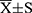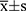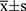Back to Journals » Journal of Inflammation Research » Volume 16
Analysis of Risk Factors and Intervention Strategies for Acute Kidney Injury After Cardiac Valve Replacement
Received 11 June 2023
Accepted for publication 11 August 2023
Published 18 August 2023 Volume 2023:16 Pages 3523—3529
DOI https://doi.org/10.2147/JIR.S425485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yi Jiang,1 Yanyan Song2
1Department of Cardiovascular Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, 710000, People’s Republic of China; 2Department of Cardiovascular Surgery, General Hospital of Ningxia Medical University, Yinchuan, 750004, People’s Republic of China
Correspondence: Yanyan Song, Department of Cardiovascular Surgery, General Hospital of Ningxia Medical University, No. 804 Shengli South Street, Xingqing District, Yinchuan, 750004, People’s Republic of China, Tel +86 951-6743014, Fax +86 951-6744302, Email [email protected]
Objective: To investigate the risk factors and intervention strategies for the development of acute kidney injury (AKI) after cardiac valve replacement with extracorporeal circulation.
Methods: Retrospective analysis of the clinical data of a total of 106 patients diagnosed with heart valve disease and undergoing extracorporeal circulation heart valve replacement surgery from January 2021 to December 2021 in the Department of Cardiac and Major Vascular Surgery of our hospital. The two groups were divided into AKI and non-AKI groups according to whether acute kidney injury occurred after surgery, and the preoperative, intraoperative and postoperative clinical data were compared. Single-factor analysis and multi-factor logistic regression analysis were used to explore the risk factors for acute kidney injury after extracorporeal heart valve replacement, and to improve the prognosis by giving kidney function protection strategies as early as possible.
Results: Univariate analysis showed that age, preoperative blood creatinine > 130umol/L, LVEF < 45%, presence of subacute infective endocarditis (SIE), concurrent coronary artery bypass grafting (CABG), time to extracorporeal circulation, time to surgery, MAP < 70mmHg, urine output < 0.5mL/(kg-h), pulmonary infection, low cardiac output, and bacteraemia were risk factors for postoperative AKI. Multi-factor regression analysis showed that preoperative blood creatinine > 130umol/l, LVEF < 45%, combined infective endocarditis, extracorporeal circulation time > 2h, intraoperative and postoperative hypotension, low cardiac output, and postoperative bacteremia were independent risk factors for postoperative AKI.
Conclusion: Active intervention strategies in the perioperative period can reduce the occurrence of postoperative AKI and improve patient prognosis.
Keywords: heart valve replacement surgery, acute kidney injury, risk factors, intervention strategies
A Letter to the Editor has been published for this article.
Introduction
With the development of medical technology, heart valve replacement surgical skills have increasingly improved, and the proportion of minimally invasive, non-extracorporeal cardiac surgery has increased. However, the occurrence of acute kidney injury (AKI) after surgery to resolve heart valve lesions cannot be ignored.1 In recent years, the occurrence of Cardio Renal Syndrome (CRS) after cardiac surgery has received increasing attention. The etiology of AKI is complex, not only with cardiopulmonary bypass (CPB), but also with intraoperative and postoperative drug use. The risk factors include chronic kidney disease, proteinuria, hypertension, diabetes, old age and obesity. Potentially nephrotoxic drugs, such as antibiotics, non-steroidal anti-inflammatory drugs (aspirin), angiotensin converting enzyme inhibitors, angiotensin II receptor antagonists and intravenous contrast agents, are commonly used in patients before cardiac valve replacement surgery with cardiopulmonary bypass. Among them, non-steroidal anti-inflammatory drugs can impair the self-regulation of renal blood flow. Preoperative use of intravenous contrast media increases the risk of perioperative AKI. Before surgery, many patients undergoing cardiac surgery will experience one or more episodes of hypotension, which can cause endothelial damage and local release of endothelin, angiotensin II and catecholamines, leading to renal vasoconstriction and exacerbating ischemia. The blood pump used in CPB causes mechanical damage to blood, which contributes to oxidative stress and systemic inflammatory response. The incidence of AKI after cardiac surgery has been reported in the literature to be 5%-40.2%,2–5 which increases the in-hospital mortality rate of patients. This study investigates the risk factors for the development of AKI after heart valve replacement and provides early interventional treatment in order to improve the prognosis.
Objects and Methods
Study Subjects and Subgroups
The clinical data of a total of 106 patients diagnosed with heart valve disease and undergoing extracorporeal circulation heart valve replacement surgery from January 2021 to December 2021 in the Department of Cardiac and Macrovascular Surgery of our hospital were selected, including 66 males and 40 females, aged 43 to 72 years, with an average of (57.8±10.9) years. Inclusion criteria: (1) Diagnosis of heart valve disease with indications for surgical heart valve replacement surgery. (2) Preoperative surgical plan based on cardiac ultrasound: aortic valve replacement, mitral valve replacement, or double valve replacement. Exclusion criteria: (1) Preoperative diagnosis of chronic renal failure stage 5 maintained on hemodialysis therapy. (2) Renal transplant patients. (3) Age <18 years. (4) Second valve replacement surgery, second intraoperative transfer, and second postoperative chest opening. (5) Intraoperative death. The AKI and non-AKI groups were divided according to whether AKI occurred within 7 days after surgery.
AKI Diagnostic Criteria
AKI diagnosis and staging is based on the 2012 KDIGO Clinical Practice Guidelines for Acute Kidney Injury, defined as a creatinine level increase of ≥26.5umol/L (0.3mg/m L) within 48 h; or creatinine exceeding 1.5 times the basal value or more, and it is clear or inferred that the above occurred within 7 d; or urine output <0.5 mL/(kg-h) for 6 h. The patient should have one of these conditions. AKI is diagnosed when one of the above conditions is present.6
Clinical Data Collection and Study Methods
(1) Preoperative clinical data: age, weight, gender, previous underlying diseases, preoperative renal function serum creatinine (SCr) level, left ventricular ejection fraction (LVEF), presence of subacute infective endocarditis (SIE) in both groups.(2) Intraoperative clinical data: surgical approach, extracorporeal circulation time, aortic clamping time, operative time, intraoperative hypotension: mean arterial pressure (MAP) <70 mmHg, urine output <0.5 mL/(kg-h).(3) Postoperative clinical data: duration of mechanical ventilation, ICU stay, postoperative hypotension within 24 h (MAP <70 mmHg), postoperative drainage, postoperative complications: hypoxemia (PaO2/FiO2 <200 mmHg), low cardiac output, infection (pulmonary infection, bacteremia). Diagnostic criteria for hypoxemia: according to the diagnostic criteria for acute respiratory distress syndrome at the Berlin Conference in 2010,7 and with reference to other domestic studies, arterial oxygen partial pressure (PaO2)/inhalation oxygen (FiO2) concentration (PaO2/FiO2) <200 mmHg within 48 hours after surgery was defined as postoperative hypoxemia. Single-factor analysis and multi-factor logistic regression analysis were used to investigate the risk factors for AKI after extracorporeal heart valve replacement and to provide interventional treatment for the risk factors.
Statistical Methods
Data were processed using SPSS 23.0 statistical software. Normally distributed measures were expressed as mean±standard deviation (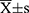 ) and t-test; comparisons between groups of count data were expressed as percentages or rates and χ2 test (fisher’s exact probability method was used when conditions were not met). Factors with statistically significant preoperative, intraoperative, and postoperative risk factors were analyzed by multi-factor logistic regression to determine independent risk factors for AKI, and P<0.05 was considered a statistically significant difference.
) and t-test; comparisons between groups of count data were expressed as percentages or rates and χ2 test (fisher’s exact probability method was used when conditions were not met). Factors with statistically significant preoperative, intraoperative, and postoperative risk factors were analyzed by multi-factor logistic regression to determine independent risk factors for AKI, and P<0.05 was considered a statistically significant difference.
Results
Univariate Analysis of Patients’ Preoperative Clinical Data
106 patients were selected, 66 males and 40 females, including 24 in the AKI group and 82 in the non-AKI group, with a postoperative AKI incidence rate of 22.64% for the whole group. The results of the univariate analysis of preoperative clinical data showed that age, preoperative SCr>130umol/L, LVEF<45%, and presence of infective endocarditis (SIE) in the AKI group were statistically significant differences compared with the non-AKI group (P<0.05) and were risk factors for AKI after heart valve replacement, as shown in Table 1.
 |
Table 1 Univariate Analysis of Preoperative Clinical Data of Patients in Both Groups [ |
Single-Factor Analysis of Intraoperative Clinical Data
The results showed that the differences in coronary artery bypass grafting (CABG), extracorporeal circulation time, operative time, intraoperative MAP <70 mmHg, and urine volume <0.5 mL/(kg.h) were statistically significant in the AKI group compared with the non-AKI group (P<0.05), and were risk factors for heart valve risk factors for the development of AKI after replacement, as shown in Table 2.
 |
Table 2 Univariate Analysis of Intraoperative Clinical Data in the Two Groups [ |
Postoperative Clinical Data Were Compared
8 cases of postoperative hemodialysis were performed in the AKI group, with an incidence of 33.33%, while there were no cases of hemodialysis in the non-AKI group. 2 cases of postoperative in-hospital death were reported in the AKI group, with a mortality rate of 8.33%, while 2 cases of in-hospital death were reported in the non-AKI group, with a mortality rate of 2.43%. The duration of postoperative ICU stay, incidence of hemodialysis, and incidence of postoperative in-hospital death were higher in the AKI group than in the non-AKI group, with statistically significant differences (P<0.05). MAP <70 mmHg, pulmonary infection, low cardiac output, and bacteremia were statistically significant (P<0.05) in the AKI group compared with the non-AKI group, and were risk factors for AKI after heart valve replacement, as shown in Table 3.
 |
Table 3 Univariate Analysis of Postoperative Clinical Data in the Two Groups [ |
Multifactorial Logistic Regression Analysis
Preoperative blood creatinine values >130umol/L, LVEF <45%, preoperative presence of infective endocarditis, extracorporeal circulation time >2h, intraoperative and postoperative MAP <70mmHg, Low cardiac output syndrome (LCOS), and postoperative bacteremia were independent risk factors for AKI after heart valve replacement (P<0.05), as shown in Table 4.
 |
Table 4 Postoperative AKI Multifactorial Logistic Regression Analysis |
Low cardiac output syndrome (LCOS) diagnostic criteria:8 LCOS can be diagnosed according to any two of the following diagnostic criteria: (1) Urine volume < 0.5mL/ (kg.H), duration time≥2h; (2) The difference between body surface temperature and core body temperature is more than 5°C, and the duration is more than 2 hours; (3) CVP > 1.73kPa (12.98mmHg) for≥2h; (4) Compared with preoperation, the systolic blood pressure decreased more than 20% after operation, and the duration was more than 2 hours; (5) cardiac index < 2.5L/min.
Discussion
In this study, the presence of preoperative renal impairment (SCr >130umol/l), LVEF <45%, co-infected endocarditis, intraoperative and postoperative hypotension, low cardiac output, and postoperative hematogenous infection were found to be risk factors for the development of AKI after surgery and were analyzed as follows.
Some studies have reported that patients with any degree of preoperative renal function abnormalities have increased postoperative renal function abnormalities and mortality.9,10 It has been estimated that for every 1 mg/dl increase in serum creatinine, there is a 4.8-fold increase in the risk of abnormal renal function after surgery.11 It has also been found that preoperative creatinine greater than 2.5 mg/dl is associated with a risk of more than 30% for postoperative dialysis.12 In this study, preoperative renal insufficiency (blood creatinine >130umol/l) was found to be a risk factor for postoperative AKI, and the incidence of postoperative dialysis and in-hospital death was higher in the AKI group than in the non-AKI group, with a statistically significant difference (P<0.05), which is consistent with the findings of the above study.
The mechanisms of renal impairment caused by infection may be as follows: (1) Severe infection leads to systemic inflammatory response, circulatory failure and shock caused by the release of various inflammatory mediators, which can directly lead to the reduction of glomerular filtration rate and result in acute renal failure. (2) Infection can lead to hypoxia, and hypoxia causes different degrees of renal insufficiency, which can lead to the development of proteinuria, tubuluria, and even oliguria and anuria. (3) The infection itself can directly damage the function of the kidney and lead to renal insufficiency. Bacterial infection can also directly lead to infection of the kidney with blood circulation, resulting in different degrees of renal insufficiency. In conclusion, infection can lead to different degrees of renal impairment through direct bacterial factors, factors of blood circulation, metabolites, and inflammatory mediators.13–15
In patients with acute heart failure, the severity of AKI is higher in patients with impaired LVEF than in those with normal LVEF. The lower the LVEF, the higher the risk of AkI,16,17 and this study found that preoperative LVEF <45% was a risk factor for postoperative AKI. Because the renal class is sensitive to ischemia and hypoxia, in patients with reduced LVEF and decreased renal function, a state of renal hypoperfusion exists preoperatively, and a further decrease in renal perfusion during extracorporeal cardiac surgery may lead to an increased risk of renal injury. Postoperative low cardiac output syndrome is a risk factor for postoperative AKI due to sympathetic hyperactivity and corresponding activation of the renin-angiotensin-aldosterone system, which increases renal vasoconstriction and leads to pre-renal renal damage. The high use of vasoactive drugs in postoperative patients with low cardiac output also leads to an increased likelihood of AKI after cardiac surgery.18
Kidney ischemia caused by low blood pressure can lead to kidney injury. Renal ischemia leads to reduced glomerular filtration rate, tubular damage, increased glomerular capillary permeability, and inflammatory factors that further aggravate renal tissue damage. Blood pressure decreases, resulting in slow blood circulation and distal capillary ischemia, which affects the supply of oxygen and nutrients to the cells and the discharge of metabolic wastes, and affects the blood supply to the kidney, which is prone to tubular necrosis in the long term and continues severely. Combined surgery, with coronary artery manipulation, further prolongs the operative time and predisposes to AKI postoperatively.19,20
Intervention strategies in the perioperative period to initiate early renal function protection are particularly important for the above risk factors, especially for those patients at high risk. The interventions are discussed as follows: (1) Preoperative identification and correction of factors causing abnormal renal function and prophylactic monitoring during and after surgery to ensure renal perfusion and tubular function, which would lead to improvement of oliguric renal impairment as a complication. These factors include: electrolyte abnormalities, abnormal pulmonary and cardiac function, bleeding, infection, hemodialysis, and other comorbidities. Preoperative correction of renal abnormalities and electrolyte disturbances, control of fluid intake, reduction of cardiac volume load, and also administration of drugs (levosimendan, etc.) to improve cardiac function, anti-infection, correction of anemia, and correction of blood glucose and blood pressure abnormalities can be performed.21,22 The surgery can also be postponed according to the condition until the patient’s preoperative abnormal indicators return to normal. (2) To address intraoperative risk factors, try to use antifibrinolytic drugs cautiously during surgery and maintain a high perfusion pressure (75–80 mmHg) during extracorporeal circulation to avoid intraoperative hypotension. (3) Adopt ultrafiltration technology to drain excess water from the body, reduce the cardiac volume load, and try to maintain normal electrolyte and acid-base levels and hemodynamic stability during the extracorporeal circulation. Shorten the duration of extracorporeal circulation as much as possible. (4) Actively control infection after surgery. If infection occurs, activate intravenous antimicrobial agent as early as possible, and parallel blood culture and sputum culture to clarify the pathogenic bacteria and control the infection as early as possible. (5) Give hemostatic agents to prevent postoperative bleeding and avoid pre-renal renal damage due to blood volume deficiency caused by acute blood loss. Blood loss leading to hypotension, actively rehydrate and transfuse blood to correct hypovolemia.
Once abnormal renal function occurs, in addition to correcting risk factors, pharmacological treatment is also important. The drugs available are: Recombinant Human Brain Natriuretic Peptide, Shenshuaining, and Bering capsule, etc. Transient oliguria is often seen within 12 hours after surgery, and fluid supplementation or low-dose vasoactive drugs are effective. Persistent oliguria, on the other hand, is a sign of acute renal impairment caused by prolonged hypotension or low cardiac output. Creatinine levels are often low in the early postoperative period after extracorporeal circulation due to hemodilution, but can rise rapidly if renal function is impaired. Therefore, postoperative monitoring of urine volume and renal function should be focused on, and if significant electrolyte or metabolic disorders occur, active measures should be taken or hemodialysis treatment should be performed.
Conclusion
The presence of preoperative renal impairment (blood creatinine >130umol/l), co-infected endocarditis, low cardiac output, intraoperative and postoperative hypotension, and postoperative hematogenous infection are independent risk factors for AKI after heart valve replacement, and active treatment strategies in the perioperative period can reduce the occurrence of postoperative AKI and improve patient prognosis.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of General Hospital of Ningxia Medical University (No. KYLL-2022-0589). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Disclosure
The authors declare that they have no any financial or nonfinancial interests that might have influenced the performance or presentation of the work described in this manuscript.
References
1. Zhuo L, Xu Y, Zhang C, et al. Research progress of minimally invasive heart valve surgery under complete thoracoscopy. Int J Cardiovasc Dis. 2021;48(04):210–213.
2. Amini S, Najafi MN, Karrari SP, et al. Risk factors and outcome of acute kidney injury after isolated CABG surgery: a Prospective Cohort Study. Braz J Cardiovasc Surg. 2019;34(1):70. doi:10.21470/1678-9741-2017-0209
3. Mao H, Katz N, Ariyanon W, et al. Cardiac surgery associated acute kidney injury. Cardio Renal Me. 2013;3(3):178. doi:10.1159/000353134
4. Fan GL, Wang ZQ, Tang Y, et al. Factors affecting acute kidney injury and prognosis after coronary artery bypass grafting. J Nephrol Dial Kidney Transplant. 2020;29(2):114.
5. Kong Y, Yu YH. Retrospective analysis of high-risk factors for acute kidney injury in neurosurgery under general intravenous anesthesia. J Tianjin Med Univ. 2018;24(1):47–48.
6. Khwaja A. KDIGO clinical practice guide lines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179. doi:10.1159/000339789
7. Definition Task Foree ARDS, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533.
8. Chinese Medical Doctor Association Heart Intensive Care Expert Committee. Low cardiac output syndrome Chinese expert consensus. PLA Med J. 2017;42(11):933–944.
9. Liu Y, Yan ZY. Analysis of risk factors associated with the occurrence of acute kidney injury after mitral aortic valve bivalve replacement. Chin J Clinic Phys. 2015;9(11):2127–2130.
10. Küllmar M, Meersch M. Perioperative acute kidney injury. Anaes Thesist. 2019;68(4):194.
11. Young EW, Diab A, Kirsh MM. Intravenous diltiazem and acute renal failure after cardiac operations. Ann Thorac Surg. 1998;65(5):1316–1319. doi:10.1016/S0003-4975(98)00157-X
12. Durmaz İ, Buket S, Atay Y, et al. Cardiac surgery with cardiopulmonary bypass in patients with chronic renal failure. J Thorac Cardiovasc Surg. 1999;118(2):306–315. doi:10.1016/S0022-5223(99)70221-7
13. Tang Y, Wang W, Song Y. Analysis of risk factors for the occurrence of acute kidney injury after heart valve surgery. J Tianjin Med Univ. 2021;27(3):247–251.
14. Li L, Wang B, Xu CN. Poor prognosis and its risk factors in patients with severe hyperbilirubinemia combined with acute kidney injury after valve replacement for rheumatic heart disease. Chin J Extracorporeal Circ. 2021;19(2):94–98.
15. Rodríguez-Cubillo B, Carnero-Alcázar M, Cobiella-Carnicer J. Impact of postoperative acute kidney failure in long-term survival after heart valve surgery. Interact Cardiovasc Thorac Surg. 2019;29(1):35–42. doi:10.1093/icvts/ivz035
16. Yue Z, Yan-meng G, Ji-Zhuang L. Prediction model for acute kidney injury after coronary artery bypass grafting: a retrospective study. Int Urol Nephrol. 2019;51(9):1605–1611. doi:10.1007/s11255-019-02173-7
17. Wang CP, Liu YQ, Wang RC, et al. Clinical analysis of early acute kidney injury after heart valve surgery. Chin J Nephrol. 2021;37(11):881–888.
18. Sasabuchi Y, Kimura N, Shiotsuka J, et al. Long-term survival in patients with acute kidney injury after acute type a aortic dissection repair. Ann Thorac Surg. 2016;102(6):2003–2009. doi:10.1016/j.athoracsur.2016.05.006
19. Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13(11):697–711.
20. Vermeulen Windsant IC, de Wit NC, Sertorio JT, et al. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front Physiol. 2014;5:340.
21. Mo RH, Shao YM. Risk factor analysis of acute kidney injury after extracorporeal cardiac surgery in adults. J Arm Police Logist Coll. 2021;30(7):55–56.
22. Zhang W, Xin FR, Yang ZL, et al. Analysis of risk factors for developing acute kidney injury after heart valve surgery in adults. Trauma Acute Crit Care Med. 2022;10(3):174–190.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.