Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Annual Body Weight Change and Prognosis in Chronic Obstructive Pulmonary Disease
Authors Abe Y, Suzuki M , Shima H, Shiraishi Y , Tanabe N , Sato S , Shimizu K, Kimura H, Makita H, Hirai T, Konno S, Nishimura M
Received 12 September 2021
Accepted for publication 23 November 2021
Published 30 November 2021 Volume 2021:16 Pages 3243—3253
DOI https://doi.org/10.2147/COPD.S338908
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Yuki Abe,1 Masaru Suzuki,1 Hiroshi Shima,2 Yusuke Shiraishi,2 Naoya Tanabe,2 Susumu Sato,2 Kaoruko Shimizu,1 Hirokazu Kimura,1 Hironi Makita,1,3 Toyohiro Hirai,2 Satoshi Konno,1 Masaharu Nishimura1,3
1Department of Respiratory Medicine, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Kita-ku, Sapporo, 060-8638, Japan; 2Department of Respiratory Medicine, Graduate School of Medicine, Kyoto University, Kyoto, 606-8507, Japan; 3Hokkaido Medical Research Institute for Respiratory Diseases, Chuo-ku, Sapporo, 060-0063, Japan
Correspondence: Masaru Suzuki
Department of Respiratory Medicine, Faculty of Medicine, Hokkaido University, Kita 15, Nishi 7, Kita-ku, Sapporo, 060-8638, Japan
Tel +81-11-706-5911
Fax +81-11-706-7899
Email [email protected]
Purpose: Low body mass index (BMI) has been reported to be associated with poor prognosis in patients with chronic obstructive pulmonary disease (COPD). In contrast, a detailed analysis of the association between body weight change over time and prognosis is not sufficient, particularly in Japanese patients with COPD who have been reported to be much thinner compared to Westerners. This study aimed to investigate the relationship between annual body weight change and long-term prognosis in Japanese patients with COPD in two independent cohorts.
Patients and Methods: We analyzed 279 patients with COPD who participated in the Hokkaido COPD cohort study as a discovery cohort. We divided participants into three groups according to quartiles of annual body weight change calculated by the data from the first 5 years: weight loss group ( 0.20 kg/year). The association between annual body weight change and prognosis was replicated in the Kyoto University cohort (n = 247).
Results: In the Hokkaido COPD cohort study, the weight loss group had significantly worse mortality than the other groups, whereas there was no difference in BMI at baseline. In the multivariate analysis, annual body weight change was an independent risk factor for all-cause mortality, which was confirmed in the Kyoto University cohort.
Conclusion: Annual body weight loss is associated with poor prognosis in Japanese patients with COPD, independent of baseline BMI. Longitudinal assessment of body weight is important for the management of COPD.
Keywords: annual body weight change, body mass index, chronic obstructive pulmonary disease, mortality, prognosis, weight loss
Corrigendum for this paper has been published.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of death worldwide and is characterized by persistent airflow limitation and systemic inflammation.1 In patients with COPD, increased ventilation costs due to obstructive ventilatory impairment and systemic inflammation cause an imbalance between energy intake and expenditure, leading to weight loss.2 Low body mass index (BMI) is a strong predictor of mortality in patients with COPD; therefore, nutritional support has been advocated as an effective approach to the care of COPD patients with low BMI.2,3 We previously reported that low BMI was also associated with shorter exacerbation-free survival, higher frequency of exacerbations, and the occurrence of recurrent exacerbations in patients with COPD.4 The BODE index, including BMI, has been proposed as a prognostic factor for COPD,5 and the importance of assessing baseline BMI is well known. In contrast, longitudinal weight loss during the clinical course is also a common and serious problem for patients with COPD regardless of baseline BMI and has been reported to be an independent predictor of mortality.6–8 We also reported that rapid annual body weight loss during the first 5 years (≤ −0.458 kg/year determined by classification and regression tree analysis) was associated with shorter survival thereafter.9 However, a detailed analysis of the association between body weight change over time and prognosis as well as causes of death is not sufficient, particularly in Japanese patients with COPD who have been reported to be much thinner compared to Westerners.10,11
In the present study, we aimed to retrospectively explore the associations between annual body weight change and long-term prognosis as well as causes of death in two independent prospective cohorts of Japanese patients with COPD: the Hokkaido COPD cohort study and the Kyoto University cohort.
Patients and Methods
Hokkaido COPD Cohort Study
Study Protocol
The details of the Hokkaido COPD cohort study have been described previously.4,9,12–14 Japanese patients with COPD were recruited at Hokkaido University Hospital (Sapporo, Japan) and nine affiliated hospitals from May 2003 to May 2005. All participants were older than 40 years of age and were current or former smokers with a smoking history of 10 pack-years or more. Patients who had a current diagnosis of asthma and any history of childhood asthma were completely excluded by a respiratory specialist. During the first year of follow-up, the diagnosis was reconfirmed based on the spirometry criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.15 A total of 279 patients with COPD (GOLD 1, 26%; GOLD 2, 45%; GOLD 3, 24%; GOLD 4, 5%) were followed up for the subsequent study. On the first visit, demographic information, including sex, age, height, weight, smoking history, comorbidities, medical history, medications, and information on pulmonary symptoms were collected. We conducted computed tomography (CT) at baseline and performed spirometry every 6 months before and after bronchodilator inhalation. The CT scanners and other technical parameters used in this study were described in previous reports.12,16 The severity of emphysema was scored using CT according to the modified Goddard scoring system.17 Three-dimensional computerized CT analysis of low attenuation volume (LAV) was performed to evaluate the severity of emphysema in participants with available three-dimensional data (n=156) in whom inspiratory CT scans with a 1.25 mm slice thickness were acquired at Hokkaido University Hospital using a Somatom Plus Volume Zoom scanner (Siemens AG, Berlin, Germany) at 140 kVp, 150 mA, 4×1 mm detector collimation, and helical pitch 6 or 7. The raw data were reconstructed using a soft algorithm (standard kernel, FC30).13,16 Until the 5th year of the study, moderate-to-severe exacerbation (requiring prescription change and/or hospital admission) was recorded,4 and body weight was measured annually. Most of the eligible individuals who dropped out of the regular follow-up program visited outpatient clinics to receive appropriate medical care. Therefore, telephone interviews and chart reviews to monitor 10-year annual mortality data were required for only a very small number of study participants. Only eight patients (2.9%) received a telephone interview to obtain data on 10-year mortality. Information about fatal events was collected as described previously.9 This study was conducted as per the Declaration of Helsinki and was approved by the ethics committee of Hokkaido University School of Medicine (med02-001). All participants provided written informed consent.
Participants
The annual change in body weight and post-bronchodilator forced expiratory volume in 1 second (FEV1) during the first 5 years were estimated using linear mixed-effects models.9 Based on the annual change in body weight, we classified the participants into the three groups as weight loss group (<-0.17 kg/year or 25th percentile), no change group (−0.17 to ≤0.20 kg/year), and weight gain group (>0.20 kg/year or 75th percentile). We examined the association between body weight change during the first 5 years and 10-year mortality, including the causes of death. The cause of death was divided into four categories: respiratory diseases (including respiratory failure, pneumonia, and lung abscess); malignancy (lung cancer or cancer in other organs); cardiovascular diseases (including heart failure, ischemic heart diseases, aortic aneurysm rupture, aortic dissection, cerebral infarction, and cerebral hemorrhage); and others (including renal failure, gastrointestinal disease, and accident). No patients died of pulmonary embolism. Additionally, the ratio of body weight during the first 5 years divided by the body weight at baseline was calculated. The annual change in this ratio was estimated using linear mixed-effects models, and the participants were divided into three groups: rate decrease group (<-1.21%/year or 25th percentile), no change group (−1.21 to ≤0.08%/year), and rate increase group (>0.08%/year or 75th percentile). The annual change in the rate of FEV1 to baseline during the first 5 years was calculated in the same manner.
Kyoto University Cohort
The Kyoto University cohort has been described previously.18–21 Briefly, 247 patients with COPD underwent chest inspiratory CT scans and pulmonary function tests and were originally followed up. Patients who had a current diagnosis asthma were excluded. For the present study, the follow-up period was extended as of October 2019, and the median of the final follow-up duration was 3277 (2331–4240) days, and information on all-cause mortality and the cause of death during the follow-up period were recorded. Information on moderate-to-severe exacerbation (requiring antibiotics and/or systemic corticosteroids and/or hospital admission) during the first 5 years was also collected. All CT images with a 0.5 mm slice thickness at full inspiration were obtained using an Aquilion 64 scanner (Toshiba, Tokyo, Japan) with a 0.5 mm collimation, a scan time of 500 ms, 120 kVp, and auto-exposure control, and reconstructed using a sharp algorithm (FC56). The severity of emphysema in all patients was assessed using the %LAV.22 Body weight was measured at least once per year. The annual change in body weight and post-bronchodilator FEV1 during the first 5 years was calculated using linear mixed-effects models, and the association between body weight change during the first 5 years and mortality was examined as in the Hokkaido COPD cohort study. Based on the cutoff values of annual change in body weight determined in the Hokkaido COPD cohort study, patients were divided into three groups: weight loss, no change, and weight gain. Additionally, patients were classified into three groups: rate decrease, no change, and rate increase using the same methods and cutoff values as in the Hokkaido COPD cohort study. This study was conducted as per the Declaration of Helsinki and was approved by the ethics committee of Kyoto University (E182 and R0311-2). All participants provided written informed consent.
Statistical Analysis
Differences among the groups were analyzed using Fisher’s exact test for categorical variables, Student’s t-test or one-way analysis of variance for continuous parametric variables, and the Mann–Whitney U-test or Kruskal–Wallis test for continuous nonparametric variables. Factors associated with mortality were analyzed using the Kaplan-Meier method with the Log rank test and multivariate Cox proportional hazards models. Two Cox proportional hazard models were used. The covariate included in the first model (Model 1) was the age at enrollment. Covariates included in the second model (Model 2) were age, sex, BMI, smoking status, FEV1, and the severity of emphysema at enrollment. For the severity of emphysema, we used the CT emphysema score in the Hokkaido COPD cohort study because three-dimensional CT data were not available in some patients in whom %LAV could not be assessed for emphysema. Covariates for inclusion in the models were selected based on potential confounding variables known to affect COPD mortality. Logistic regression analysis was performed as required. Statistical significance was defined as p <0.05. Statistical analyses were performed using JMP (SAS Institute Inc., Cary, NC, USA) and EZR version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R software (The R Foundation for Statistical Computing, Vienna, Austria).23
Results
Hokkaido COPD Cohort Study
The characteristics of the participants in the Hokkaido COPD cohort study are shown in Table 1. Figure 1 illustrates the distribution of annual body weight change, and the patients were classified into three groups according to the quartiles of the distribution of annual body weight change: weight loss group (<-0.17 kg/year, −0.50 ± 0.25 kg/year: mean ± standard deviation); no change group (−0.17 to ≤0.20 kg/year, 0.03 ± 0.10 kg/year); and weight gain group (>0.20 kg/year, 0.43 ± 0.22 kg/year). Table 2 shows the clinical characteristics of the three groups. There were significant differences in age and the annual decline in FEV1 among the three groups, whereas there were no significant differences in sex, smoking status, pulmonary function test results, CT emphysema score, or annual decline in the rate of FEV1 to baseline. The height and BMI at baseline did not differ among the three groups. The weight loss group had a higher mortality rate than the other groups (p = 0.01, Figure 2A), and the association was similar only for participants who survived during the first 5 years (p=0.006, Figure 2B).
 |
Table 1 Characteristics of Subjects in the Hokkaido COPD and Kyoto University Cohorts |
 |
Table 2 Characteristics of Groups Divided by Annual Weight Change in the Hokkaido COPD Cohort |
 |
Figure 1 Distribution of annual body weight change in the Hokkaido COPD cohort study. |
The multivariate Cox proportional hazards models showed that annual body weight change was significantly associated with mortality after adjustments for age as well as adjustments for both BMI and emphysema score on CT at baseline (Table 3). Regarding the cause of death, the prevalence of respiratory diseases was higher in the weight loss group (Table 4). Even after excluding patients who died of malignancy, there was a significant association between the weight loss group and poor prognosis (p=0.04, Figure 2C).
 |
Table 3 Multivariable Cox Proportional Hazards Analysis of All-Cause Mortality in the Hokkaido COPD and Kyoto University Cohorts |
 |
Table 4 Causes of Death in the Hokkaido COPD and Kyoto University Cohorts |
Kyoto University Cohort
The characteristics of the participants in the Kyoto University cohort are shown in Table 1. The FEV1 and forced vital capacity (FVC) were significantly lower and carbon monoxide diffusion capacity (DLCO) and %LAV were higher in the Kyoto University cohort than in the Hokkaido COPD cohort study. Patients were divided into three groups based on the cutoff values used in the Hokkaido COPD cohort study: weight loss group (<-0.17 kg/year, −0.55 ± 0.29 kg/year); no change group (−0.17 to ≤0.20 kg/year, −0.006 ± 0.11 kg/year); and weight gain group (>0.20 kg/year, 0.46 ± 0.24 kg/year). Table 5 shows the clinical characteristics of the three groups in the Kyoto University cohort. There were significant differences in the severity of airflow limitation (GOLD stages), FEV1, and FEV1/FVC among the three groups, whereas height and BMI at baseline did not differ. The weight loss group had a higher mortality rate than the other groups (p = 0.005, Figure 3A), and the association was similar only for participants who survived during the first 5 years or when participants who died of malignancy were excluded (p=0.04, Figure 3B; p=0.007, Figure 3C). There was no difference in the causes of death among the three groups (Table 4). The multivariate Cox proportional hazards models showed that annual body weight change was significantly associated with mortality, independent of BMI or %LAV at baseline (Table 3).
 |
Table 5 Characteristics of Groups Divided by Annual Weight Change in the Kyoto University Cohort |
Factors Associated with Annual Body Weight Loss
To identify the factors that contribute to annual body weight loss, a multivariate logistic regression model for the weight loss group was performed using the combined participants from the two cohorts. Consequently, older age and a higher frequency of exacerbations during the first 5 years were independent predictors for the weight loss group (Table 6).
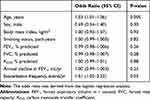 |
Table 6 Relative Risk for Weight Loss Group versus Others Using Logistic Regression Analysis |
Association of Annual Change in the Rate of Body Weight to Baseline with Mortality
Since annual changes in absolute body weight values may be affected by body weight at baseline, the association between the annual change in the rate of body weight to baseline and mortality was also examined using combined participants from the two cohorts. Participants were divided into three groups based on the quartiles of annual change in the rate of body weight to the baseline of the Hokkaido COPD cohort study: rate decrease group (<-1.21%/year, −2.0 ± 0.8%/year), no change group (−1.21 to ≤0.08%/year, −0.5 ± 0.3%/year), and rate increase group (>0.08%/year, 0.9 ± 0.8%/year). There were significant differences in BMI at baseline among the three groups (Supplementary Table 1). The rate decrease group had higher mortality compared with that of the other groups, and the association was similar only for participants who survived during the first 5 years or when participants who died of malignancy were excluded (Supplementary Figure 1). The multivariate Cox proportional hazards models showed that annual changes in the rate of body weight to baseline were significantly associated with mortality (Supplementary Table 2).
Discussion
In this study, we first examined the association between annual body weight change and mortality in Japanese patients with COPD using data from the Hokkaido COPD cohort study. We found that annual body weight loss assessed over 5 years was associated with poor prognosis, independent of baseline BMI. The strength of this study is that the major findings of the Hokkaido COPD cohort study were confirmed in another independent cohort, the Kyoto University cohort. Additionally, multivariate logistic regression analysis demonstrated that body weight loss over time was independently associated with a higher frequency of exacerbations. These results suggest that the rate of weight loss would be a useful indicator of future mortality risk in patients with COPD, regardless of their BMI.
Low BMI has been reported to be associated with higher long-term mortality in patients with COPD.3,24,25 Underweight is associated with reduced lung function,26 decreased metabolically and functionally active fat-free mass (FFM),27 and a decrease in diaphragm muscle strength.28 Low BMI is also associated with frequent exacerbations,4 which leads to a faster decline in FEV1 and higher mortality.29,30 On the other hand, weight loss in the clinical course of COPD is due to multiple factors, such as oxidative stress causing systemic inflammation,31,32 higher metabolic rate, and increased ventilation costs.33 Increased levels of acute-phase reactant proteins and inflammatory cytokines are related to increased resting energy expenditure and decreased FFM.33 Previous reports have shown that the loss of skeletal muscle mass, particularly the erector spinae muscles, was correlated with BMI and was significantly associated with poor prognosis.34,35
Regarding factors associated with annual body weight loss in patients with COPD, we showed that a higher frequency of exacerbations during the first 5 years was a significant predictor for the weight loss group in this study. It has been reported that an impaired endogenous oxidant-antioxidant balance associated with evidence of increased oxidative stress has been reported during COPD exacerbations.36 Since oxidants enhance muscle proteolysis,37 this may be relevant for the pathogenesis of skeletal muscle mass loss in patients with COPD and may contribute to weight loss in frequent exacerbators.38 Additionally, there was a significant difference in the annual decline in FEV1 among the three groups in the Hokkaido COPD cohort study. The slower annual FEV1 decline in the weight loss group may be due to the lower baseline FEV1 in the weight loss group. The annual decline in the rate of FEV1 to baseline also tended to be slower in the weight loss group, but there was no significant difference among the three groups.
The prognostic value of weight loss may be reflected by cachexia in the terminal phase of COPD. Additionally, the increased mortality in participants with weight loss could be due to other terminal illnesses, such as advanced cancer. However, if the prognostic value of weight loss in COPD is merely a reflection of terminal disease, it would be limited primarily to the first few months or years after evaluation.39 In the present study, we confirmed that the annual body weight loss during the first 5 years was associated with all-cause mortality among participants who survived during the first 5 years. In the weight loss group, the most frequent cause of death was a respiratory disease, but not malignancy in both cohorts. We also confirmed that there was a relationship between weight loss and poor prognosis, even when patients who died of malignancy were excluded. Consequently, the association between weight loss and poor prognosis is not only due to terminal illness or the end stage of COPD. It has been reported that the increased rate of body weight loss started around 9 years before death for all-cause mortality in the Baltimore Longitudinal Study of Aging.40 Therefore, the rate of body weight loss may reflect a poor long-term prognosis. However, another report has shown improved prognosis with weight gain in patients with COPD,6 weight loss may be a contributing factor to poor prognosis.
We used the first and last quartiles, but not tertiles, of annual change in body weight and classified the participants into three comparison groups to be clearer about the relative impact of annual weight change in patients with COPD. This study showed that COPD patients with an annual weight loss of less than 0.17 kg/year have a poor prognosis; however, this value may not be useful for defining individual patients with COPD in practical clinical settings. Additionally, more than half of the patients in the Kyoto University cohort were categorized into this group. This value is interpreted as data indicating that the prognosis is poor for patients with relative longitudinal weight loss in the population of patients with COPD, rather than the importance of this value itself. In both cohorts, the initial age, initial FEV1 values, and distribution of the patients into GOLD stages were worse in the weight loss groups. Therefore, it is possible that weight change is a just surrogate marker reflecting other factors. However, we have applied the multivariate Cox proportional hazards models and confirmed that annual body weight change was significantly associated with all-cause mortality independent of these factors. Thus, annual body weight change is considered to be important as a comprehensive and easily evaluated indicator for the prognosis of patients with COPD.
This study has several limitations. First, the sample size was relatively small compared to that in previous large-scale clinical studies.7,41 However, we confirmed the major finding that annual body weight change predicts mortality in two independent cohorts, which enhances the validity of our study. Second, there are differences in patient characteristics and methods between the two cohorts. The definition of exacerbation slightly differed between the two cohorts. In addition, the FEV1 was higher but DLCO was lower in the Hokkaido COPD cohort study than in the Kyoto University cohort. This may be due to that ventilation heterogeneity is more frequent in the Hokkaido COPD cohort study, but it is not definitive because not all patients have the same conditions for evaluation of emphysema. However, each of the two cohorts was well designed, and similar results were found using different cohorts in the present study. Third, this result may not be applicable to all patients with COPD. Patients in both cohorts are predominantly male, and the generalizability of the results to women is unclear. Moreover, the lifestyle of the Japanese patients with COPD may have changed since 2003 when the Hokkaido COPD cohort study began. Nevertheless, the characteristics of Japanese patients with COPD, such as older, thinner, and better health-related quality of life,42 have been unchanged in recent clinical trials.43 Finally, no information on body composition was available. Losing fat tissue in obese patients is not expected to have the same implication as losing FFM in lean patients with COPD. We confirmed that the annual change in the rate of body weight to baseline during the first 5 years was also significantly associated with mortality. Additionally, mean baseline BMI was 22.3 ± 3.2 kg/m2 in the Hokkaido COPD cohort and 22.1 ± 2.9 kg/m2 in the Kyoto University cohort, and the number of obese patients with COPD (BMI ≥30 kg/m2) was only three (1.1%) and one (0.4%) in each cohort, respectively. This suggests that weight loss in our study participants was primarily due to a decrease in FFM, but not fat tissue. Additionally, a previous report has shown that the decline in FFM varies by body part in patients with COPD;44 therefore, an analysis based on total body weight would also be useful.
Conclusion
Two well-established Japanese cohorts demonstrated an association between annual weight loss and poor prognosis in patients with COPD. It was also suggested that weight loss not only reflects the end stage of COPD or other diseases. The findings of the present study emphasize the importance of longitudinal assessment of body weight change in the management of COPD. Further research is needed to determine whether weight gain improves the prognosis of patients with COPD.
Abbreviations
COPD, chronic obstructive pulmonary disease; BMI, body mass index; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CT, computed tomography; LAV, low attenuation volume; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, carbon monoxide diffusion capacity; FFM, fat-free mass.
Data Sharing Statement
The data sets analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Hokkaido University School of Medicine (med02-001) and the ethics committee of Kyoto University (E182 and R0311-2). All participants provided written informed consent.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The Hokkaido COPD cohort study was supported by a scientific research grant from the Japan Society for the Promotion of Science (17390239 and 2139053); a grant to the Respiratory Failure Research Group from the Ministry of Health, Labor and Welfare, Japan; and from Boehringer-Ingelheim and Pfizer. The Kyoto University cohort was supported by the Japan Society for the Promotion of Science (16390234, 21590964, and 17H06807) and a grant to the Respiratory Failure Research Group from the Ministry of Health, Labour and Welfare, Japan. The sponsors had no role in the design of the study, collection and analysis of data, or preparation of the manuscript.
Disclosure
MS, KS, HM, SK, and MN have received grants from Boehringer-Ingelheim and Pfizer during the conduct of the study. MS has received grants from Boehringer-Ingelheim, Novartis, GlaxoSmithKline, and AstraZeneca outside the submitted work. MN has received personal fees from Nippon Boehringer-Ingelheim, Pfizer, Novartis, and AstraZeneca outside the submitted work. NT, SS, and TH report a grant from Fujifilm Medical outside the current work. YA, HS, YS, and HK declare that they have no conflicts of interest in this work.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
2. Agusti AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–360. doi:10.1183/09031936.03.00405703
3. Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi:10.1164/ajrccm.160.6.9902115
4. Suzuki M, Makita H, Ito YM, Nagai K, Konno S, Nishimura M. Clinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort study. Eur Respir J. 2014;43(5):1289–1297. doi:10.1183/09031936.00110213
5. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322
6. Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1791–1797. doi:10.1164/ajrccm.157.6.9705017
7. Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J. 2002;20(3):539–544. doi:10.1183/09031936.02.00532002
8. Engelen MP, Schols AM, Baken WC, Wesseling GJ, Wouters EF. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J. 1994;7(10):1793–1797. doi:10.1183/09031936.94.07101793
9. Makita H, Suzuki M, Konno S, et al. Unique Mortality Profile in Japanese Patients with COPD: an Analysis from the Hokkaido COPD Cohort Study. Int J Chron Obstruct Pulmon Dis. 2020;15:2081–2090. doi:10.2147/COPD.S264437
10. Fukuchi Y, Fernandez L, Kuo HP, et al. Efficacy of tiotropium in COPD patients from Asia: a subgroup analysis from the UPLIFT trial. Respirology. 2011;16(5):825–835. doi:10.1111/j.1440-1843.2011.01982.x
11. Ichinose M, Taniguchi H, Takizawa A, et al. The efficacy and safety of combined tiotropium and olodaterol via the Respimat(®) inhaler in patients with COPD: results from the Japanese sub-population of the Tonado(®) studies. Int J Chron Obstruct Pulmon Dis. 2016;11:2017–2027.
12. Makita H, Nasuhara Y, Nagai K, et al. Characterisation of phenotypes based on severity of emphysema in chronic obstructive pulmonary disease. Thorax. 2007;62(11):932–937. doi:10.1136/thx.2006.072777
13. Nishimura M, Makita H, Nagai K, et al. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(1):44–52. doi:10.1164/rccm.201106-0992OC
14. Suzuki M, Makita H, Konno S, et al. Asthma-like Features and Clinical Course of Chronic Obstructive Pulmonary Disease. An Analysis from the Hokkaido COPD Cohort Study. Am J Respir Crit Care Med. 2016;194(11):1358–1365. doi:10.1164/rccm.201602-0353OC
15. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2021. Available from: https://goldcopd.org.
16. Shimizu K, Tanabe N, Tho NV, et al. Per cent low attenuation volume and fractal dimension of low attenuation clusters on CT predict different long-term outcomes in COPD. Thorax. 2020;75(2):116–122. doi:10.1136/thoraxjnl-2019-213525
17. Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed tomography in pulmonary emphysema. Clin Radiol. 1982;33(4):379–387. doi:10.1016/S0009-9260(82)80301-2
18. Terada K, Muro S, Sato S, et al. Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation. Thorax. 2008;63(11):951–955. doi:10.1136/thx.2007.092858
19. Terada K, Muro S, Ohara T, et al. Abnormal swallowing reflex and COPD exacerbations. Chest. 2010;137(2):326–332. doi:10.1378/chest.09-0482
20. Tanabe N, Muro S, Hirai T, et al. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(12):1653–1659. doi:10.1164/rccm.201009-1535OC
21. Tanabe N, Muro S, Tanaka S, et al. Emphysema distribution and annual changes in pulmonary function in male patients with chronic obstructive pulmonary disease. Respir Res. 2012;13(1):31. doi:10.1186/1465-9921-13-31
22. Tanabe N, Muro S, Sato S, Oguma T, Sato A, Hirai T. Fractal analysis of low attenuation clusters on computed tomography in chronic obstructive pulmonary disease. BMC Pulm Med. 2018;18(1):144. doi:10.1186/s12890-018-0714-5
23. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi:10.1038/bmt.2012.244
24. Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153(3):961–966. doi:10.1164/ajrccm.153.3.8630580
25. Hallin R, Gudmundsson G, Suppli Ulrik C, et al. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD). Respir Med. 2007;101(9):1954–1960. doi:10.1016/j.rmed.2007.04.009
26. Coxson HO, Chan IH, Mayo JR, Hlynsky J, Nakano Y, Birmingham CL. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med. 2004;170(7):748–752. doi:10.1164/rccm.200405-651OC
27. Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(1):53–59. doi:10.1093/ajcn/82.1.53
28. Laaban JP, Kouchakji B, Dore MF, Orvoen-Frija E, David P, Rochemaure J. Nutritional status of patients with chronic obstructive pulmonary disease and acute respiratory failure. Chest. 1993;103(5):1362–1368. doi:10.1378/chest.103.5.1362
29. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi:10.1136/thorax.57.10.847
30. Connors AF
31. MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429(1–3):195–207. doi:10.1016/S0014-2999(01)01320-6
32. Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am J Respir Crit Care Med. 1997;156(2 Pt 1):341–357. doi:10.1164/ajrccm.156.2.9611013
33. Schols AM, Buurman WA. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51(8):819–824. doi:10.1136/thx.51.8.819
34. Tanimura K, Sato S, Fuseya Y, et al. Quantitative Assessment of Erector Spinae Muscles in Patients with Chronic Obstructive Pulmonary Disease. Novel Chest Computed Tomography-derived Index for Prognosis. Ann Am Thorac Soc. 2016;13(3):334–341. doi:10.1513/AnnalsATS.201507-446OC
35. Tanabe N, Sato S, Tanimura K, et al. Associations of CT evaluations of antigravity muscles, emphysema and airway disease with longitudinal outcomes in patients with COPD. Thorax. 2021;76(3):295–297. doi:10.1136/thoraxjnl-2020-215085
36. Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154(4 Pt 1):1055–1060. doi:10.1164/ajrccm.154.4.8887607
37. Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335(25):1897–1905. doi:10.1056/NEJM199612193352507
38. Reid MB. COPD as a muscle disease. Am J Respir Crit Care Med. 2001;164(7):1101–1102. doi:10.1164/ajrccm.164.7.2108039a
39. Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173(1):79–83. doi:10.1164/rccm.200506-969OC
40. Alley DE, Metter EJ, Griswold ME, et al. Changes in weight at the end of life: characterizing weight loss by time to death in a cohort study of older men. Am J Epidemiol. 2010;172(5):558–565. doi:10.1093/aje/kwq168
41. Montes M, Tálamo C, Perez-Padilla R, et al. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med. 2008;102(5):642–650. doi:10.1016/j.rmed.2007.12.025
42. Abe Y, Suzuki M, Makita H, et al. One-year clinically important deterioration and long-term clinical course in Japanese patients with COPD: a multicenter observational cohort study. BMC Pulm Med. 2021;21(1):159. doi:10.1186/s12890-021-01510-w
43. Kato M, Tomii K, Hashimoto K, et al. The IMPACT study - single inhaler triple therapy (FF/UMEC/VI) versus FF/VI and UMEC/VI in patients with COPD: efficacy and safety in a Japanese population. Int J Chron Obstruct Pulmon Dis. 2019;14:2849–2861. doi:10.2147/COPD.S226601
44. Machado FVC, Spruit MA, Coenjaerds M, Pitta F, Reynaert NL, Franssen FME. Longitudinal changes in total and regional body composition in patients with chronic obstructive pulmonary disease. Respirology. 2021;26(9):851–860. doi:10.1111/resp.14100
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


