Back to Journals » Vascular Health and Risk Management » Volume 19
Anatomical Distribution Patterns of Peripheral Arterial Disease According to Patient Characteristics: A Unicentral Cohort Study
Authors Alghanimi IA, Al-Sharydah AM , Alqutub AA, Zeidan N, Bukhamseen F, Alradhi A , Alqassab AT, Al-Aftan MS
Received 13 April 2023
Accepted for publication 20 June 2023
Published 17 July 2023 Volume 2023:19 Pages 447—457
DOI https://doi.org/10.2147/VHRM.S416967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Ibrahim Abobaker Alghanimi,1 Abdulaziz Mohammad Al-Sharydah,1 Afnan Amro Alqutub,2 Nehal Zeidan,2 Fatimah Bukhamseen,2 Alzahra Alradhi,2 Aqilah Taleb Alqassab,2 Mohammed Saad Al-Aftan1
1Diagnostic and Interventional Radiology Department, King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal University, AlKhobar City, Eastern Province, Saudi Arabia; 2College of Medicine, King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal University, AlKhobar City, Eastern Province, Saudi Arabia
Correspondence: Ibrahim Abobaker Alghanimi, Assistant Professor and Consultant of Radiology, Vascular and Interventional Radiologist, Faculty of Medicine, Imam Abdulrahman Bin Faisal University, Radiology Department, King Fahd Hospital of the University, Khobar City, Eastern Province, Saudi Arabia, Tel +138957999 Ext 2007, Email [email protected]
Purpose: Peripheral arterial disease (PAD) is a common disease with multiple risk factors and affects patients worldwide. Several international studies have established correlations between anatomical topography/distribution of atherosclerosis and comorbidities in patients with PAD. In this cohort study, we aimed to analyze the patterns of atherosclerosis (site, distribution, and degree) in patients who underwent lower limb computed tomography angiography and arterial angiography by identifying the atherosclerotic plaque(s) that were possibly responsible for thrombi. Additionally, we aimed to determine any relationship between comorbidities and identified patterns.
Patients and Methods: Between January 2015 and January 2021, we retrospectively recruited 140 patients at King Fahd Hospital of the University of Saudi Arabia. Data collected included patient characteristics, risk factors, and metabolic disorders, such as hypertension (HTN), diabetes mellitus (DM), dyslipidemia, and chronic kidney disease. Patients with incomplete records or unavailable radiological images were excluded.
Results: The infrapopliteal territory was the most common segment that was affected. HTN, DM, and dyslipidemia were found in 81.4%, 77.9%, and 62.9% of patients, respectively. Correlation analyses revealed that DM was the only independent metabolic disorder associated with a PAD distribution pattern in the femoropopliteal segment (p=0.039), thus denoting distal involvement. No significant association was found between PAD distribution and the severity of stenosis.
Conclusion: Segmental involvement in PAD varies with the risk factors and metabolic comorbidities present in patients. DM is an independent predictor of the anatomical distribution of PAD. The identification of such an anatomical distribution is paramount for screening procedures, early detection of disease, and prevention of complications, particularly limb amputation.
Keywords: angiography, computed tomography, diabetes mellitus, peripheral arterial disease, stenosis
Plain Language Summary
This study focused on determining the relationship between site-selectivity in peripheral artery disease (PAD) and risk factors, such as age, sex, smoking, chronic kidney disease, diabetes mellitus, hypertension, and hyperlipidemia. This is one of the few studies from Saudi Arabia on the topic of correlation between the anatomical distribution of PAD and risk factors. The results demonstrated that among three studied segments, PAD in the infrapopliteal segment was most frequently associated with all explored comorbidities. Particularly, patients with diabetes were found to be affected by PAD in the femoropopliteal segment with a tendency of distal involvement. Diabetes was the only comorbidity that demonstrated a significant independent correlation with personalized PAD patterns. Identification of anatomical distribution is paramount in screening procedures, early detection of disease, and prevention of complications, particularly limb amputation. Confirming the effect of a risk factor on the anatomical distribution of atherosclerosis can possibly provide evidence for developing targeted screening guidelines aimed at early detection of PAD to prevent adverse outcomes, such as limb amputation.
Introduction
Peripheral arterial disease (PAD) is an occlusive vascular disease caused by the deposition of adipose tissue within arterial walls, which results in a plaque and subsequent narrowing of the affected arterial segment and a wide range of manifestations.1,2 The burden of PAD is increasing globally, and has particularly increased in the last decade, with an estimated additional prevalence of 34 million between 2010 and 2015.2 In patients with diabetes, PAD has an estimated prevalence which is twice as much as that in patients without diabetes and is considered a potent predictor of coronary artery disease, stroke, and mortality.2 Therefore, screening procedures are critical for the early detection of PAD and the prevention of complications, particularly limb amputation.2,3
Globally, an estimated 200 million people suffer from occlusive arteriosclerosis, which can be life-threatening.3 The estimated prevalence of PAD in the Asian continent is 6–10%.4,5 The prevalence of PAD also increases with age (20% at 40 years and 29% in those aged over 50 years).5 National prevalence rates have estimated that 11.7% of the Saudi population aged ≥45 years are affected by PAD,6 and the prevalence of PAD in Saudis was also noticed to increase with age.7–9
Risk factors of PAD and its related comorbidities can be classified as non-modifiable factors6–8 and modifiable risk factors and manageable comorbidities, such as diabetes mellitus (DM), hypertension (HTN), dyslipidemia, obesity, chronic kidney disease (CKD), and smoking.7,8 In 2019, the World Health Organization estimated that 11% of Saudis aged 15 and older were daily smokers.10 In Saudi Arabia, these risk factors and comorbidities are especially prevalent in patients with PAD; 95% of patients with PAD had at least one contributing factor, and 69% presented with two or more factors.6,7
If chronic arterial insufficiency of the lower limbs results in symptoms, patients may experience pain, mostly in the calves, or intermittent claudication. Additionally, if the arterial flow cannot meet the needs of resting tissue metabolism, critical ischemia may develop with resting pain or tissue loss (eg, ulceration or gangrene).9 Atherosclerotic obstruction of the aortoiliac arterial segment or anywhere in the arterial tree of the lower limbs can result in clinical manifestations of PAD. The most commonly affected anatomical sites are the superficial femoral and popliteal arteries.9
Several studies have discussed the risk factors of PAD and its related comorbidities as well as their effects on the incidence of the disease. However, limited studies have evaluated the anatomical distribution patterns of PAD using computed tomographic angiography (CTA) and catheter angiography (CA) and whether specific factors demonstrate any relationship with the site of atherosclerotic stenosis and obstruction.6,7,9 It has recently been proposed that multiple atherosclerotic risk factors and related comorbidities are associated with varying distribution patterns of PAD.11 To understand the pattern of PAD distribution, several international studies have highlighted the anatomical variations in arterial involvement based on a wide range of risk factors.12,13 Since patient characteristics vary between populations, this study could provide valuable insights into how various risk factors affect arterial involvement in the Saudi population.
Therefore, in this study, we aimed to determine the relationship between the anatomical site in PAD and the associated risk factors and comorbidities (eg, DM, HTN, dyslipidemia, and CKD). Elucidating this relationship could significantly improve the screening procedures by aiding early disease detection, reducing its chronicity, and preventing subsequent complications, such as limb amputation.
Materials and Methods
The study was performed in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines and regulations for cohort studies.
Study Setting and Design
This retrospective chart review study was performed at the Radiology Department of King Fahd Hospital of the University (KFHU; Khobar City, Saudi Arabia) between January 2015 and January 2021. KFHU is one of the largest academic hospitals in Al Khobar City in the Eastern Province of Saudi Arabia, with the Radiology Department being operational since 1981.
Study Population and Participants
The sample size was determined (1–β=0.80) using G*Power v3.1.9.7. (RRID: SCR_013726, available at http://www.gpower.hhu.de/) to ensure sufficient statistical power and prioritize outcomes based on these parameters for independent means for an α error of 0.05 and effect size of 0.5. We included all adult patients (aged > 18 years) who presented to our health facility with an initial clinical suspicion of PAD and whose presumptive diagnosis was confirmed using lower limb CTA and/or CA. We excluded patients with normal angiograms (n=16), non-atherosclerotic lesions (eg, dissection; n=68), and iatrogenic etiologies including previous vascular intervention (n=15) or lower extremity amputation (n=21). Young patients (aged <18 years) (n=10) and patients with poor documentation and incomplete data (n=30) were also excluded. Consequently, 140 patients were included in the study (Figure 1).
 |
Figure 1 Flowchart of patient selection showing the inclusion and exclusion criteria and final sample size. |
Study Variables
The collected data were divided into (a) patient characteristics as independent variables and (b) patterns of segmental artery involvement as dependent variables. The patient characteristics included basic demographic information, such as age and sex, and the associated factors, such as risk factors and metabolic comorbidities. Information regarding the patterns of arterial involvement included the affected arterial segments (name of the artery and its segmental localization [proximal/middle/distal]) and the severity of stenosis (mild=30%, moderate=30–70%, severe=70–99%, or total occlusion=100%). Hypertension was defined as either (i) receipt of antihypertensive therapy or (ii) systolic blood pressure > 130–139 mmHg or diastolic blood pressure > 80–89 mmHg.12 Obesity was quantified using body mass index (BMI), which was calculated as the weight in kilograms divided by the height in meters squared (kg/m2); it was categorized as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (>30 kg/m2).12 Dyslipidemia was defined as (i) receipt of antihyperlipidemic therapy or (ii) an abnormal lipid profile, especially serum triglycerides > 150 mg/dL or low-density lipoprotein cholesterol > 140 mg/dL.12,13 DM was defined as (i) receipt of antidiabetic therapy or (2) serum hemoglobin A1c > 6.5%.12 Dialysis dependence, ie, end-stage renal disease on dialysis, included both hemodialysis and peritoneal dialysis with an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine protein-to-creatinine ratio > 0.5. Smoking was recorded as “yes” or “no” based on whether the patient smoked at the time of enrollment.12
Data Collection
Consultants of Vascular and Interventional Radiology (IAA, AMA, and MSA) reviewed the radiological studies and reported the presence of stenotic atherosclerotic lesions. The lesions were classified into the following three categories based on previous studies:13,14 (a) aortoiliac arteries, including the infrarenal aorta and the common, external, and internal iliac arteries; (b) femoropopliteal arteries, including the common femoral, superficial femoral, deep femoral, and popliteal arteries; and (c) infra-popliteal arteries, including the anterior tibial, posterior tibial, and peroneal arteries.
Statistical Analysis
Statistical analyses were performed using SPSS v23.0 (IBM Inc., Armonk, NY, USA). A descriptive analysis of the patient characteristics at the time of the first radiological study was performed. Categorical variables were expressed as percentages, and continuous variables were expressed as means ± standard deviations. Unpaired t-tests and chi-square tests were used to compare continuous and categorical variables, respectively. The hypotheses were tested at a significance level of 5%.
Univariate logistic regression analysis was used to evaluate independent relationships of risk factors and related comorbidities with the anatomical distribution of lesions. Non-significant predictive variables for PAD were excluded from the multivariate analysis. Significant variables were entered into a multivariate logistic regression analysis to identify the most parsimonious model of independent predictors of PAD distribution. Similarly, they were tested for correlation with the severity of involvement. Significant associations are expressed as odds ratio (OR), and statistical significance was determined at p≤0.05.
Ethical Statement
The Imam Abdulrahman Bin Faisal University Institutional Review Board approved the study to be conducted at King Fahd Hospital of the University (IRB-UGS-2020-01-367). This study was performed in accordance with the Helsinki Declaration of 1975 (revised in 1983).
Results
A total of 140 lower limb CTA and CA images were analyzed between January 2015 and January 2021. An average of 38 newly diagnosed cases of PAD presented to our hospital each year during the study period, which included an average of 9.4, 13.4, and 15 cases of aortoiliac, femoropopliteal, and infrapopliteal PAD, respectively. An annual summary of the trends in the prevalence of PAD is shown in Figure 2.
 |
Figure 2 Annual prevalence of patients who were diagnosed with peripheral arterial disease at our hospital per 1000 people screened. |
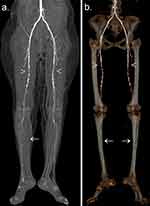
Demographic analysis revealed that most patients were male, with an estimated male-to-female ratio of 2:1. The overall mean age was 59.29 ± 13.97 years (range, 24–94 years). Analysis of different risk factors and comorbidities of PAD according to the sites involved is summarized in Table 1. DM was the only statistically significant independent contributor according to the arterial sites involved (p=0.048). Additionally, a tendency of distal involvement (ie, below-knee disease) was evident in all investigated risk factors and related comorbidities (Table 1, Figures 3 and 4). The aortoiliac segment was the least involved, followed by the femoropopliteal and infrapopliteal segments (47%, 67.1%, and 75%, respectively) (Figure 5).
 |
Table 1 Summary of Risk Factors and Related Comorbidities in Patients with Peripheral Arterial Disease |
Further characterization of the PAD pattern, severity, and sites of arterial involvement in patients with and without DM is summarized in Table 2. The femoropopliteal segment was the most common site of arterial involvement in patients with DM (p=0.039). A greater proportion of patients with DM had occluded arteries among the involved segments compared with those without DM (108 vs 32 lesions, respectively).
 |
Table 2 Characterization of the Pattern and Severity of Peripheral Artery Disease and Sites of Involvement |
Likewise, the incidence of stenotic lesions was higher in patients with DM than in those without DM (114 vs 11 lesions, respectively). However, univariate logistic regression analysis revealed no relationship between the severity of femoropopliteal involvement and DM (p=0.138) (Table 2 and Figure 6). Multivariate logistic regression revealed that DM was the only independent predictor of femoropopliteal involvement (OR=4.063, 95% confidence interval: 1.393–11.850, p=0.010) (Table 3).
 |
Table 3 Independent Predictors Associated with Different Risk Factors and Comorbidities on Multivariate Analysis |
Discussion
Our results revealed, for the first time, that PAD of the femoropopliteal segment is independently associated with DM. This retrospective analysis found that the prevalence of PAD in men is twice that in women. These results are consistent with those of most studies, which highlighted that male sex is a significant non-modifiable risk factor for PAD, particularly in Saudi Arabia.7,9,15
Genetic factors, especially mitochondrial DNA mutations, have recently been shown to be involved in the risk of atherosclerotic lesions. Several such genes have provided new opportunities for developing novel therapies.16,17
Among the risk factors investigated in our study, we identified a significant association between DM and atherosclerotic obstruction of the femoropopliteal arteries. This finding contradicts those of a recent report by Takahara et al13 in which they reported that DM was not significantly related to lesion localization.
Diabetic lower extremity arterial disease usually manifests as thickened arterial intima and some atherosclerotic plaques of different sizes protruding into the lumen, thus resulting in different degrees of arterial stenosis.1,18 However, most studies contradict this finding and suggest a significant correlation between DM and infrageniculate or distal arterial involvement.19–21 Notwithstanding these contradictions, patients with distal disease have a greater risk of amputation and a shorter survival rate than patients without distal disease.14 Additionally, DM affects both PAD presentation and post-revascularization outcomes.14,22
Variations in population characteristics might explain the differences in the anatomical distribution and the level of glycemic control. Chronic hyperglycemia can accelerate atherosclerotic disease and lead to ischemic macrovascular events, including symptomatic PAD and cardiovascular events.23,24 Poor glycemic control has been demonstrated to be associated with the involvement of the infrapopliteal region in patients with DM.20 In our study, the analysis did not include the glycemic control status, which might have affected the results. Recently, Lowry et al14 conducted a systematic review and meta-analysis that included 14 studies and reported that patients with DM tend to have a more distal distribution in PAD compared to healthy individuals. However, the cause of the distal involvement in the lower extremity remains unknown.14 This observation can be interpreted based on the available evidence through positron emission tomography study with fluorine-18-sodium, which suggests patients with DM have increased levels of plasma total cholesterol and hemoglobin A1C, which tends to have a higher propensity for femoropopliteal vascular disease than patients without DM.25
In severe infrapopliteal occlusion, femoropopliteal disease correlates significantly with DM and smoking is an additional risk factor.19 Most patients in our study had multiple comorbidities, which might have affected the site of atherosclerosis. Among our study participants, 20% were smokers, and 65.5% of them had femoropopliteal disease. This result suggests that smoking might have a strong effect on the pattern of PAD in patients with DM and might explain the disparity between our results and those reported previously. Smoking usually causes proximal PAD (eg, aortoiliac lesions) rather than infrapopliteal lesions due to more elastic elements in the iliac artery.11,13 In contrast, CKD usually causes distal involvement in uremia via vascular calcification, which was demonstrated to be attributable to the osteogenic differentiation of vascular smooth muscle cells.11 Our results are consistent with those of Kourtidou et al26 who found that DM and CKD are independently associated with PAD. Keller et al27 who reviewed profiles and outcomes of hospitalized patients with PAD revealed an unfavorable association with DM, including a higher risk of adverse events, higher amputation rates, and higher hospital mortality.
Other cardiovascular risk factors in our study, such as HTN and hyperlipidemia, were not significantly associated with any distribution pattern in PAD. Similar results were found in other studies.21,28 Another study conducted in a Saudi population in Jeddah15 demonstrated a clear relationship between atherosclerosis in the legs and two risk factors—HTN and metabolic syndrome. However, that study did not specify the arterial segments.15 Recent studies by Huo et al3 and Rencüzoğulları et al29 support the role of serum high-sensitivity C-reactive protein and endothelin-1 as useful biomarkers for impaired vessel patency secondary to arterial occlusion.
Multiple studies have suggested a significant relationship between the distribution of atherosclerotic lesions and patient-related risk factors, such as age and smoking. The results from these studies collectively suggest that older age is associated with distal arterial involvement and smoking is significantly correlated with proximal atherosclerotic arterial disease.19–21 However, none of these risk factors demonstrated statistically significant results in our study. The lack of significant age-related findings might have resulted from the heterogeneity of our study population, the relatively small sample size (140 patients), and wide age range (24–94 years). Although smoking is an important risk factor, data regarding smoking history in our patients were difficult to obtain and were not recorded clearly. This limitation might be the reason for finding no significant correlation between the site-selectivity of atherosclerosis and smoking.
Although various studies have reported the effects of certain risk factors on the distribution pattern of atherosclerosis, the results remain inconclusive due to the variations in the observed populations and their characteristics. Additionally, the precise pathophysiological mechanisms underlying such observations have not yet been identified. Further research in this field is needed to validate the findings regarding the anatomical distribution in PAD.
Strengths and Limitations
Our study is one of the few studies in Saudi Arabia regarding the anatomical distribution of PAD and its correlation with risk factors and comorbidities; therefore, we believe that our study makes a significant contribution to the literature. However, this study has some limitations. First, because of its retrospective design, it is subject to substantial observer bias, which might have contributed to an underestimation of some clinical data due to inaccurate reporting of medical histories. Another possible source of bias in our results is the small number of patients from a single academic care center. Lastly, the logistic regression analysis applied a forward parsimonious stepwise model that is not suitable for studying the relationship between DM and other outcomes of comorbidities (ie, with or without PAD), which was considered beyond the scope of this study.
Recommendations for Future Studies
Confirming the effects of a risk factor on the anatomical distribution of atherosclerosis can provide a basis for developing targeted screening guidelines for the early detection of PAD and preventing further adverse outcomes, such as limb amputation. Given the substantial cardiovascular morbidity associated with the comorbidities in PAD, identifying patients with the highest risk of cardiovascular events is pivotal in preventing such events.26 The authors suggest prospective screening studies using Doppler ultrasonography to diagnose PAD while collecting more data on the risk factors. Furthermore, randomized controlled interventional trials for treating PAD with highly calcified vessels are needed to clarify this issue. Regular screening of lower extremity vessels in patients with DM is recommended.
Conclusion
In this cohort study, we analyzed the prevalence and anatomical distribution of PAD and the effects of risk factors on the site of the disease in patients who underwent lower limb CTA and peripheral arterial angiography. The infrapopliteal segment was the most frequently involved segment among the three segments evaluated. The femoropopliteal segment is commonly involved in patients with DM. Furthermore, DM was the only factor that was significantly correlated with personalized PAD patterns. Future research on lower limb PAD with a larger sample size is necessary to distinguish the relative risk factors and comorbidities regarding the distribution patterns and identify the pathophysiological mechanisms underlying these effects.
Abbreviations
CT, computed tomography; CTA, computed tomography angiography; DM, diabetes mellitus; HTN, hypertension; CKD, chronic kidney disease; AI, aortoiliac; FP, femoropopliteal; IP, infrapopliteal.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available due to restrictions (eg, information that could compromise the privacy of participants). Anonymized data are available from the corresponding author on reasonable request. IAA is the principal investigator and corresponding author for this project. IAA should be contacted for data pertaining to this study ([email protected]).
Ethical Approval and Informed Consent
The non-experimental study protocol was approved by the Imam Abdulrahman Bin Faisal University licensing committee of Institutional Review Board (IRB-UGS-2020-01-367) and granted approval for the study to be conducted at King Fahd Hospital University. This study was performed in accordance with the Helsinki Declaration of 1975 (revised in 1983). Anonymized data were collected, analyzed, and reported only in aggregate form, and no identifiable participant information (image, face, name etc.) was revealed in the study. Informed consent was obtained from all participants and/or their legal guardian(s).
Acknowledgments
The authors gratefully acknowledge Dr. Mohamed Selim (Vascular Surgeon) for his initial assistance with the study proposal.
Author Contributions
All authors made a significant contribution to the work reported in terms of the conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas; drafting, revising or critically reviewing the article; and final approval of the version. The authors have agreed to the submission of the article to the current journal and agree to be accountable for all aspects of the work.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare no competing interests.
References
1. Kiss LZ, Bagyura Z, Vadas R, et al. Signs of subclinical atherosclerosis in asymptomatic patients at increased risk of type 2 diabetes mellitus. J Diabetes Complications. 2017;31(8):1293–1298. doi:10.1016/j.jdiacomp.2017.05.007
2. Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi:10.1016/S0140-6736(13)61249-0
3. Huo J, Wu Z, Jiang H, Zhang H. Multislice computed tomography angiography imaging diagnosis of lower extremity arteriosclerosis in patients with hypertension and its correlation with the level of high-sensitivity C-reactive protein. Comput Math Methods Med. 2022;2022:1768208. doi:10.1155/2022/1768208
4. Tang T, Kum S, Ho P, Tan Y. Peripheral vascular disease and endovascular therapy in Singapore. In: Dardik A, editor. Vascular Surgery. Cham: Springer; 2017:81–90.
5. Soon SXY, Patel A, Chong TT, et al. Distribution of peripheral arterial disease in patients undergoing endovascular revascularization for chronic limb threatening ischaemia: insights from the vascular quality initiative in Singapore. Vasc Specialist Int. 2021;37:13. doi:10.5758/vsi.210016
6. Al-Sheikh SO, Aljabri BA, Al-Ansary LA, Al Khayal LA, Al Salman MM, Al Omran MA. Prevalence of and risk factors for peripheral arterial disease in Saudi Arabia. A pilot cross-sectional study. Saudi Med J. 2007;28(3):412–414.
7. Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health Risk Manag. 2007;3(2):229–234. doi:10.2147/vhrm.2007.3.2.229
8. Ness J, Aronow WS, Newkirk E, McDanel D. Prevalence of symptomatic peripheral arterial disease, modifiable risk factors, and appropriate use of drugs in the treatment of peripheral arterial disease in older persons seen in a university general medicine clinic. J Gerontol a Biol Sci Med Sci. 2005;60(2):255–257. doi:10.1093/gerona/60.2.255
9. Aronow WS. State of the art paper Peripheral arterial disease of the lower extremities. Arch Med Sci. 2012;8(2):375–388. doi:10.5114/aoms.2012.28568
10. World Health Organization. WHO report on the global tobacco epidemic, 2021: addressing new and emerging products; 2021. Available from: https://www.who.int/teams/health-promotion/tobacco-control/global-tobacco-report-2021.
11. Ohtake T, Mitomo A, Yamano M, et al. Impact of arterial calcification of the lower limbs on long-term clinical outcomes in patients on hemodialysis. J Clin Med. 2023;12(4):1299. doi:10.3390/jcm12041299
12. Ogunwole SM, Chen X, Mitta S, et al. Interconception care for primary care providers: consensus recommendations on preconception and postpartum management of reproductive-age patients with medical comorbidities. Mayo Clin Proc Innov Qual Outcomes. 2021;5(5):872–890. doi:10.1016/j.mayocpiqo.2021.08.004
13. Takahara M, Soga Y, Fujihara M, Iida O, Kawasaki D. Association of smoking, diabetes, and dialysis with the presence of popliteal lesions in femoropopliteal artery disease. J Atheroscler Thromb. 2022;64007. doi:10.5551/jat.64007
14. Lowry D, Saeed M, Narendran P, Tiwari A. A review of distribution of atherosclerosis in the lower limb arteries of patients with diabetes mellitus and peripheral vascular disease. Vasc Endovascular Surg. 2018;52(7):535–542. doi:10.1177/1538574418791622
15. Mufti AAI, Subki AH, Abushanab RH, et al. Peripheral artery disease risk factors in Jeddah, Saudi Arabia: a retrospective study. Int J Gen Med. 2019;12:49–54. doi:10.2147/IJGM.S176451
16. González-Becerra K, Ramos-López O, Barrón-Cabrera E, et al. Fatty acids, epigenetic mechanisms and chronic diseases: a systematic review. Lipids Health Dis. 2019;18(1):178. doi:10.1186/s12944-019-1120-6
17. Markin AM, Sobenin IA, Grechko AV, Zhang D, Orekhov AN. Cellular mechanisms of human atherogenesis: focus on chronification of inflammation and mitochondrial mutations. Front Pharmacol. 2020;11:642. doi:10.3389/fphar.2020.00642
18. Yang Z, Han B, Zhang H, Ji G, Zhang L, Singh BK. Association of lower extremity vascular disease, coronary artery, and carotid artery atherosclerosis in patients with type 2 diabetes mellitus. Comput Math Methods Med. 2021;2021:6268856. doi:10.1155/2021/6268856
19. Motsumi MJ, Naidoo NG. Pattern and distribution of peripheral arterial disease in diabetic patients with critical limb ischemia (Rutherford clinical category 4–6). S Afr J Surg. 2017;55(3):48–54.
20. Chung J, Modrall JG, Knowles M, et al. Arteriographic patterns of atherosclerosis and the association between diabetes mellitus and ethnicity in chronic critical limb ischemia. Ann Vasc Surg. 2017;40:198–205. doi:10.1016/j.avsg.2016.11.003
21. Diehm N, Shang A, Silvestro A, et al. Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur J Vasc Endovas Surg. 2006;31(1):59–63. doi:10.1016/j.ejvs.2005.09.006
22. Arya S, Binney ZO, Khakharia A, et al. High hemoglobin A1c associated with increased adverse limb events in peripheral arterial disease patients undergoing revascularization. J Vasc Surg. 2018;67(1):217–228.e1. doi:10.1016/j.jvs.2017.06.101
23. Shatnawi NJ, Al-Zoubi NA, Hawamdeh HM, et al. The relation of anatomical distribution of symptomatic peripheral arterial disease (PAD) with HbA1c level in patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metab. 2021;12:20420188211000504. doi:10.1177/20420188211000504
24. Thiruvoipati T, Kielhorn CE, Armstrong EJ. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J Diabetes. 2015;6(7):961–969. doi:10.4239/wjd.v6.i7.961
25. Chou TH, Rimmerman ET, Patel S, et al. Vessel-by-vessel analysis of lower extremity 18F-NaF PET/CT imaging quantifies diabetes-and chronic kidney disease-induced active microcalcification in patients with peripheral arterial disease. EJNMMI Res. 2023;13(1):1. doi:10.1186/s13550-023-00951-0
26. Kourtidou C, Rafailidis V, Varouktsi G, et al. Evaluation of subclinical vascular disease in diabetic kidney disease: a tool for personalization of management of a high-risk population. J Pers Med. 2022;12(7):1139. doi:10.3390/jpm12071139
27. Keller K, Schmitt VH, Vosseler M, et al. Diabetes mellitus and its impact on patient-profile and in-hospital outcomes in peripheral artery disease. J Clin Med. 2021;10(21):5033. doi:10.3390/jcm10215033
28. Haltmayer M, Mueller T, Horvath W, Luft C, Poelz W, Haidinger D. Impact of atherosclerotic risk factors on the anatomical distribution of peripheral arterial disease. Int Angiol. 2001;20(3):200–207.
29. Rencüzoğulları I, Çınar T, Karabağ Y. Endothelin-1 and C reactive protein as potential biomarkers for restenosis in patients with arteriosclerosis obliterans. J Invest Surg. 2021;34(7):771–772. doi:10.1080/08941939.2019.1693668
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.