Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Analysis of Multi-Part Phenotypic Changes in Skin to Characterize the Trajectory of Skin Aging in Chinese Women
Authors Zhang Y, Liu X, Wang J, Du L, Ma Y, Liu W, Ye R, Yang Y, Xu H
Received 21 November 2021
Accepted for publication 27 March 2022
Published 13 April 2022 Volume 2022:15 Pages 631—642
DOI https://doi.org/10.2147/CCID.S349401
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yan Zhang,1,* Xiaoyu Liu,2,3,* Jingpu Wang,3 Le Du,3 Yanyun Ma,4– 6 Wei Liu,7 Rui Ye,3 Yali Yang,1,* Hui Xu1
1Department of Dermatology, Shanghai Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Molecular Diagnostics & Endocrinology, The Core Laboratory in Medical Center of Clinical Research, State Key Laboratory of Medical Genomics, Shanghai Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Inertiabiotech Co., LTD, Shanghai, People’s Republic of China; 4Human Phenome Institute, Fudan University, Shanghai, People’s Republic of China; 5State Key Laboratory of Genetic Engineering, Collaborative Innovation Center for Genetics and Development, School of Life Sciences, Fudan University, Shanghai, People’s Republic of China; 6Institute for Six-Sector Economy, Fudan University, Shanghai, People’s Republic of China; 7Department of Dermatology, The Medical Center of Air Force PLA, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Xu, Department of Dermatology, Shanghai Ninth People’s Hospital, No. 639, Manufacturing Bureau Road, Shanghai, People’s Republic of China, Email [email protected] Rui Ye, Inertiabiotech Co., LTD, Shanghai, People’s Republic of China, Email [email protected]
Purpose: As the human body’s largest organ exposed to the external environment, the skin suffers from internal and external aging factors, leading to wrinkles, loss of elasticity, sagging, and rough appearance. However, little is known of the characteristics of skin aging of different body parts in Chinese women. Here, we study the signs of extrinsic skin aging in different body parts to identify the knowledge map of manifestations of aging in Chinese women.
Patients and Methods: Wrinkle and texture phenotypes and collagen samples from the face, neck, hands, and arms of 326 Chinese women were collected. The correlations between phenotypes and ages and the differences in phenotypes by age were evaluated.
Results: The wrinkle and texture phenotypes around the eyes and mouth and of the hands were strongly correlated with age. Ages 32 and 58 showed the largest number of differentially changed aging phenotypes. The number of aging phenotypes increased sharply between the ages of 24 and 30, suggesting that the skin was undergoing rapid aging. Eye aging was the most rapidly changing phenotype between 19 and 30 years old. Wrinkles at the corner of the eyes showed a significant difference in the older group, suggesting an early onset and long-term effects.
Conclusion: This is the first study to be performed on the characteristics of skin aging among Chinese women that takes account of multiple areas of the body. It was found that 24 years old was the time point at which the skin begins to age in Chinese women. This provides important clues for aging-related research and personalized skin care.
Keywords: aging, Chinese women, phenotypes, skin, wrinkles
Introduction
The skin is the largest organ of the human body and is directly exposed to the external environment, thus playing an important role in maintaining the physiological functions of the human body, such as protection (providing a barrier), sensation, regulation of body temperature, and immunity. Because aging is a predominant risk factor for several chronic diseases that limit health span,1 numerous studies of the mechanisms, clinical manifestations, and prevention of skin aging have been conducted. Increasing numbers of people, particularly women, are pursuing a youthful appearance for their skin.2 Therefore, research on skin aging is of great significance for the development of skin care treatments and products to satisfy individuals’ needs.
Skin aging is generally divided into intrinsic and extrinsic aging.3,4 Intrinsic aging is induced by irresistible factors in the body (such as aging-induced changes in the endocrine and immune functions) and genetic factors. Extrinsic aging is caused by environmental exposure, such as solar ultraviolet (UV) radiation, smoking, air pollution, and so on, manifesting in the form of coarse wrinkles, loss of elasticity, laxity, pigmentation, and rough-textured appearance.5,6 Long-term exposure to UV radiation produces what is called photoaging, a major factor in extrinsic skin aging.6 Skin aging studies have generally concentrated on facial skin.7,8 Because the neck, arms, and hands are, after the face, among the body parts most exposed to sunlight, the risk for photoaging is higher than that of other parts of the body.9,10 Moreover, head movement accelerates the formation of neck wrinkles.9,11,12 Therefore, skin scientists and dermatologists have shown greater interest in skin aging in these areas, and people with a desire for body rejuvenation also focus on this area.9,13–15 Skin aging is mainly manifested as loss of skin elasticity and further wrinkle formation due to the progressive disorganization of collagen and elastin during aging.16 Skin aging shows different features in distinct age segments. It has been reported that facial wrinkles increase sharply from 40 to 50 years old in Chinese women.17,18 The onset of skin aging in different body parts is also inconsistent among gender, as marionette lines are reported to be more marked and to progress more rapidly among Chinese women compared with man.19
However, previous studies have usually focused on a single body part, and studies of the characteristics of skin aging in different parts were still deficient, and aging-related phenotypes were unclear. Moreover, objective aging-related biophysical parameters have not been well used to classify the different stages of skin aging. The onset of early aging remains vague. In this study, we recruited 326 healthy Chinese women from 18 to 66 years old. The biophysical parameters of skin aging in all subjects were collected to identify the aging characteristics in certain body parts and the phenotypes related to skin aging.
Materials and Methods
Participants and Questionnaire
In all, 326 healthy Chinese women aged 18–66 were recruited into this study, which was performed from May 2020 to June 2020. All subjects had lived in Shanghai, China for more than 2 years, and the mean body mass index was 22.33 ± 3.66 kg/m2. The exclusion criteria were as follows: subjects with severe diseases (eg, severe hypertension, diabetes, hepatitis, kidney disease, autoimmune diseases, cancer, and immunosuppression); subjects with moderate and severe acne in the facial area; those who have potential diseases or other dermatosis and received treatment within half a year before the study, such as systemic or topical corticosteroid therapy, vasoconstrictors, antibiotics, anti-inflammatory drugs; those who underwent cosmetic surgery within 1 year; those who were pregnant or breastfeeding.
The study included non-invasive measurements of the face, neck, dorsal and volar forearms, and dorsal hand area under controlled environmental conditions (room temperature: 20 ± 1°C; relative humidity: 55% ± 3%). After wiping the regions to be measured with a cotton pad soaked in distilled water, the participants were asked to relax under the same conditions for 30 minutes. To explore the relationship between lifestyle and aging phenotypes, we conducted a questionnaire survey on the participants. In total, 324 questionnaires contain information of demographics, skin type, air conditioning, and lifestyle were collected. Details are shown in Table 1.
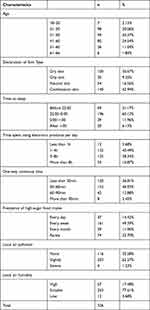 |
Table 1 Description of Study Population Characteristics |
Noninvasive Measurements
On the day that measurements were taken, the participants were instructed not to apply any cosmetic or skin care products. When measuring the face and neck area, participants were asked to maintain a natural sitting posture, looking straight ahead, and the chin and neck were naturally stretched, showing an obtuse angle. Participants were asked to maintain a natural standing position with their hands at their sides during the measurement of the forearm and dorsal hand area. The biophysical parameters including the depth of the wrinkles, the skin texture, and the melanin content were measured using Antera 3D®. The wrinkle mode of this device allows the measurement of parameters related to fine lines and wrinkles, including their maximum depth. The texture mode evaluates skin texture by measuring roughness, including average roughness (Ra), root mean squared roughness (Rq), and elevation span (R1) for small and medium-sized wrinkles. The melanin mode allows the average concentration of melanin per unit area to be measured.
Instant assessment of low echogenic band (LEB), intensity and skin thickness were measured using DermaScan® C USB (Cortex, Denmark). LEB represents the gap between the epidermis and dermis. The larger the LEB value, the more serious the aging. Intensity represents the skin collagen level. Skin thickness represents the thickness of the dermis.
The measurement areas are illustrated in Figure 1B, and all abbreviations of the measured phenotypes are shown in Supplementary Table 1. The method and software used for data processing were detailed in a previous study.20
Statistical Analyses
Spearman correlation analyses were conducted to detect possible relationships between skin phenotypes and physiological age. To evaluate the relationships between aging phenotypes, skin type, air conditioning, and lifestyle, a univariate regression model was used in the study. Estimates and p values for each category within the reference group were derived from the same regression model after adjustment for confounders. Differential expression-sliding window analyses (DE-SWAN) were to quantify changes in skin phenotypes in individuals 18 to 66 years old by comparing the phenotype levels of groups in parcels of 10 years within a window of 20 years.21 Phenotypes that changed significantly different were identified (False Discovery Rate (FDR) adjusted p value < 0.05). Principal component analysis (PCA) was conducted to study the difference among all age groups. Phenotype normality was evaluated using the Shapiro-Wilk test. The statistical differences in the normally distributed phenotypes between different age groups were compared with an unpaired Student t test (two‐tailed). The Mann-Whitney U-test was used to compare phenotypes. P values < 0.05 was considered to indicate statistical significance. All statistical analyses were conducted using R (version 3.6.2).
Results
Screening of Aging-Related Phenotypes
The age groups are shown in Table 1. A total of 82 biophysical phenotypes related to wrinkles, texture, melanin, LEB, intensity, and skin thickness were measured in 10 skin areas of 326 females.
To screen aging-related skin phenotypes and compare their characteristics at different points on the skin, we calculated the Spearman correlation coefficients between physiological age and all measured phenotypes. It was found that 66 of the 82 skin phenotypes were significantly correlated with age (p values < 0.05), with data shown in Figure 2 and Supplementary Table 2. The phenotypes for right corner of the eye (RET), right corner of the mouth (RM), and back of the right hand (HB) were strongly correlated with age (ρ > 0.6), and HB had the most aging-related phenotypes. Of all of the phenotypes, those related to wrinkles and texture were strongly correlated with age. Specifically, the volume of depressions of fine lines (WSO) of RET (RETWSO) and maximum depth of fine lines (WSMD) of RET (RETWSMD), were strongly positively correlated with age. The volume of depressions of wrinkles (WMO) of RM (RMWMO) and maximum depth of wrinkles (WMMD) of RM (RMWMMD), were strongly positively correlated with age. The WSO of HB (HBWSO), WSMD of HB (HBWSMD), WMO of HB (HBWMO), root mean squared roughness of fine lines (TSRq), average roughness of fine lines (TSRa), elevation span of fine lines (TSR1) (HBTSRq, HBTSRa, HBTSR1, respectively) and roughness of wrinkles (HBTMRq, HBTMRq) had a strong positive correlation with age.
Because the skin phenotypes of the face and hands are highly correlated with lifestyle, we selected the most aging-related phenotypes (RETWSO, RMWMMD, and HBWSO, respectively) among RET, RM, and HB, and constructed a multivariate regression model to explore association of lifestyle with aging phenotypes. To exclude the influence of confounding factors, we adjusted for the interaction factors of age, BMI, age and questionnaire questions in the model. It was found that the time to sleep was strongly associated with RETWSO, RMWMMD, and HBWSO (p values < 0.001). The frequency of high-sugar food intake was strongly associated with RETWSO and HBWSO (p values < 0.001). Meanwhile, the time spent using electronic products per day was strongly associated with HBWSO (p values < 0.001), as shown in Table 2. Furthermore, lifestyle appeared to have a greater impact on HBWSO and less on RMWMMD.
 |
Table 2 Relationships of Ageing Phenotypes with Skin Type, Air Conditioning, and Lifestyle |
Quantification of Aging-Phenotypic Changes Uncovered the Onset of Presenile Stage
To quantitatively understand aging-related phenotypic changes in Chinese women occurring from 18 to 66 years old, DE-SWAN software was used to analyze phenotypes levels within a window of 20 years and compare two groups in parcels of 10 years (eg, 20–25 years old was compared to 25–30 years old), while sliding the window in increments of 1 year from young to old. Using DE-SWAN, we identified phenotypes changing in waves throughout aging periods. All phenotypes that have significantly differences were shown (FDR adjusted p value < 0.5) (Figure 3). Crests at ages 32 and 58 were identified by summing the number of differentially changed phenotypes at each age (Figure 3A). Further crests in facial phenotypes appeared at ages 35 and 53 (Figure 3B). In addition, it was found that the number of differentially changed phenotypes increased rapidly from 24 to 30 years old, suggesting that the presenile stage of skin aging on the face, neck, hands, and arms may occur at this time. Meanwhile, phenotypes changes across aging were identified (Figure 3C). It was found that as age increased, the HB skin gradually roughened and the wrinkles deepened; the wrinkles at RET deepened, and melanin deposits appeared on right cheek (RF) after 22 years old. A loss of collagen was seen on RET, HB, right side of the neck (NS), and front of the neck (NF) after 25 years old. The skin on NS, HF, right volar forearm (VF), and right dorsal forearm (DF) became rough gradually, and the wrinkles deepened after 40 years old.
Characteristics of Aging Manifestation in Different Body Parts
To study the onset and rate of skin aging in different body parts, we summed the number of differentially changed phenotypes in different parts (Figure 3D and E). All significantly different phenotypes were identified by DE-SWAN, as mentioned above (FDR adjusted p value < 0.5). For the facial phenotype, the aging rate of the eyes was the fastest between the ages of 19 and 32 years, followed by the cheeks and corner of mouth and then forehead. Skin around the eyes aged rapidly between the ages of 19 and 32 years, and then the aging rate slowed down. The aging of cheeks and corner of mouth crested at the ages of 30 and 58. The forehead appeared to age slowly before the age of 40, and then tended to flatten out (Figure 3D and E). Neck aging reached its peak around the age of 50. The aging characteristics of the hands were similar to those of the eyes. Rapid aging occurred between the ages of 19 and 30, after which the rate slowed. Arm aging was similar to the aging in the cheeks and at the corner of mouth, peaking at 33 and 56 years old (Figure 3D).
We summarized the aging map of certain body parts according to the age of the earliest significant aging in each part. All significantly different phenotypes identified by DE-SWAN were involved (FDR adjusted p value < 0.5) (Figure 4). Wrinkles around the eye appeared the earliest (21 years old), followed by wrinkles on the face (22 years old), on the hands (22 years old), on the neck (25 years old), and on the forehead (30 years old). Collagen loss occurred first on the cheeks and at the corners of the mouth (19 years old), followed by the neck (21 years old), the hands (22 years old), the eyes (26 years old), and the forehead (46 years old). Wrinkles appeared around the eye at 21 years old, collagen loss occurred in the area at 26 years old, and the gap between the dermis and epidermis widened at 30 years old in this area, with the dermis thickening at 32 years old.
Comparison of Skin Phenotypes in Different Stage of Aging
For difference analyses of skin phenotypes in subjects in different age windows, we detected changes at particular stages of life. To identify differential phenotypes at different stages of aging, we divided all subjects into four groups, namely, a young group (≤25 years old), a presenile group (26–32 years old), a middle group (33–57 years old), and an old group (≥58 years old), according to the time points identified above. Using PCA, it was found that the young group and the presenile group could not be separated, but they could easily be separated from the middle group and old group (Figure 5).
 |
Figure 5 Skin phenotypes differ in the young group, the presenile group, the middle group, and the old group. (A) PCA results for in different groups. (B) Density of PC1 in different groups. |
To identify the different phenotypes in different age groups, we tested the phenotypes of the presenile group, the middle group, the old group, and the young group, respectively. For the presenile group, there were few differential phenotypes compared to the young group (n = 5, adjusted p < 0.05), mainly focused on the eyes, neck, and arms. The definite manifestations were that the RETWSO were deeper in the presenile group; the fine lines (NFWSO, NFWSMD) and wrinkles (NFWMO) on the front of the neck (NF) were deeper; and the gap between epidermis and dermis (LEB) on the right dorsal forearm (DF) (DF.LEB) was larger. For the middle group, numerous differential phenotypes were identified (n = 52, adjusted p < 0.05). The top 10 were mainly focused on HB, RET, RM, and RF. Specifically, the skin texture on HB (HBTSRa, HBTSRq, HBTSR1) was rougher; the fine lines (HBWSO, HBWSMD) and wrinkles (HBWMO) on HB were deeper; the fine lines on RET (RETWSO) were deeper; the wrinkles on RM (RMWMO, RMWMMD) were deeper; and the melanin content on the right cheek (RF) (RF.melanin) was greater. For the old group, the differential phenotypes were greater (n = 62, adjusted p <0.05). The top 10 were mainly focused on the HB, RF, and RET. HB had the most differential phenotypes. Specifically, the HB skin texture (HBTSRa, HBTSRq, HBTMRa, HBTMRq, HBTSR1) was rougher, the fine lines (HBWSO, HBWSMD) and wrinkles (HBWMO) were deeper; the RF melanin content (RF.melanin) was greater; and the RET fine lines (RETWSMD) were deeper. Details are shown in Supplementary Table 3.
Discussion
The time of aging occurrence and the severity of age-related features differ among populations. Although the population of East Asia is the largest population of any world region, little is known of the aging characteristics of Chinese women.8,22
Because the face is the area of the body most likely to be a social concern, most studies have focused on facial wrinkles, particularly crow’s feet. People do not strongly recognize the aging characteristics of other body parts.14 The neck and hands are among the parts of the body most visible to others’ eyes after the face.23 To identify aging-related features of face, neck, and hands, we evaluated the relationships between all skin phenotypes and physiological age. It was found that the wrinkles and texture-related phenotypes of RET, RM, and HB were strongly correlated with age. HB featured the most aging-related phenotypes, but this may also be due to the fact that the largest number of phenotypes in this study were measured on the back of the hand. To evaluate the differences in the aging phenotypes across different ages, we quantified the changes in aging-related phenotypes from 18 to 66 years old using DE-SWAN. The number of differentially changed aging phenotypes reached a peak at 32 and 58 years old. This number of phenotypes increased sharply between the ages of 24 and 30, suggesting that the presenile stage of the skin of the face, neck, and hands may occur at this point.
In addition, we created a knowledge map of manifestations of aging. Changes in phenotype and the aging characteristics of different body parts were identified. For facial aging, the rate of eye aging was the fastest between the ages of 19 and 30, followed by the cheeks, the corners of mouth, and the forehead. Neck aging reached its peak around age 50. The hands aged rapidly between the ages of 19 and 30, and then the rate of aging slowed. Arm aging peaked at around 33 and 56 years old. We identified the ages at which aging manifestations appeared at different body parts. This information makes it easy to understand, for example, the body parts where wrinkles appear first. We also identified the earliest signs of aging-related biochemical phenotypes of a given body part. For example, the phenotypes for eyes and wrinkles appeared earlier than collagen loss.
From the identified time points, the subjects were grouped, and differences in phenotypes were identified between the old group and the young group. RET wrinkles were significantly different in the presenile group from those in the young group, the middle group, and the old group, suggesting an early onset and long-term effects of wrinkles on the corners of the eyes. It has been reported that the severity of eye wrinkles has a significant impact on the perception of age.24 Chinese women have been found to have significantly more severe wrinkles in the area around the eyes than Japanese women.8,25 Effective anti-wrinkle chemicals have been found, including Argireline and hyaluronic-acid. A study of 60 Chinese subjects found that Argireline had a significant anti-wrinkle effect on the wrinkles around the eye.26 Another study showed that Argireline can improve the histological structure of the skin tissue in aged mice and rejuvenate aging skin.27 Moreover, the regular use of anti-wrinkle creams containing hyaluronic acid for over 3 months shows clear and positive effects on wrinkle-depth and skin-tightness.28 PCA results indicated that the young group and the presenile group could not be separated, suggesting that existing skin phenotypes are insufficient to describe the characteristics of these two groups. Follow-up studies are needed to increase the measurement dimensions of biochemical phenotypes and multi-omics data (eg, epigenomic, proteomic, metabolome, metagenome) to identify presenile features. What’s more, corresponding epidemiological investigations and relevant molecular biology experiments were also needed to discover the mechanisms behind different aging features.
Conclusion
This is the first study on the aging characteristics of Chinese women assessed in multiple skin areas. The aging-related skin features were identified by correlation analyses. Using sliding window analyses, we characterized the different stages of aging and identified the onset of the presenile stage. Wrinkle-related phenotypes at the corner of the eyes had strong correlations with age and showed significant differences in all age groups, characterized by early onset and long-term effects. In addition, we drew a knowledge map of aging manifestations in different parts to provide new clues for studies on Chinese female aging. Our results are of great significance for the specific skin care and development of effective treatment plans for individuals at different ages.
Data Sharing Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was examined and approved by the Ethics Committee on Human Research at Shanghai Ninth People’s Hospital, and written informed consent was provided by all study subjects. The study was conducted in accordance with the Declaration of Helsinki.
Funding
This work was supported by the industry Support Foundation of Huangpu District, Shanghai, Big Data Construction and Application of Artificial Intelligence Skin Diagnosis and Treatment (XK2020007).
Disclosure
The authors declare that they have no conflicts of interest to report.
References
1. Harman D. The aging process: major risk factor for disease and death. Proc Natl Acad Sci USA. 1991;88(12):5360–5363. doi:10.1073/pnas.88.12.5360
2. Kazanci AKM, Atasever A, Atasever A. Analyses of changes on skin by aging. Skin Res Technol. 2016;23(1):48–60. doi:10.1111/srt.12300
3. Puizina-Ivic N. Skin aging. Acta Dermatoven APA. 2008;17(2):47.
4. Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi:10.1177/0963689717725755
5. Krutmann JBA, Sore G, Bernard BA, Passeron T, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–161. doi:10.1016/j.jdermsci.2016.09.015
6. Mora Huertas AC, Hoehenwarter W, Heyroth F, Heinz A, Heinz A. Molecular-level insights into aging processes of skin elastin. Biochimie. 2016;128–129:163–173. doi:10.1016/j.biochi.2016.08.010
7. Dobos G, Blume-Peytavi U, Kottner J, Kottner J. Evaluation of skin ageing: a systematic review of clinical scales. Br J Dermatol. 2015;172(5):1249–1261. doi:10.1111/bjd.13509
8. Andrea Vierkötter AH, Yamamoto A, Yamamoto A, et al. Extrinsic skin ageing in German, Chinese and Japanese women manifests differently in all three groups depending on ethnic background, age and anatomical site. J Dermatol Sci. 2016;83(3):219–225. doi:10.1016/j.jdermsci.2016.05.011
9. Kim ECG, Won NG, Cho J, Cho J. Age‐related changes in skin bio‐mechanical properties: the neck skin compared with the cheek and forearm skin in Korean females. Skin Res Technol. 2013;19:236–241. doi:10.1111/srt.12020
10. Sattler G, Carruthers J, Flynn TC, Geister TL. Validated assessment scale for neck volume. Dermatol Surg. 2012;38:343–350. doi:10.1111/j.1524-4725.2011.02253.x
11. Lee SK. Correction of horizontal neck lines: our preliminary experience with hyaluronic acid fillers. J Cosmet Dermatol. 2017;17:1–6.
12. Xie X, Wang Y, Zeng Q, et al. Characteristic features of neck skin aging in Chinese women. J Cosmet Dermatol. 2018;17(5):935–944. doi:10.1111/jocd.12762
13. Wang Y, Hu L-W, Wang Y, et al. Skin texture parameters of the dorsal hand in evaluating skin aging in China. Skin Res Technol. 2011;17(4):420–426. doi:10.1111/j.1600-0846.2011.00513.x
14. Yoo MA, Shin MK, Koh JS, Koh JS. How much related to skin wrinkles between facial and body site? Age-related changes in skin wrinkle on the knee assessed by skin bioengineering techniques. Skin Res Technol. 2016;11(1):69–74. doi:10.1111/srt.12230
15. Nam J-H, Choi Y-J, Lim JY, Min JH, Kim W-S. Synergistic effect of high-intensity focused ultrasound and low-fluence Q-switched Nd: yAGlaser in the treatment of the aging neck and décolletage. Lasers Med Sci. 2017;32(1):109–116. doi:10.1007/s10103-016-2092-7
16. DiBernardo BE. The aging neck: a diagnostic approach to surgical and nonsurgical options. J Cosme Laser Ther. 2013;15:56–64. doi:10.3109/14764172.2012.758383
17. Fang R-Y, Zhang H-C, Liu Y-G, Sun Q-N. Quantitative evaluation of rejuvenation treatment of nasolabial fold wrinkles by regression model and 3D photography. J Cosmet Dermatol. 2021;20(1):338–345. doi:10.1111/jocd.13486
18. Nouveau-Richard S, Mac-Mary S, Mac-Mary S, et al. Skin ageing: a comparison between Chinese and European populations. A pilot study. J Dermatol Sci. 2005;40(3):187–193. doi:10.1016/j.jdermsci.2005.06.006
19. Frederic Flament AA, Amar D, Amar D. Gender-related differences in the facial aging of Chinese subjects and their relations with perceived ages. Skin Res Technol. 2020;26(6):905–913. doi:10.1111/srt.12893
20. Ye R, Chang W, Hu J, Qiao L, Wang Q. A new method for skin aging evaluation of Chinese women. J Cosmet Dermatol. 2021;20(1):256–262. doi:10.1111/jocd.13461
21. Lehallier B, Gate D, Schaum N, et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019;25(12):1843–1850. doi:10.1038/s41591-019-0673-2
22. Zhang J, Hou W, Feng S, Chen X, Wang H. Classification of facial wrinkles among Chinese women. J Biomed Res. 2017;31(2):108–115. doi:10.7555/JBR.31.20150175
23. Messaraa C, Doyle L, Mansfield A, O’Connor C, Mavon A. Ageing profiles of Caucasian and Chinese cohorts: focus on hands skin. Int J Cosmet Sci. 2019;41(1):79–88. doi:10.1111/ics.12514
24. Porcheron A, Latreille J, Jdid R, Tschachler E, Morizot F. Influence of skin ageing features on Chinese women’s perception of facial age and attractiveness. Int J Cosmet Sci. 2014;36(4):312–320. doi:10.1111/ics.12128
25. Tsukahara K, Sugata K, Osanai O. Comparison of age-related changes in facial wrinkles and sagging in the skin of Japanese, Chinese and Thai women. J Dermatol Sci. 2007;47(1):19–28. doi:10.1016/j.jdermsci.2007.03.007
26. Wang Y, Wang M, Xiao S, Pan P, Li P, Huo J. The anti-wrinkle efficacy of argireline, a synthetic hexapeptide, in Chinese subjects: a randomized, placebo-controlled study. Am J Clin Dermatol. 2013;14(2):147–153. doi:10.1007/s40257-013-0009-9
27. Wang Y, Wang M, Xiao XS, Huo J, Zhang WD. The anti-wrinkle efficacy of argireline. J Cosmet Laser Ther. 2013;15(4):237–241. doi:10.3109/14764172.2013.769273
28. Poetschke J, Schwaiger H, Steckmeier S, et al. Anti-wrinkle creams with hyaluronic acid: how effective are they? MMW Fortschr Med. 2016;158(Suppl 4):1–6.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.