Back to Journals » Journal of Inflammation Research » Volume 16
Andrographolide Attenuates Inflammation Due to Intra-Abdominal Sepsis by Enhancing Bacterial Clearance in Mice
Authors Yu L, Liu Y, Cao C, Yang L, Liu H, Wang C
Received 26 June 2023
Accepted for publication 3 October 2023
Published 6 October 2023 Volume 2023:16 Pages 4413—4423
DOI https://doi.org/10.2147/JIR.S422342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Lechang Yu,1 Ying Liu,2 Chao Cao,3 Liheng Yang,4 Haijing Liu,5 Chunli Wang5
1Department of Geriatrics, Tianjin Medical University General Hospital; Tianjin Geriatrics Institute, Tianjin, 300052, People’s Republic of China; 2Department of Integrated Traditional Chinese and Western Medicine, Tianjin First Central Hospital, Tianjin, 300192, People’s Republic of China; 3Department of Emergency Medicine, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Tianjin Chest Hospital, Tianjin, 300222, People’s Republic of China; 5Department of General Surgery, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China
Correspondence: Haijing Liu; Chunli Wang, Department of General Surgery, Tianjin Medical University General Hospital, No. 154, Anshan Road, Heping District, Tianjin, 300052, People’s Republic of China, Tel +86-22-60363333, Email [email protected]; [email protected]
Purpose: Intra-abdominal infection is a complex pathophysiological process involving multiple systems and organs of the body. Abdominal infections complicated by severe sepsis or septic shock have a high mortality rate of 30– 50%. Therefore, novel strategies to treat sepsis are urgently needed.
Methods: Andrographolide (AD), the main active ingredient of Andrographis paniculata, reportedly exerts beneficial effects on mice with sepsis. However, its exact mechanism of action in attenuating inflammation due to intra-abdominal sepsis remains unclear to date. Hence, this study aimed to examine the therapeutic effects of AD on cecal ligation and puncture (CLP)-induced sepsis and elucidate the underlying mechanisms.
Results: Results showed that AD therapy could significantly improve the 7-day survival rate and alleviate pathological organ injury in mice with CLP. In addition, AD treatment decreased the levels of proinflammatory factors, such as tumor necrosis factor-α and interleukin (IL)-6 in the peritoneal cavity fluid and blood and increased the level of anti-inflammatory factor IL-10 in the peritoneal cavity fluid of mice with CLP. Moreover, bacterial counts in the blood and peritoneal lavage fluid were lower in the mice treated with AD than in those untreated. Mechanistically, AD treatment increased the percentage and phagocytic activity of macrophages in the peritoneal cavity.
Conclusion: These data showed that AD can improve the survival of mice with intra-abdominal sepsis by enhancing bacterial clearance, as evidenced by the increased percentages and phagocytic activity of macrophages in the peritoneal cavity. This study is the first to demonstrate the protective effects of AD against intra-abdominal sepsis.
Plain Language Summary: Intra-abdominal infection is a complex pathophysiological process involving multiple systems and organs of the body. Abdominal infections complicated by severe sepsis or septic shock have a high mortality rate of 30– 50%. Therefore, novel strategies for treatment of sepsis are urgently needed. Andrographolide (AD), the main active ingredient of Andrographis paniculata, reportedly exerts beneficial effects on mice with sepsis. However, its exact mechanism of action in attenuating inflammation due to intra-abdominal sepsis remains unclear to date. Here, the study aimed to examine the therapeutic effects of AD on cecal ligation and puncture (CLP)-induced sepsis and elucidate the underlying mechanisms. Our findings showed that AD therapy could significantly improve the 7-day survival rate and alleviate pathological organ injury in mice with CLP. In addition, AD treatment decreased the levels of proinflammatory factors, such as tumor necrosis factor-α and interleukin (IL)-6 in the peritoneal cavity fluid and blood and increased the level of anti-inflammatory factor IL-10 in the peritoneal cavity fluid of mice with CLP. Moreover, bacterial counts in the blood and peritoneal lavage fluid were lower in the mice treated with AD than in those untreated. Mechanistically, AD treatment increased the percentage and phagocytic activity of macrophages in the peritoneal cavity. These data showed that AD can improve the survival of mice with intra-abdominal sepsis by enhancing bacterial clearance, as evidenced by the increased percentages and phagocytic activity of macrophages in the peritoneal cavity. This study is the first to demonstrate the protective effects of AD against intra-abdominal sepsis.
Keywords: andrographolide, bacterial clearance, inflammatory response, 7-day survival rate, intra-abdominal sepsis
Introduction
Intra-abdominal infection (IAI) is defined as a common clinical disease that usually occurs secondary to gastrointestinal perforation, necrosis, or gangrene.1 Once perforation occurs, pathogenic microorganisms and their metabolites colonize the digestive tract and enter the abdominal cavity, which immediately initiates the host’s immune inflammatory response to remove pathogenic bacteria and control the infection. However, the disease progresses and worsens when the infection is not effectively controlled; common symptoms of disease progression include fever, tachycardia, and shortness of breath, leading to organ function damage and sepsis. Sepsis caused by IAI is termed intraperitoneal sepsis.2 Although significant clinical improvements in surgical and intensive care treatment, patients with IAI still have a fatality rate of 20%, and those complicated with septic shock have a mortality rate of 30–50%.3 Food and Drug Administration-approved therapies for sepsis are currently lacking, highlighting the need for novel treatment modalities.
Andrographolide (AD), the main active ingredient extracted from Andrographis paniculata, exhibits antibacterial, antiviral, antiplatelet aggregation, liver- and gallbladder-protective, antitumor, and immune regulatory effects.4 AD treatment reportedly exerts beneficial effects on mice with sepsis. Zhu et al showed that AD reduces lung inflammation and pulmonary edema in lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the activation of the NF-κB signaling pathway.5 Moreover, Lee et al reported that AD could inhibit the cecal ligation and puncture (CLP)-mediated release of HMGB1, suppresses the secretion of pro-inflammatory cytokines, and reduces the mortality of mice with sepsis.6 However, the exact mechanism of action of AD in attenuating inflammation due to intra-abdominal sepsis remains unclear to date. Therefore, in the present study, we aimed to examine the therapeutic effects of AD on CLP-induced sepsis and elucidate the underlying mechanisms. This study is the first to demonstrate the protective effects of AD against intra-abdominal sepsis.
Materials and Methods
Animals and Grouping
Forty male wild-type C57BL/6 mice, aged 8–10 weeks and weighing 22–28 g from Beijing Vital River Laboratory Animal Technology Co. were used in whole experiments. The animals were placed in cages under specific pathogen-free (SPF) conditions and nourished with a standard laboratory diet. Before starting, all experimental operations were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Scientific Investigation Committee of Tianjin Medical University General Hospital.
CLP-Induced Mouse Model of Sepsis
To induce sepsis models, cecal ligation and puncture (CLP) was performed as previously described.7 Briefly, the mice were deeply anesthetized with isoflurane. A 2 cm-long incision was made in the lower abdomen of mice to expose the cecum under sterile conditions. The cecum was ligated 1 cm from the tip with a 4–0 silk suture and punctured thoroughly once with a 20-gauge needle. Then the cecum was returned to the peritoneal cavity and squeezed to place a small portion of its contents into the peritoneum. Thereafter, the peritoneal wall and skin were closed with silk sutures. The mice were subcutaneously injected with 1 mL of sterile saline (0.9%) and warmed on a heating mat until they were resuscitated from anesthesia.
The mice were randomly divided into two groups: CLP (n = 20) and treatment group (CLP+AD 5 mg/kg, n = 20). The mice in the treatment group were injected with AD (MedChem Express, USA) through the tail vein immediately and 24 h after surgery, whereas the mice in the CLP group were injected with the same volume of sterile normal saline via the tail vein at the same time points. To explore the effect of AD in clearing bacteria, we excluded other confounding factors, including the absence of antibiotic therapy. The 7-day survival rates of the two groups of mice were observed, and a survival curve was drawn.
Additional mice were sacrificed 24 or 48 h after surgery. Peritoneal lavage fluid (PLF), blood plasma, and tissue samples were collected from the mice.
Histopathological Analysis
The livers and lungs of the mice were collected and fixed with 4% formaldehyde. The tissues were embedded in paraffin and serially sectioned (4 μm) in toto. Then the paraffin section was treated with antigen retrieval solution (DAKO) under 96 °C for 20 min, and then cooled at room temperature for 20 min. The slides were stained with hematoxylin and eosin for histopathological analysis. Liver and lung injuries were independently scored by two double-blind pathologists.
Enzyme-Linked Immunosorbent Assay
At the indicated time-points after CLP, blood samples were drawn and gathered from the mice by cardiac puncture with 1 mL syringes,8 and then centrifuged at 1000×g for 15 min to separate serum. The peritoneal cavities were washed with 3 mL of PBS, and the PLF was harvested and centrifuged at 300 × g for 15 min to collect the supernatant. The levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-10 (IL-10) in the peripheral blood and peritoneal cavity were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits in accordance with the manufacturer’s instructions (Dakewe, China).
Determination of Bacterial Colony-Forming Units
The bacterial colony-forming units (CFUs) in the peripheral blood and peritoneum were analyzed, we obtained whole blood by cardiac puncture (0.5 mL) and harvested the peritoneal lavage by washing the cavity with 3 mL of sterile PBS in accordance with previously described methods.9 Whole blood was immediately mixed with EDTA (final concentration 4 mM) to restrain coagulation. All samples were placed on ice and serially diluted 1:10–1:100 in sterile PBS. Then, 100 µL of each sample was placed on trypticase soy agar plates with 5% sheep blood and hatched in a moist chamber at 37 °C for 24 h. The number of aerobic bacterial colonies was carefully counted, and the results are expressed as CFUs per milliliter.
Cell Segregation from the Peritoneal Cavity
Peritoneal cells were garnered by washing the peritoneal cavity. In short, the peritoneal cavities of the mice were injected with 5 mL of ice-cold PBS, and then gently massaged to extract loosely attached cells. The PLF was drawn and washed twice with PBS by centrifugation at 300 × g for 10 min at 4 °C. The resulting pellet was suspended in 1 mL of ice-cold PBS.
Flow Cytometry
Cells present in the PLF were pretreated on ice for 20 min with anti-mouse CD16/32 antibodies to intercept FC receptors. For cell surface staining, the collected cells were stained on ice for 30 min with the following fluorescently conjugated antibodies: isothiocyanate (FITC)-anti-Ly6G (1A8), peridinin-chlorophyll-protein complex (PerCP)-anti-CD45 (30-F11), phycoerythrin (PE)-anti-CD11b (M1/70), FITC-anti-CD11b (M1/70), allophycocyanin (APC)-anti-F4/80 (BM8), and PerCP-anti-CD86 (BU63) (entirely from BioLegend). Neutrophils were identified as CD11b+Ly6G+, and macrophages were identified as marker of CD11b+F4/80+. For intracellular staining, a Cytofix/Cytoperm kit (BD Biosciences) was utilised to fix and permeabilize the cells. APC–anti-TNF-α (MP6-XT22) and APC–anti-iNOS (CXNFT) antibodies were used for intracellular staining. FACS analysis was performed using a BD Accuri C6 Plus cell analyzer (BD Biosciences). All data were analyzed employing FlowJo software (Tree Star). For all samples, leastways 5×104 cells were collected to emanate scatter plots.
Macrophage Segregation and in vitro Phagocytosis Assays
Peritoneal cells were gathered as described above. The collected PLF was filtered to dislodge debris through a 100 mM filter. Macrophages were separated using a Macrophage Isolation Kit (Miltenyi Biotec). In brief, the resuspended cells were incubated with a macrophage biotin-antibody cocktail for 10 min in a refrigerator (2–8 °C), and then anti-biotin microbeads were appended. After incubation for 15 min, the cell suspension was applied to the MS column, and then flow-through cells were gathered. The purity of the collected macrophages was greater than 90%, as confirmed by flow cytometry staining with CD11b and F4/80 antibodies. For in vitro phagocytosis assays, the isolated macrophages were incubated with 1×106 CFUs of fluorescently labeled Escherichia coli (Abcam) in 24-well culture plates. After being incubated for 2 h at 37 °C in 5% CO2, the cells were reaped and washed with PBS to remove unphagocytosed bacteria. For the bacterial phagocytosis assay, FITC-labeled E. coli were measured using flow cytometry. FACS analysis was performed using a BD Accuri C6 Plus cell analyzer (BD Biosciences). All data were analyzed employing FlowJo software (Tree Star). For all samples, leastwise 5×104 cells were collected to emanate scatter plots.
Statistical Analysis
All statistical comparisons were performed using GraphPad Prism 8 (GraphPad Software). Statistical difference was analyzed using a 2-tailed t-test. Nonparametric Mann–Whitney U-test was used to compare bacterial CFUs among groups. Survival curves were dissected using the Log rank test. All values are expressed as mean ± SEM apart from bacterial counts. For all experiments, statistical significance was considered at P < 0.05.
Results
Single-Cell Transcriptomes and Cell Typing of BALF Cells
We first studied the role of AD in experimental sepsis, and abdominal sepsis was induced as previously described.10 The survival rate 7 days after surgery was investigated. On day 7 after CLP surgery, the survival rate of the mice in the CLP+AD group was significantly higher (P < 0.05) than that of the mice in the CLP group (Figure 1).
AD Alleviates Histopathological Damages in Intra-Abdominal Sepsis
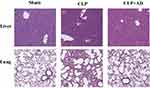
The liver and lung tissues of the two groups of mice were obtained 48 h after CLP surgery. As shown in Figure 2, tissues in the sham group exhibited normal structures with no histopathological changes, but the liver cells of the mice were swollen after CLP surgery, and the nuclei were enlarged and hyperchromatic, with a huge number of inflammatory cells infiltrating the hepatic portal area, which presented as damaged tissue. After treatment with AD (CLP+AD group), the edema in liver cells and inflammatory cell infiltration in the portal area were reduced. Similarly, the alveolar spaces widened significantly and were accompanied by inflammatory cell infiltration in the mice after CLP surgery compared with the healthy mice, with improvement of lung tissue edema and significant reduction of cellular infiltration in CLP+AD group. These results indicated that AD treatment significantly alleviated the pathological lung damage caused by CLP-induced sepsis in mice (Figure 2).
AD Mitigates Pro-Inflammatory Responses in Intra-Abdominal Sepsis
We detected the levels of proinflammatory factors TNF-α and IL-6 and anti-inflammatory factor IL-10 in the PLF and peripheral blood of the two groups of mice 24 and 48 h after CLP surgery. Compared with those of the mice in the CLP group, the TNF-α levels in the abdominal cavity and peripheral blood of the mice in the CLP+AD group decreased 24 and 48 h after surgery. Furthermore, the IL-6 levels in the abdominal cavity and peripheral blood of the mice in the CLP+AD group decreased 48 h after surgery. However, the intraperitoneal IL-10 levels of the mice in the CLP+AD group obviously increased 48 h after surgery, without statistically significant differences in other components (Figure 3).
AD Improves Bacterial Clearance in Intra-Abdominal Sepsis
We measured the number of colonies in the PLF and peripheral blood of the two groups of mice 24 and 48 h after CLP surgery to verify whether AD improves the survival rate in mice with sepsis and whether this phenomenon is associated intraperitoneal bacterial clearance. Compared with those of the mice in the CLP group, the PLF and peripheral blood colony counts of the mice in the CLP+AD group significantly decreased 48 h after surgery (Figure 4).
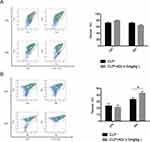
AD Increases the Proportion and Number of Peritoneal Macrophages in Intra-Abdominal Sepsis
During an infection, peritoneal immune cells, especially neutrophils and macrophages, increase in number. Therefore, we detected the changes in the proportion and number of neutrophils and macrophages in the PLF after CLP surgery. No significant differences in the proportion and number of peritoneal neutrophils were found between the mice in the CLP+AD and CLP groups 24 and 48 h after surgery, but the proportion and number of peritoneal macrophages significantly increased in the mice from the CLP+AD group 48 h after surgery (Figure 5).
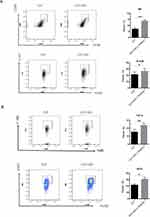
AD Increases the Proportion of Peritoneal M1-Type Macrophages and Enhances Their Phagocytic and Bactericidal Activities in Intra-Abdominal Sepsis
We verified the functional activity of macrophages by detecting the proportion of M1-type macrophages and the ability of cells to phagocytose bacteria and secrete iNOS and TNF-α in the CLP+AD and CLP groups. The proportion of peritoneal M1-type macrophages, the ability to phagocytose E. coli, and the levels of secreted iNOS and TNF-α were all higher in the CLP+AD group than in the CLP group 48 h after surgery (Figure 6).
Discussion
When the integrity of the digestive tract is destroyed, numerous pathogenic microorganisms and their metabolites colonize the digestive tract and enter the peritoneal cavity, immediately initiating the host’s immune inflammatory response to remove pathogenic bacteria. However, the disease progresses and worsens when the infection is not effectively controlled; common symptoms of disease progression include fever, tachycardia, and shortness of breath, leading to organ function damage and sepsis.2 Therefore, effective control of infection and prevention of the propagation and spread of pathogenic bacteria are undoubtedly key to blocking the progression of abdominal infection to sepsis. In the present study, AD significantly attenuated excessive inflammatory responses, alleviated pathological organ damage, and effectively improved survival during intra-abdominal sepsis infection by decreasing the levels of proinflammatory factors TNF-α and IL-6 and increasing the level of anti-inflammatory factor IL-10 in the abdominal cavity and peripheral blood, which may be related to enhanced bacterial clearance in mice. This result provides evidence supporting the clinical potential of herbal treatments for sepsis and sheds light on their potential effector targets.
AD is the main ingredient of A. paniculata, which is currently widely used in China and other Asian countries. Several studies analyzed the protective effects of AD against sepsis. Guo et al showed that andrographolide improves the 100 h survival rate of mice with LPS-induced sepsis.11 Another study found that AD treatment improves the 126 h survival rate of mice with CLP-induced sepsis.6 Consistent with previous studies, the present study showed that AD can improve the postoperative 7-day survival of mice with intra-abdominal sepsis.
TNF-α and IL-6 are the main pro-inflammatory factors involved in sepsis, and IL-10 is an anti-inflammatory factor.12 TNF-α is the earliest cytokine released in the inflammatory response after a trauma or infection, and its synergistic effect with IL-1β is important in activating the cytokine cascade in the inflammatory response.13–15 IL-6 is an important monitoring indicator for the prognosis of sepsis, and a sustained increase in IL-6 levels often indicates a poor prognosis.16 AD treatment could reduce the serum levels of TNF-α and IL-1β and alleviate the pathological damage to lung and liver tissue by inhibiting the activation of the p38MAPK, STAT3, and NF-κB signaling pathways in mice with LPS-induced sepsis.11 Moreover, AD can downregulate the levels of TNF-α, IL-1β, and IL-6 and the number of neutrophils and macrophages in bronchoalveolar lavage fluid in LPS-induced acute lung injury.5 It’s known that AD could exert beneficial effects on immune balance and regulation of inflammatory response, so a lethal sepsis model was introduced in the study. In the present study, AD administration through the tail vein in mice with CLP-induced sepsis decreased the levels of TNF-α and IL-6 in the abdominal cavity and peripheral blood, increased the level of IL-10 in the abdominal cavity, and alleviated pathological organ damage. These results illustrate that AD can regulate the inflammatory factor network to maintain the proinflammatory and anti-inflammatory balance and reduce the inflammatory response in the viscera.
Under physiological conditions, the peritoneal cavity contains approximately 50–100 mL of buffering serous fluid, which contains a number of macrophages that initiate the immune inflammatory response of the peritoneal cavity.17 Once the integrity of the digestive tract is destroyed, the invading pathogenic microorganisms are first recognized by macrophages, which then initiate an inflammatory immune response.3 While phagocytosing and eliminating pathogenic microorganisms, macrophages alter the local immune microenvironment by releasing proinflammatory cytokines, which recruit neutrophils and monocytes via circulation to migrate into the peritoneal infection site. In the present study, the colony counts in the peritoneal cavity and peripheral blood were reduced. Macrophages are an important component of innate immunity. As major cells involved in infection immunity, macrophages have a wide range of biological effects, including phagocytosis of invading pathogens, virus-infected cells, ruptured cell debris, and dead cells.18 Macrophages polarize into M1-type or M2-type under different pathophysiological conditions, in which M1-type macrophages initiate and maintain inflammatory responses and exhibit antimicrobial and antitumor effects.19 M1-type macrophages can also release potent proinflammatory factors, such as TNF-α, to recruit neutrophils into the peritoneal cavity and exert anti-infection effects. In addition, iNOS secreted by M1-type macrophages can induce a large amount of nitric oxide to exhibit bactericidal and cytotoxic effects. Moreover, macrophages play an important role in clearing peritoneal bacteria.20
The present study aimed to elucidate the changes in macrophages and their functional status during infection. We found that the AD administration of mice with CLP via the tail vein increased the number of peritoneal macrophages, proportion of M1-type macrophages, their ability to phagocytose E. coli, and production of iNOS and TNF-α, but it did not significantly affect the proportion and number of peritoneal neutrophils. These results suggest that AD can improve the prognosis of sepsis by increasing the number of macrophages, strengthening their phagocytosis and bactericidal activities, enhancing bacterial clearance, and mitigating inflammatory immune responses.
Taken together, our data demonstrate that AD exerts potential protective effects on mice with sepsis by enhancing bacterial clearance. However, the exact mechanism warrants further exploration. This study provides evidence for the clinical application of AD in the treatment of sepsis and other inflammatory diseases.
Ethics Approval Statement
Animal protocols were approved by Scientific Investigation Board of Tianjin Medical University General Hospital (No. IRB2023-DWFL-275), Tianjin, China, and complied with the revised Animals (Scientific Procedures) Act 1986 in the UK and Directive 2010/63/EU in Europe.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81902007 to CC, Grant No. 82202399 to YL), the Natural Science Foundation of Tianjin (Grant No. 19JCQNJC10000 to CC), and Binhai New District Health Commission Science Foundation of Tianjin (Grant No. 2019BWKY012 to CC).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sartelli M, Chichom-Mefire A, Labricciosa FM, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12(1):29.
2. Volakli E, Spies C, Michalopoulos A, et al. Infections of respiratory or abdominal origin in ICU patients: what are the differences? Crit Care. 2010;14(2):R32. doi:10.1186/cc8909
3. Girard JP, Moussion C, Rster F. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12(11):762–773.
4. Varma A, Padh H, Shrivastava N. Andrographolide: a new plant-derived antineoplasticentity on horizon. Evid Based Compl Alt. 2011;2011(5):1–9. doi:10.1093/ecam/nep135
5. Zhu T, Wang D-X, Zhang W, et al. Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-kB. PLoS One. 2013;8(2):e56407. doi:10.1371/journal.pone.0056407
6. Lee W, Ku S, Yoo H, Song K, Bae J. Andrographolide inhibits HMGB1-induced inflammatory responses in human umbilical vein endothelial cells and in murine polymicrobial sepsis. Acta Physiol. 2014;211(1):176–187. doi:10.1111/apha.12264
7. Zhao J, Liu Y, Hu JN, et al. The autocrine regulation of Interleukin-3 on the activity of regulatory T cells and its effectiveness in the pathophysiology of sepsis. J Infect Dis. 2020;223(5):893–904. doi:10.1093/infdis/jiaa441
8. Aziz M, Holodick NE, Rothstein TL, Wang P. B-1a cells protect mice from sepsis: critical role of cAMP-response Element Binding Protein (CREB). J Immunol. 2017;199(2):750–760. doi:10.4049/jimmunol.1602056
9. Gao X, Yan X, Yin Y, et al. Therapeutic targeting of Apoptosis Inhibitor of Macrophage (AIM)/CD5L in sepsis. Am J Respir Cell Mol Biol. 2018;60(3):323–334. doi:10.1165/rcmb.2018-0272OC
10. Liu Y, Hu JN, Luo N, et al. The essential involvement of the omentum in the peritoneal defensive mechanisms during intra-abdominal sepsis. Front Immunol. 2021;12:631609. doi:10.3389/fimmu.2021.631609
11. Guo W, Liu W, Chen G, et al. Water-soluble andrographolide sulfonate exerts anti-sepsis action in mice through down-regulating p38 MAPK, STAT3 and NF-κB pathways. Int Immunopharmacol. 2012;14(4):613–619. doi:10.1016/j.intimp.2012.09.002
12. Calvano SE, Xiao W, Richards DR, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437(7061):1032–1037. doi:10.1038/nature03985
13. Kox WJ, Volk T, Kox SN, Volk HD. Immunomodulatory therapies in sepsis. Intensive Care Med. 2000;26(Suppl1):S124–S128. doi:10.1007/s001340051129
14. Zanotti S, Kumar A, Kumar A. Cytokine modulation in sepsis and septic shock. Expert Opin Investig Drugs. 2002;11(8):1061–1075. doi:10.1517/13543784.11.8.1061
15. Alexander JJ, Jacob A, Cunningham P, et al. TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int. 2008;52(3):447–456. doi:10.1016/j.neuint.2007.08.006
16. Van den Boogaard M, Ramakers BP, van Alfen N, et al. Endotoxemia-induced inflammation and the effect on the human brain. Crit Care. 2010;14(3):R81. doi:10.1186/cc9001
17. Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30(5):731–743. doi:10.1016/j.immuni.2009.03.014
18. Wen S, Jiang Y, Liang S, Cheng Z, Zhu X, Guo Q. opioids regulate the immune system: focusing on macrophages and their organelles. Front Pharmacol. 2021;12:814241. doi:10.3389/fphar.2021.814241
19. Unuvar Purcu D, Korkmaz A, Gunalp S, et al. Effect of stimulation time on the expression of human macrophage polarization markers. PLoS One. 2022;17(3):e0265196. doi:10.1371/journal.pone.0265196
20. Serafini N, Dahdah A, Barbet G, et al. The TRPM4 channel controls monocyte and macrophage, but not neutrophil, function for survival in sepsis. J Immunol. 2012;189(7):3689–3699. doi:10.4049/jimmunol.1102969
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.