Back to Journals » International Journal of General Medicine » Volume 16
Analysis of Related Risk Factors and Prognostic Factors of Gastric Cancer with Liver Metastasis: A SEER and External Validation Based Study
Authors An W , Bao L, Wang C, Zheng M , Zhao Y
Received 23 August 2023
Accepted for publication 12 December 2023
Published 19 December 2023 Volume 2023:16 Pages 5969—5978
DOI https://doi.org/10.2147/IJGM.S434952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dinesh Vyas
Wenxiu An,1,2 Lijie Bao,1,2 Chenyu Wang,1,2 Mingxin Zheng,3 Yan Zhao1,2
1Cancer Hospital of Dalian University of Technology (Liaoning Cancer Hospital & Institute), Shenyang City, Liaoning Province, People’s Republic of China; 2Liaoning Provincial Key Laboratory of Interdisciplinary Research on Gastrointestinal Tumor Combining Medicine with Engineering, Shenyang City, Liaoning Province, People’s Republic of China; 3Neusoft Research of Intelligent Healthcare Technology, Co. Ltd, Shenyang City, Liaoning Province, People’s Republic of China
Correspondence: Yan Zhao, Liaoning Cancer Hospital & Institute, No. 44 Xiaoheyan Road, Dadong District, Shenyang City, Liaoning Province, 110042, People’s Republic of China, Tel +86-24-81916608, Email [email protected]
Background: Gastric cancer (GC) has a poor prognosis, particularly in patients with liver metastasis (LM). This study aims to identify relevant factors associated with the occurrence of LM in GC patients and factors influencing the prognosis of gastric cancer with liver metastasis (GCLM) patients, in addition to developing diagnostic and prognostic nomograms specifically.
Patients and Methods: Overall, 6184 training data were from the Surveillance, Epidemiology, and End Results (SEER) database from 2011 to 2015. 1527 validation data were from our hospital between January 2018 and December 2022. Logistic regression was used to identify the risk factors associated with the occurrence of LM in GC patients, Cox regression was used to confirm the prognostic factors of GCLM patients. Two nomogram models were established to predict the risk and overall survival (OS) of patients with GCLM. The performance of the two models was evaluated using the area under the curve (AUC), concordance index (C-index), and calibration curves.
Results: A nomogram included five independent factors from multivariate logistic regression: sex, lymph node removal, chemotherapy, T stage and N stage were constructed to calculate the possibility of LM. Internal and external verifications of AUC were 0.786 and 0.885, respectively. The other nomogram included four independent factors from multivariate Cox regression: surgery at primary site, surgery at other site, chemotherapy, and N stage were constructed to predict OS. C-index for internal and external validations were 0.714 and 0.702, respectively, and the calibration curves demonstrated the robust discriminative ability of the models.
Conclusion: Based on the SEER database and validation data, we defined effective nomogram models to predict risk and OS in patients with GCLM. They have important value in clinical decision-making and personalized treatment.
Keywords: gastric cancer, liver metastasis, nomogram, SEER database, external validation
Introduction
Gastric cancer (GC) is one of the most common types of digestive tract tumors in humans and is the fourth leading cause of cancer-related deaths worldwide. In 2020, it was estimated that there were 1.1 million new cases of gastric cancer and 800,000 deaths globally,1 indicating significant challenges in its treatment of gastric cancer. Many patients with gastric cancer do not exhibit significant early symptoms and are often diagnosed at advanced stages or with metastasis to other organs, which severely affects their health and quality of life.2,3 Therefore, understanding the status of cancer metastasis can help reduce damage to the body and improve the prognosis of patients with cancer. Distant organ metastasis is a critical factor affecting the prognosis of patients with gastric cancer, and the liver is one of the most common sites of distant metastasis in gastric cancer. The clinical incidence of liver metastasis in gastric cancer can reach as high as 13.5%, which is much higher than that of other types of metastasis, such as lung or bone metastasis.4–6 Gastric cancer with liver metastasis is considered a systemic disease, and there is still considerable controversy regarding its treatment and prognosis, with a focus on comprehensive treatment to extend patient survival.7 Therefore, patients with gastric cancer and liver metastasis, as a special population, deserve further research.
The Tumor Node Metastasis (TNM) system proposed by American Joint Commission on Cancer (AJCC) and the International Alliance Against Cancer is widely used to evaluate tumor staging and predict the prognosis of patients. However, using the TNM system alone for prediction is insufficient. Patient factors such as sex, age, and surgery are also risk factors that influence patient prognosis.8,9 However, most of the current research on risk factors and prognostic factors for gastric cancer liver metastasis is based on small sample sizes,10,11 requiring further studies to confirm or supplement the accuracy of the results. Prediction models are multifactorial models that can assess the probability of developing a certain disease,12,13 and they can be visualized using nomograms.14,15 Nomograms have been used as prediction tools to assess survival and prognosis of patients with multiple diseases, especially patients with tumors.16–18 Additionally, the SEER database contains a large amount of data on cancer incidence and prevalence, making it an effective method to construct a prediction model based on the SEER database and validate it with other data. This study built two models based on the SEER database and used data from Liaoning Cancer Hospital & Institute for external validation to analyze and explore the relevant risk factors of gastric cancer, liver metastasis and factors affecting the prognosis. The goal is to provide evidence for the diagnosis and treatment of gastric cancer liver metastasis and improve patient prognosis.
Materials and Methods
Data Source and Characteristics
The SEER database provides detailed information on various types of patients in different states of the United States since 1973, including age, sex, race, year of diagnosis, marital status, insurance, tumor size, TNM stage, and distant organ metastasis.19 Based on the publicly available data published in the SEER database, all patients are required to remove their personal identification without the approval of the ethics committee, and since 2010, relevant information on tumor metastasis has been improved. In this study, we used SEER * Stat 8.4.1 version and included patients with primary gastric cancer from 2011 to 2015 in the SEER database, exclusion criteria were as follows: (1) the primary tumor site was not located in the stomach, (2) non-malignant behavior based on ICD-O-3, (3) unknown surgery or chemotherapy status, (4) metastasis in other parts except the liver, and (5) missing survival status or time data. Finally, 6184 patients (including 858 liver metastasis patients) as training data. A total of 1527 gastric cancer patients (including 102 liver metastasis patients) at Liaoning Cancer Hospital & Institute from January 2018 to December 2022 were selected as validation data. The exclusion criteria were the same as those used for the SEER database, which were approved by the ethics committee, met the requirements of the Declaration of Helsinki, and followed the principles of medical ethics.
Demographic characteristics included sex (male and female) and age at initial diagnosis (<60 and ≥60 years). Tumor characteristics included American Joint Committee for Cancer T stage (T1/T2, T3/T4, TX) and AJCC N stage (N0, N1/N2/N3, NX). Treatment-related characteristics included surgery at primary site (yes or no), surgery at other site (yes or no), lymph node removal (yes or no), and chemotherapy (yes or no). Overall survival (OS) was defined as the time from the initial cancer diagnosis to death or last follow-up.
Statistical Analysis
All data are presented as frequencies and percentages. Logistic regression analysis was used to analyze the risk factors related to liver metastasis of gastric cancer, and the Cox regression proportional hazard model was used to analyze the impact of various variables on OS. Differences were considered statistically significant at P < 0.05. Based on the results of the logistic regression and Cox regression analyses, we constructed two nomograms to predict liver metastasis and the 2-year, 3-year, 5-year survival rates of patients with liver metastasis from gastric cancer. We then use the validation data to validate the two models. The nomogram model based on logistic regression uses the AUC values for validation and the nomogram model based on Cox regression uses the C-index for validation. Calibration curves were plotted for both parts to compare predicted and observed probabilities. Statistical analyses were performed using R version 3.6.3 (R Project for Statistical Computing, Vienna, Austria) and SPSS version 23.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline Characteristics
According to the inclusion and exclusion criteria, 6184 patients from the SEER database and 1527 patients from Liaoning Cancer Hospital & Institute were enrolled in this study. Table 1 shows the baseline patient characteristics. Among the patients in the SEER database, 3815 (61.69%) were male, 2369 (38.31%) were female, 1763 (28.51%) were aged <60 years, and 4421 (71.49%) were aged ≥60 years. Regarding treatment-related variables, approximately half of the patients underwent primary site surgery (49.19%), half did not (50.81%), and most patients did not undergo surgery at other site (94.91%), with only 315 patients (5.09%) undergoing surgery at other site; 3926 (63.49%) patients did not undergo lymph node removal, while 2258 (36.51%) patients received chemotherapy (51.12%), and those who did not receive chemotherapy (48.88%) also accounted for approximately half of them. Regarding tumor characteristics T stage T1/T2, T3/T4, and TX were found in 2278 (36.84%), 2551 (41.25%), and 1355 (21.91%), respectively, N stage N0, N1/N2/N3, and NX were found in 3287 (53.15%), 2389 (38.64%), and 508 (8.21%), respectively. There were 858 patients (13.87%) with liver metastasis, and 5326 patients (86.13%) without liver metastasis. For patients in Liaoning Cancer Hospital & Institute, 1001 (65.55%) males and 526 (34.45%) females; 854 (55.93%) were under the age of 60 years, and 673 (44.07%) were aged 60 years or above. Regarding treatment-related variables, 1088 (71.25%) patients underwent primary site surgery and 439 (28.75%) did not, and the proportion of patients was higher than that in the SEER database; only 50 (3.27%) patients underwent surgery at another site, which was similar to the SEER database. A total of 465 (30.45%) patients did not undergo lymph node removal, 1062 (69.55%) underwent lymph node removal, 1318 (86.31%) underwent chemotherapy, and 209 (13.69%) did not. Regarding tumor characteristics, T stage, T1/T2, T3/T4, and TX were found in 411 (26.92%), 1068 (69.94%), and 48 (3.14%), respectively, N stage, N0, N1/N2/N3, NX were found in 339 (22.20%), 943 (61.76%), 245 (16.04%), respectively. There were 102 patients with liver metastasis (6.68%) and 1425 patients without liver metastasis (93.32%). The proportion of patients with liver metastasis was slightly lower than the SEER database.
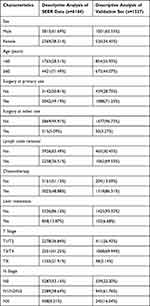 |
Table 1 Baseline Characteristics of Patients in Training and Validation Cohorts |
Nomogram Model Developed to Predict Liver Metastasis in Gastric Cancer Patients and Validation
For patients with liver metastasis in gastric cancer, a consensus has been reached among most clinicians that surgery is not typically the preferred initial treatment modality. This is attributed to its inability to address the systemic nature of the cancer and the potential for increased risk. Instead, there is a predilection towards utilizing systemic treatment approaches, such as chemotherapy and targeted therapy, aimed at controlling disease progression. Hence, no surgery of primary site is a consequence of the presence of liver metastasis in gastric cancer, and it should not be considered as a predictor. Univariate analysis showed that sex, lymph node removal, chemotherapy, T stage, and N stage were related to liver metastasis in gastric cancer (P < 0.05). Multivariate logistic regression analysis indicated that all univariate results were independent risk factors for liver metastasis (see Table 2). Based on this result, we constructed a nomogram to calculate the probability of liver metastasis by accumulating the relevant variables. Figure 1 shows that being male, having a higher T Stage and N Stage, undergoing chemotherapy, and lymph node removal were associated with a higher risk of liver metastasis in gastric cancer patients. Internal validation was conducted using bootstrapping with 1000 self-sampling times. The AUC values of the training and validation data were 0.786 and 0.885, respectively. The calibration curve (Figure 2) shows that the predicted probability of the nomogram is consistent with the actual observed probability.
 |
Table 2 Univariate and Multivariate Analysis for Factors Associated with Liver Metastasis |
 |
Figure 1 Nomogram for predicting liver metastasis in gastric cancer. |
 |
Figure 2 Evaluation of calibration plots using logistic nomogram model. Training data (a), validation data (b). |
Nomogram Model Developed to Predict Survival of Gastric Cancer Patients with Liver Metastasis and Validation
We conducted a prognostic analysis of patients with liver metastasis from gastric cancer. The training data consisted of all liver metastasis data in the training dataset (858 cases), and validation data were obtained from all liver metastasis data in the validation dataset (102 cases). The results of Cox univariate analysis showed that age, surgery of primary site, surgery of other site, lymph node removal, chemotherapy, T stage, and N stage were associated with OS (P < 0.05), as shown in Table 3, the factors found to be significant in univariate analysis were used in the multivariate Cox regression, and it demonstrated that surgery at primary site, surgery at other site, chemotherapy, N stage were independently associated with OS. Subsequently, we constructed a visual nomogram model for OS of patients at 2-years, 3-years, and 5-years. As shown in Figure 3, patients receiving chemotherapy, primary site surgery, other site surgery, and those with lower N stage achieved a longer survival period. We then performed internal validation of the model and used the validation data for external validation. Internal validation was conducted using the bootstrap method with a self-service sampling frequency of 1000. The calibration plots are shown in Figure 4. The C-index for the training and validation data were 0.714 and 0.702, respectively.
 |
Table 3 Univariate and Multivariate Analysis for Factors Associated with OS |
 |
Figure 3 Nomogram for predicting overall survival of gastric cancer liver metastasis. |
 |
Figure 4 Evaluation of calibration plots using OS nomogram model. (a–c) 2, 3, 5 years in training data, (d–f) 2,3,5 years in validation data. |
Discussion
Gastric cancer is a highly invasive cancer with a 5-year overall survival rate of only approximately 5–20%.7 The prognosis of gastric cancer patients mainly depends on tumor-related information, such as the presence of lymph nodes and other distant metastases. Currently, the AJCC staging system is the most widely used for evaluating the prognosis of patients with cancer. However, focusing solely on the location of the primary tumor, lymph node involvement, and distant metastasis is insufficient. Some studies have also shown that the prognosis of patients is closely related to clinical and pathological parameters and tumor characteristics.20,21 Therefore, this study constructed logistic and Cox regression models based on the SEER database and validated them with data from Liaoning Cancer Hospital & Institute, integrating different clinical features to evaluate the influencing factors and prognosis of gastric cancer patients with liver metastasis, thereby providing a reference for personalized comprehensive treatment. A visual nomogram is a graphical representation of complex formulas. Compared with the traditional analysis mode, it has many advantages, such as high accuracy, flexible use, and ease of promotion, and has been widely used in the medical field.22 This study established two nomogram models: one to predict the occurrence of gastric cancer liver metastasis, and the other to predict the OS of patients with gastric cancer liver metastasis.
Univariate and multivariate analyses of relevant clinicopathological factors affecting liver metastasis revealed that sex, lymph node removal, chemotherapy, T Stage, and N stage were independent risk factors for liver metastasis of gastric cancer (P < 0.05). Consistent with Wei’s research,23 sex was an independent risk factor for liver metastasis of gastric cancer. Men are more likely to develop liver diseases, including liver cancer, due to alcoholism and various unhealthy lifestyle habits.24 Surgical treatments, such as lymph node dissection, can help suppress the spread of cancer cells and effectively reduce the probability of liver metastasis. The preferred treatment for advanced gastric cancer is chemotherapy, and liver metastasis of gastric cancer usually occurs in late stages. Therefore, during chemotherapy, patients are usually diagnosed with advanced gastric cancer, which increases the risk of liver metastasis. T Stage and N stage were independent influencing factors, which were consistent with the research.9 Studies have pointed out a close relationship between T Stage, N stage, and liver metastasis in gastric cancer. Patients with a higher N stage were more likely to develop metastatic lesions because of the large number of tumor cells in the lymphatic system and blood circulation,25–27 this is consistent with the recommendation of this study for lymph node removal.
Based on the results of survival analysis, four indicators were considered. Acceptance of primary site surgery results in improved survival. A study on primary site surgery showed that the median cancer-specific survival (CSS) of patients with gastric cancer liver metastasis who underwent primary site surgery was 12 months, whereas the median CSS of patients with gastric cancer liver metastasis who did not undergo primary site surgery was 7 months. Additionally, primary site surgery is an independent diagnostic factor in multivariate Cox regression.28 This is consistent with our findings. Surgery at other sites can also improve a patient's prognosis. Liao et al showed that the 1-year, 2-year, and 3-year survival rates of gastric cancer patients with liver metastasis after liver resection surgery were 69%, 40%, and 33%, respectively, which were significantly higher than the 27%, 8%, and 4% in the palliative treatment group.29 Chemotherapy is also one of the ways to improve patient prognosis, which is consistent with most studies. At the same time,28 it was pointed out that patients who underwent both primary site surgery and chemotherapy had better OS than those who received chemotherapy alone (22.9 vs 17 months). The results of our study showed that surgery and chemotherapy were good factors that influenced patient prognosis. These findings suggest that active treatment provides relatively good survival benefits to patients. Ueda et al indicated that N stage was an independent prognostic factor for survival,30 the earlier the N stage, the better the prognosis, which is consistent with the results of this study.
Conclusion
In conclusion, we comprehensively identified factors related to the occurrence of gastric cancer in liver metastasis, including sex, lymph node removal, chemotherapy, T stage, and N stage. We then identified prognostic factors for gastric cancer liver metastasis, including surgery at primary site, surgery at other site, chemotherapy, and N stage. We then establish and validate two nomograms. This study provides a reference for future clinical research.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by Ethics Committee of the Liaoning Cancer Hospital & Institute (approval number:20200818YG). Informed consent was obtained from participants and/or their legal guardian for participation in the study, and all methods were carried out in accordance with relevant guidelines and regulations.
Funding
This research was funded by the National Key Research and Development Program of China (grant number 2021YFF1201102).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung HA-O, Ferlay J, Siegel RA-O, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
2. Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134(3):622–628. doi:10.1002/ijc.28373
3. Ganguly S, Biswas B, Ghosh J, Dabkara D. Metastatic gastric cancer: real world scenario from a developing country. South Asian J Cancer. 2018;7:171–174. doi:10.4103/sajc.sajc_2_18
4. D’Angelica M, Gonen M, Fau - Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;15:808–816. doi:10.1097/01.sla.0000143245.28656.15
5. Kong JH, Lee J, Yi C-A, et al. Lung metastases in metastatic gastric cancer: pattern of lung metastases and clinical outcome. Gastric Cancer. 2012;15(3):292–298. doi:10.1007/s10120-011-0104-7
6. Nakamura K, Tomioku M, Fau - Nabeshima K, Nabeshima K, Fau - Yasuda S, Yasuda S. Clinicopathologic features and clinical outcomes of gastric cancer patients with bone metastasis. Tokai J Exp Clin Med. 2014;39:193–198.
7. Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2019;18:534–542. doi:10.1016/j.cgh.2019.07.045
8. Alshehri A, Alanezi H, Kim BS. Prognosis factors of advanced gastric cancer according to sex and age. World J Clin Cases. 2020;8:1608–1619. doi:10.12998/wjcc.v8.i9.1608
9. Xiao Y, Zhang B, Wu Y. Prognostic analysis and liver metastases relevant factors after gastric and hepatic surgical treatment in gastric cancer patients with metachronous liver metastases: a population-based study. Ir J Med Sci. 2019;188:415–424. doi:10.1007/s11845-018-1864-4
10. Nonaka YA-O, Hiramatsu K, Kato T, et al. Evaluation of hepatic resection in liver metastasis of gastric cancer. Indian J Surg Oncol. 2019;10:204–209. doi:10.1007/s13193-018-0827-6
11. Hori SA-O, Honda M, Kobayashi H, et al. A grading system for predicting the prognosis of gastric cancer with liver metastasis. Jpn J Clin Oncol. 2021;51(11):1601–1607. doi:10.1093/jjco/hyab140
12. Bakhti SZ, Latifi-Navid SA-O, Safaralizadeh RA-O. Helicobacter pylori-related risk predictors of gastric cancer: the latest models, challenges, and future prospects. Cancer Med. 2020;9:4808–4822. doi:10.1002/cam4.3068
13. Xiaobin CA-O, Zhaojun X, Tao L, et al. Analysis of related risk factors and prognostic factors of gastric cancer with bone metastasis: a SEER-Based Study. J Immunol Res. 2022;2022:1–10. doi:10.1007/s00423-008-0311-9
14. Wang Y, Li J, Fau - Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi:10.1200/JCO.2012.41.5984
15. Wang ZX, Qiu MZ, Jiang YM, Zhou ZW, Li GX, Xu RH. Comparison of prognostic nomograms based on different nodal staging systems in patients with resected gastric cancer. J Cancer. 2017;8:950–958. doi:10.7150/jca.17370
16. Li W, Zhao K, Wang ZA-OX. Prognostic nomograms based on immune scores for head-neck squamous cell carcinoma patients. Eur Arch Otorhinolaryngol. 2021;278:2493–2500. doi:10.1007/s00405-020-06358-0
17. Caulfield S, Menezes G, Marignol L, Poole C. Nomograms are key decision-making tools in prostate cancer radiation therapy. Utol Oncol. 2018;36:283–292.
18. Nieder C, Mehta MP, Geinitz H, Grosu AL. Prognostic and predictive factors in patients with brain metastases from solid tumors: a review of published nomograms. Crit Rev Oncol Hematol. 2018;126:13–18. doi:10.1016/j.critrevonc.2018.03.018
19. Qiu MA-O, Shi SM, Chen ZH, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: a SEER-based study. Cancer Med. 2018;7:3662–3672. doi:10.1002/cam4.1661
20. Ramos MA-O, Ribeiro Júnior U, Viscondi JKY, Zilberstein B, Cecconello I, Eluf-Neto J. Risk factors associated with the development of gastric cancer - case-control study. Rev Assoc Med Bras. 2018;64:611–619. doi:10.1590/1806-9282.64.07.611
21. Katoh H, Ishikawa S. Lifestyles, genetics, and future perspectives on gastric cancer in east Asian populations. J Hum Genet. 2021;66:887–899. doi:10.1038/s10038-021-00960-8
22. Chen T, Xu L, Ye L, et al. A new nomogram for recurrence-free survival prediction of gastrointestinal stromal tumors: comparison with current risk classification methods. Eur J Surg Oncol. 2019;45:1109–1114. doi:10.1016/j.ejso.2018.12.014
23. Wei X, Yu H. Retrospective study based on SEER database: influencing factors and prognostic analysis of liver metastasis in gastric adenocarcinoma patients. J Med Res. 2020;49:19–24.
24. Kerkar SP, Kemp Cd Fau - Duffy A, Duffy A, et al. The GYMSSA trial: a prospective randomized trial comparing gastrectomy, metastasectomy plus systemic therapy versus systemic therapy alone. Trials. 2009;10:121–128. doi:10.1186/1745-6215-10-121
25. Kumagai K, Tanaka T, Fau - Yamagata K, et al. Liver metastasis in gastric cancer with particular reference to lymphatic advancement. Gastric Cancer. 2001;4(3):150–155. doi:10.1007/PL00011738
26. Maehara Y, Orita H, Fau - Okuyama T, et al. Predictors of lymph node metastasis in early gastric cancer. Br J Surg. 1992;79:245–247. doi:10.1002/bjs.1800790320
27. Saito H, Tsujitani S, Fau - Kondo A, et al. Expression of vascular endothelial growth factor correlates with hematogenous recurrence in gastric carcinoma. Surgery. 1999;125(2):195–201. doi:10.1016/S0039-6060(99)70265-5
28. Wu J, Yu J, Chen Z, et al. Survival benefit of primary tumor resection for gastric cancer with liver metastasis: a propensity score-matched, population-based study. Front Oncol. 2022;12. doi:10.3389/fonc.2022.1039086
29. Liao YY, Peng NF, Long D, et al. Hepatectomy for liver metastases from gastric cancer: a systematic review. BMC Surg. 2017;17:103–114. doi:10.1186/s12893-017-0215-0
30. Ueda K, Iwahashi M, Fau - Nakamori M, et al. Analysis of the prognostic factors and evaluation of surgical treatment for synchronous liver metastases from gastric cancer. Langenbecks Arch Surg. 2009;394:647–653. doi:10.1007/s00423-008-0311-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
