condition, the level of TNF-α highly observed in the serum and inflamed area. The increased concentration of TNF-α induces the joint tissue injury and the onset of other clinical symptoms. IL-1β is present in the joint cavity and promotes endothelial cell growth and cell migration. T lymphocytes secrete IL-16, which is essential in the progression of arthritis disease. IL-16 does not have an impact on cartilage collagen during arthritis, which inhibits bone synthesis and improves osteoclast differentiation.9,57 Previous report suggests that IL-17 play a crucial role in the activation of T cell driven inflammation.3 The various cytokines like IL-17 and others, monocytes, and B cells were found in RA patients.58 In this study, we have observed that the boosted level of IL-17 and ED treatment remarkably suppressed the level of IL-17. In this experiment, rats in the CFA group had higher levels of inflammatory-related cytokines, while rats in the ED group had lower levels of inflammatory-related cytokines, implying an anti-inflammatory effect.
NF-κB pathway commonly used for treating various diseases. According to previous research, the NF-κB signalling pathway is widely used to treat arthritis.40,54 NF-κB plays a crucial role in the regulation of immune-related inflammatory responses. During the arthritis, NF-κB boosts various genes including cytokines, histocompatibility complexes, and chemokines. NF-κB migration immune cell and induces cellular proliferation and apoptosis.38,54 The degree of NF-κB was found to be higher in human and rodent biopsy samples. ED treatment remarkably diminished the level of NF-κB.
In this experimental study, we assessed arthritis assessments and biochemical parameters. Compared to normal group rats, the CFA animal model induces erythema, joint swelling, pannus, erythema, cartilage, and bone damage, and all of these indices steadily decreased after ED treatment, demonstrating that our animal models were well-constructed and our drugs had beneficial effects. In this study, we determined the possible mechanism of ED via reducing the angiogenesis parameters (HIF-1α–VEGF–ANG-1 axis) of CFA-induced arthritis rats. We estimated the level of VEGF, ANG-1, and HIF-1α in the serum of RA rats. CFA-induced arthritis rats showed augmented levels of VEGF, ANG-1, and HIF-1α in the serum and ED treatment remarkably suppressed the level. The findings show that ED can reduce CFA by inhibiting angiogenesis, and the mechanism is thought to be linked to the HIF-1α–VEGF–ANG-1 axis.
The synovial demand oxygen in the inflamed joint by excessive migration and proliferation of immune cells such as fibroblasts, endothelial cells, leukocytes, and macrophages. This process causes increased synovial membrane hyperplasia and a wider gap between synoviocytes and blood vessels, extending the oxygen diffusion range.16 The RA joint is unable to supply oxygen to support synoviocyte proliferation at the same time, creating a hypoxic and ischemic microenvironment. This increases the amount of HIF-1 in RA synovial fibroblasts, thymus-based lymphocytes (T-cells), and macrophages during RA conditions, controlling the cellular response to hypoxia and acting as an oxygen homeostasis regulator. Moreover, hypoxic microenvironment plays an important role and pioneer condition in early RA condition.16,59 VEGF, a downstream target of HIF-1α, has been connected to the production of pro-angiogenic mediators. Angiogenesis is regulated by a mixture of anti- and pro-angiogenic factors, with VEGF being the primary inducer of pro-angiogenic factors, according to prior studies.16,60 VEGF expression is improved by HIF-1α during the expansion of RA angiogenesis in hypoxia. Endothelial cells (ECs), which provide nutrients and oxygen to the tissue, often line the vascular system, which is a heavily branched network.16,59,61 Differentiation and proliferation of endothelial cells (ECs) occur during angiogenesis. Quiescent ECs protect blood vessels in human tissue, which are unsheathed by pericytes or mural cells when ANG-1 levels are high. Adult ECs are dormant for long periods of time, but when stimulated with pro-angiogenic factors like VEGF, they can quickly turn to growth.16,62 When ANG-1, ANG-2, and VEGF (all antagonists of Tie2) are present, they become overexpressed. By boosting EC survival and decreasing vascular permeability, ANG-1 has been shown to affect vessel development and stabilisation.16,63 VEGF increases vascular permeability, while ANG-1 increases vessel maturation while suppressing vascular permeability, a process known as angiogenesis. CFA-induced RA rats exhibited the boosted level of HIF-1α, VEGF, and ANG-1, and ED treatments suppressed their levels, suggesting protection against RA disease.
Conclusion
In this experimental study, ED treatment suppresses the arthritis index and improves the body weight. Additionally, ED treatment significantly altered the haematological and antioxidant parameters. ED treatment considerably reduced the level of inflammatory-related cytokines and inflammatory mediators in the serum and suggested an anti-arthritic effect. We also looked at the levels of VEGF, ANG-1 and HIF-1α, and found that ED could reduce the expression of angiogenesis parameters in the synovium and serum, indicating that the HIF-1α–VEGF–ANG-1 axis is involved in the angiogenesis process in CFA. The current study findings suggest that ED could recover from arthritis and its symptoms, protect bones from destruction and suppress osteoclast differentiation. This study may provide a novel approach to treating RA disease. ED treatment effectively relieved the symptoms of CFA-induced arthritis in rats.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bakheet SA, Ansari MA, Nadeem A, et al. CXCR3 antagonist AMG487 suppresses rheumatoid arthritis pathogenesis and progression by shifting the Th17/Treg cell balance. Cell Signal. 2019;64:1. doi:10.1016/j.cellsig.2019.109395
2. Ahmad SF, Ansari MA, Nadeem A, et al. STA-21, a STAT-3 inhibitor, attenuates the development and progression of inflammation in collagen antibody-induced arthritis. Immunobiology. 2017;222(2):206–217. doi:10.1016/j.imbio.2016.10.001
3. Ahmad SF, Ansari MA, Nadeem A, et al. The tyrosine kinase inhibitor tyrphostin AG126 reduces activation of inflammatory cells and increases Foxp3+ regulatory T cells during pathogenesis of rheumatoid arthritis. Mol Immunol. 2016;78:65–78. doi:10.1016/j.molimm.2016.08.017
4. Abd-Allah ARA, Ahmad SF, Alrashidi I, et al. Involvement of histamine 4 receptor in the pathogenesis and progression of rheumatoid arthritis. Int Immunol. 2014;26(6):325–340. doi:10.1093/intimm/dxt075
5. Mahdi HJ, Khan NAK, Bin AMZ, Mahmud R, Murugaiyah V. In vivo anti-arthritic and anti-nociceptive effects of ethanol extract of Moringa oleifera leaves on complete Freund’s adjuvant (CFA)-induced arthritis in rats. Integr Med Res. 2018;7(1):85–94. doi:10.1016/j.imr.2017.11.002
6. Zhu L, Zhang Z, Xia N, et al. Anti-arthritic activity of ferulic acid in complete Freund’s adjuvant (CFA)-induced arthritis in rats: JAK2 inhibition. Inflammopharmacology. 2020;28(2):463–473. doi:10.1007/s10787-019-00642-0
7. He P, Hu Y, Huang C, et al. N -Butanol extract of gastrodia elata suppresses inflammatory responses in lipopolysaccharide-stimulated macrophages and Complete Freund’s Adjuvant- (CFA-) induced arthritis rats via inhibition of MAPK signaling pathway. Evid Based Complement Altern Med. 2020;2020:1–11. doi:10.1155/2020/1658618
8. Gutiérrez-Rebolledo GA, Garduño-Siciliano L, Chávez-Rueda AK, Siordia-Reyes AG, Zamilpa A, Jiménez-Arellanes MA. In vivo anti-arthritic and antioxidant effects from the standardized ethanolic extract of Moussonia deppeana. Rev Bras Farmacogn. 2018;28(2):198–206. doi:10.1016/j.bjp.2018.02.004
9. Tong Z, Cheng L, Song J, et al. Therapeutic effects of Caesalpinia minax Hance on complete Freund’s adjuvant (CFA)-induced arthritis and the anti-inflammatory activity of cassane diterpenes as main active components. J Ethnopharmacol. 2018;226:90–96. doi:10.1016/j.jep.2018.08.011
10. Saad MA, El-Sahhar AE, Arab HH, Al-Shorbagy MY. Nicorandil abates arthritic perturbations induced by complete Freund’s adjuvant in rats via conquering TLR4-MyD88-TRAF6 signaling pathway. Life Sci. 2019;218:284–291. doi:10.1016/j.lfs.2019.01.002
11. Patel MG, Pundarikakshudu K. Anti-arthritic activity of a classical ayurvedic formulation Vatari Guggulu in rats. J Tradit Complement Med. 2016;6(4):389–394. doi:10.1016/j.jtcme.2015.08.007
12. Abadi SSH, Gangadharappa HV, Balamuralidhara V. Development of colon-specific mucoadhesive meloxicam microspheres for the treatment of CFA-induced arthritis in rats. Int J Polym Mater Polym Biomater. 2021;70(12):849–869. doi:10.1080/00914037.2020.1765359
13. Liu JY, Hou YL, Cao R, et al. Protodioscin ameliorates oxidative stress, inflammation and histology outcome in Complete Freund’s adjuvant induced arthritis rats. Apoptosis. 2017;22(11):1454–1460. doi:10.1007/s10495-017-1420-0
14. Zhang Z, Chinnathambi A, Ali Alharbi S, Bai L. Copper oxide nanoparticles from Rabdosia rubescens attenuates the complete Freund’s adjuvant (CFA) induced rheumatoid arthritis in rats via suppressing the inflammatory proteins COX-2/PGE2. Arab J Chem. 2020;13(6):5639–5650. doi:10.1016/j.arabjc.2020.04.005
15. Koo ST, Lee CH, Choi H, et al. The effects of pressure on arthritic knees in a rat model of CFA-induced arthritis. Pain Phys. 2013;16(2):95–102.
16. Feng Z, Yang T, Hou X, et al. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomed Pharmacother. 2019;113:108759. doi:10.1016/j.biopha.2019.108759
17. Ali EAI, Barakat BM, Hassan R. Antioxidant and angiostatic effect of spirulina platensis suspension in complete Freund’s adjuvant-Induced arthritis in rats. PLoS One. 2015;10(4). doi:10.1371/journal.pone.0121523
18. Byram K, Chinratanalab S, Sergent J. Rheumatoid Arthritis. In: Essentials of Physical Medicine and Rehabilitation: Musculoskeletal Disorders, Pain, and Rehabilitation. UK: Oxford University Press; 2018:876–881.
19. Barua CC, Bodduluru LN, Haloi P, et al. Anti-arthritic and anti-inflammatory activity of a polyherbal formulation against Freund’s complete adjuvant induced arthritis in Wistar rats. Indian J Tradit Knowl. 2017;16(3):482–489.
20. Ruckmani A, Meti V, Vijayashree R, et al. Anti-rheumatoid activity of ethanolic extract of Sesamum indicum seed extract in Freund’s complete adjuvant induced arthritis in Wistar albino rats. J Tradit Complement Med. 2018;8(3):377–386. doi:10.1016/j.jtcme.2017.06.003
21. Veale DJ, Fearon U. Inhibition of angiogenic pathways in rheumatoid arthritis: potential for therapeutic targeting. Best Pract Res. 2006;20:941–947. doi:10.1016/j.berh.2006.05.004
22. Hanlon MM, Rakovich T, Cunningham CC, et al. STAT3 mediates the differential effects of oncostatin M and TNFα on RA synovial fibroblast and endothelial cell function. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.02056
23. Noort AR, van Zoest KPM, Weijers EM, et al. NF-κB-inducing kinase is a key regulator of inflammation-induced and tumour-associated angiogenesis. J Pathol. 2014;234(3):375–385. doi:10.1002/path.4403
24. Wu G, Nie W, Wang Q, et al. Umbelliferone ameliorates complete Freund adjuvant–induced arthritis via reduction of NF-κB signaling pathway in osteoclast differentiation. Inflammation. 2021;44(4):1315–1329. doi:10.1007/s10753-021-01418-x
25. Luo XH, Guo LJ, Xie H, et al. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21(10):1648–1656. doi:10.1359/jbmr.060707
26. Kobayashi T, Notoya K, Naito T, et al. Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist reduces inflammatory-induced alteration of bone structure in rat adjuvant polyarthritis: evidence for a bone protecting effect in inflammatory conditions. Osteoarthr Cartil. 2009;17:S93–4. doi:10.1016/S1063-4584(09)60178-3
27. Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68(5):744–750. doi:10.1136/ard.2007.086066
28. Berardi S, Corrado A, Maruotti N, Cici D, Cantatore FP. Osteoblast role in the pathogenesis of rheumatoid arthritis. Mol Biol Rep. 2021;48:2843–2852. doi:10.1007/s11033-021-06288-y
29. Komatsu N. Immune molecules and the mechanism of joint destruction. Clin Calcium. 2016;26:683–689.
30. Cecchi I, Arias de la Rosa I, Menegatti E, et al. Neutrophils: novel key players in rheumatoid arthritis. Current and future therapeutic targets. Autoimmun Rev. 2018;17:1138–1149. doi:10.1016/j.autrev.2018.06.006
31. Wei J, Li Y, Liu Q, et al. betulinic acid protects from bone loss in ovariectomized mice and suppresses RANKL-associated osteoclastogenesis by inhibiting the MAPK and NFATc1 pathways. Front Pharmacol. 2020;11. doi:10.3389/fphar.2020.01025
32. Posa Krishnamoorthy P, Muthukumaran S. In vitro studies to determine the effect of boeravinone B on human dendritic cells. Pharmacogn Mag. 2018;14(56):465–470. doi:10.4103/pm.pm_625_17
33. Huang Y, Sun Y, Wang WW, Zhang L. Boeravinone B a natural rotenoid exerts anticancer activity via inducing internalization and degradation of inactivated EGFR and ERBB2 in human colon cancer cells. Am J Transl Res. 2018;10(12):4183–4192.
34. Zhang J, Zong L, Bai D. Boeravinone b promotes fracture healing in ovariectomy-induced osteoporotic rats via the regulation of NF-κB p65/IκB-α/SIRT-1 signaling pathway. Trop J Pharm Res. 2019;18(5):955–960. doi:10.4314/tjpr.v18i5.7
35. Singh S, Kalia NP, Joshi P, et al. A novel dual inhibitor of Nora bacterial efflux pump of Staphylococcus aureus and human P-Glycoprotein, reduces the biofilm formation and intracellular invasion of bacteria. Front Microbiol. 2017;8. doi:10.3389/fmicb.2017.01868
36. Weng W, Wang F, He X, Zhou K, Wu X, Wu X. Protective effect of corynoline on the CFA induced rheumatoid arthritis via attenuation of oxidative and inflammatory mediators. Mol Cell Biochem. 2021;476(2):831–839. doi:10.1007/s11010-020-03948-8
37. Zeng Z, Yan K, Liu W. Specneuzhenide ameliorate complete Freund adjuvant induced arthritis in rats: involvement of NF-κB and HO-1/Nrf-2 pathway. J Oleo Sci. 2022;71(4):551–561. doi:10.5650/jos.ess21413
38. Kumar V, Bhatt PC, Rahman M, et al. Melastoma malabathricum Linn attenuates complete Freund’s adjuvant-induced chronic inflammation in Wistar rats via inflammation response. BMC Complement Altern Med. 2016;16(1). doi:10.1186/s12906-016-1470-9
39. Kumar V, Anwar F, Verma A, Mujeeb M. Therapeutic effect of umbelliferon-α-D-glucopyranosyl-(2I→1II)-α-D-glucopyranoside on adjuvant-induced arthritic rats. J Food Sci Technol. 2015;52(6):3402–3411. doi:10.1007/s13197-014-1403-x
40. Kumar V, Al-Abbasi FA, Verma A, Mujeeb M, Anwar F. Umbelliferone β-d-galactopyranoside exerts an anti-inflammatory effect by attenuating COX-1 and COX-2. Toxicol Res. 2015;4(4):1072–1084. doi:10.1039/C5TX00095E
41. Yeom J, Yim DJ, Ma S, Lim YH. Propionibacterium freudenreichii inhibits rankl-induced osteoclast differentiation and ameliorates rheumatoid arthritis in collagen-induced arthritis mice. Microorganisms. 2022;10(1):1.
42. Wang T, Qiao H, Zhai Z, et al. Plumbagin ameliorates collagen-induced arthritis by regulating Treg/Th17 cell imbalances and suppressing osteoclastogenesis. Front Immunol. 2019;10:1.
43. Ling Y, Yang J, Hua D, et al. ZhiJingSan inhibits osteoclastogenesis via regulating RANKL/NF-κB signaling pathway and ameliorates bone erosion in collagen-induced mouse arthritis. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.693777
44. Jia X, Zhu H, Li G, et al. Anti-osteoporotic effects of alisol C 23-acetate via osteoclastogenesis inhibition. Biomed Pharmacother. 2021;137:111321. doi:10.1016/j.biopha.2021.111321
45. Rahman M, Kumar V, Beg S, Sharma G, Katare OP, Anwar F. Emergence of liposome as targeted magic bullet for inflammatory disorders: current state of the art. Artif Cells Nanomed Biotechnol. 2016;44(7):1597–1608. doi:10.3109/21691401.2015.1129617
46. Tanaka S. Emerging anti-osteoclast therapy for rheumatoid arthritis. J Orthop Sci. 2018;23:717–721. doi:10.1016/j.jos.2018.06.001
47. Wang Y, Chen S, Du K, et al. Traditional herbal medicine: therapeutic potential in rheumatoid arthritis. J Ethnopharmacol. 2021;279:1.
48. Shen W, Guan YY, Wu RM, et al. Protective effects of Wang-Bi tablet on bone destruction in collagen-induced arthritis by regulating osteoclast-osteoblast functions. J Ethnopharmacol. 2019;238:111861. doi:10.1016/j.jep.2019.111861
49. Kim KW, Kim HR, Kim BM, La CM, Lee SH. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis. Am J Pathol. 2015;185(11):3011–3024. doi:10.1016/j.ajpath.2015.07.017
50. Uehara IA, Soldi LR, Silva MJB. Current perspectives of osteoclastogenesis through estrogen modulated immune cell cytokines. Life Sci. 2020;256:117921. doi:10.1016/j.lfs.2020.117921
51. Chen Z, Buki K, Vääräniemi J, Gu G, Väänänen HK. The critical role of IL-34 in osteoclastogenesis. PLoS One. 2011;6(4):5.
52. Tanaka U, Kajioka S, Finoti LS, Palioto DB, Kinane DF, Benakanakere MR. Decitabine inhibits bone resorption in periodontitis by upregulating anti-inflammatory cytokines and suppressing osteoclastogenesis. Biomedicines. 2021;9(2):1–13. doi:10.3390/biomedicines9020199
53. Nirbhavane P, Sharma G, Singh B, et al. Preclinical explorative assessment of celecoxib-based biocompatible lipidic nanocarriers for the management of CFA-induced rheumatoid arthritis in Wistar rats. AAPS Pharm Sci Tech. 2018;19(7):3187–3198. doi:10.1208/s12249-018-1148-3
54. Kumar V, Al-Abbasi FA, Ahmed D, Verma A, Mujeeb M, Anwar F. Paederia foetida Linn. Inhibits adjuvant induced arthritis by suppression of PGE2 and COX-2 expression via nuclear factor-κB. Food Funct. 2015;6(5):1652–1666. doi:10.1039/C5FO00178A
55. Chauhan S, Devi U, Kumar VR, Kumar V, Anwar F, Kaithwas G. Dual inhibition of arachidonic acid pathway by mulberry leaf extract. Inflammopharmacology. 2015;23(1):65–70. doi:10.1007/s10787-014-0223-y
56. Alolga RN, Opoku-Damoah Y, Alagpulinsa DA, et al. Metabolomic and transcriptomic analyses of the anti-rheumatoid arthritis potential of xylopic acid in a bioinspired lipoprotein nanoformulation. Biomaterials. 2021;268:120482. doi:10.1016/j.biomaterials.2020.120482
57. Liu FC, Yu HP, Chen PJ, et al. A novel NOX2 inhibitor attenuates human neutrophil oxidative stress and ameliorates inflammatory arthritis in mice. Redox Biol. 2019;26:101273. doi:10.1016/j.redox.2019.101273
58. Ahmad SF, Zoheir KMA, Bakheet SA, Ashour AE, Attia SM. Poly (ADP-ribose) polymerase-1 inhibitor modulates T regulatory and IL-17 cells in the prevention of adjuvant induced arthritis in mice model. Cytokine. 2014;68(2):76–85. doi:10.1016/j.cyto.2014.04.006
59. Lai W-Q, Irwan AW, Goh HH, et al. Anti-inflammatory effects of sphingosine Kinase modulation in inflammatory arthritis. J Immunol. 2008;181(11):8010–8017. doi:10.4049/jimmunol.181.11.8010
60. Murakami M, Iwai S, Hirasuka S, Iwakura Y, Maru Y, Shibuya M. VEGFR-1(Flt-1) tyrosine kinase signaling enhances hematopoiesis, proliferation/differentiation and immunity of monocyte/macrophage from bone marrow hematopoietic stem cells, and promotes rheumatoid arthritis. Blood. 2004;104(11):778. doi:10.1182/blood.V104.11.778.778
61. Guo X, Ji J, Jose Kumar Sreena GS, et al. Computational prediction of antiangiogenesis synergistic mechanisms of total saponins of panax japonicus against rheumatoid arthritis. Front Pharmacol. 2020;11. doi:10.3389/fphar.2020.566129
62. Xu J, Feng Z, Chen S, et al. Taxol alleviates collagen-induced arthritis in mice by inhibiting the formation of microvessels. Clin Rheumatol. 2019;38(1):19–27.
63. Han CC, Liu Q, Zhang Y, et al. CP-25 inhibits PGE2-induced angiogenesis by down-regulating EP4/AC/cAMP/PKA-mediated GRK2 translocation. Clin Sci. 2020;134(3):331–347. doi:10.1042/CS20191032
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.