Back to Journals » Infection and Drug Resistance » Volume 16
Analysis of Drug-Resistant Tuberculosis in Children in Shenyang, China, 2017–2021
Authors Sun J, Fan L, Zhao Y, Wu H, Li R, Tian Y, Cheng M, Ma X, Ma Y, Yang X, Shen A, Yu Y, Chen Y
Received 3 August 2023
Accepted for publication 19 October 2023
Published 1 November 2023 Volume 2023:16 Pages 6983—6998
DOI https://doi.org/10.2147/IDR.S428720
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jiao Sun,1,* Lichao Fan,2,* Yanping Zhao,3,* Haoyu Wu,2 Ran Li,2 Yao Tian,2 Moxin Cheng,2 Xin Ma,1 Yingying Ma,1 Xinru Yang,1 Adong Shen,4 Yanhong Yu,1 Yu Chen2
1Tuberculosis Laboratory, Shenyang Tenth People’s Hospital/Shenyang Chest Hospital, Shenyang, People’s Republic of China; 2Department of Tuberculosis, Shenyang Tenth People’s Hospital/Shenyang Chest Hospital, Shenyang, People’s Republic of China; 3School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Special Administrative Region, People’s Republic of China; 4National Clinical Research Center for Respiratory Disease, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanhong Yu; Yu Chen, Email [email protected]; [email protected]
Objective: Drug-resistant tuberculosis (DR-TB) in children seriously threatens TB control. Information on the epidemiology and characteristics of DR-TB in children in China is limited. We studied data in Shenyang Tenth People’s Hospital to understand the DR-TB epidemiology in children in Shenyang.
Design or Methods: We retrospectively analyzed drug resistance testing data of pediatric TB patients between 2017 and 2021, and included 2976 clinically-diagnosed pediatric TB patients. We described the epidemiology of DR-TB and analyzed the trends of DR-TB incidence. The Kappa value was calculated to assess the agreement between MGIT 960 DST and Xpert MTB/RIF for detecting rifampicin resistance. Multivariate logistic regression was used to identify the risk factors for DR-TB in pediatric patients.
Results: Of the 2976 TB patients, 1076 were confirmed by MGIT 960 culture and/or Xpert MTB/RIF. Among the 806 patients identified by MGIT 960 culture, 232 cases (28.78%) were DR-TB. Resistance to the six drugs was in the following order: streptomycin (21.09%), isoniazid (9.35%), rifampin (15.01%), levofloxacin (6.20%), ethambutol (4.22%), and amikacin (3.23%). Alarmingly, 12.90% were MDR-TB (104/806), including 28 (3.47%) pre-XDR-TB. Of the 1076 pediatric TB patients, 295 (27.4%) developed DR-TB to any one drug (including 69 rifampicin-resistant cases identified by Xpert MTB/RIF only). No difference was found in the incidence of pediatric DR-TB between 2017 and 2021. Among 376 patients who were positive for both methods, using the MGIT 960 DST results as the gold standard, Xpert MTB/RIF’s sensitivity for detecting rifampicin resistance was 91.38% and its specificity was 94.65%.
Conclusion: Between 2017 and 2021, the DR-TB incidence in children remained unchanged in Shenyang. RR-TB, MDR-TB, and even Pre-XDR-TB require attention in children with drug-resistant TB. Xpert MTB/RIF helped to detect more rifampicin-resistant pediatric patients; thus Xpert MTB/RIF should be widely used as an important complementary tool to detect rifampicin-resistant TB in children.
Keywords: tuberculosis, drug-resistant, children, rifampicin, Xpert MTB/RIF, drug susceptibility testing
Introduction
Children account for approximately 11% of all TB cases globally.1 Among the estimated 1.1 million pediatric TB patients, only about one-third or 36.5% (399,000) were notified to the national TB control programs, and an estimated 226,000 children under 15 years died because of TB in 2020.2 The expanding global TB epidemic, especially drug-resistant tuberculosis (DR-TB) in children, remains one of the leading causes of childhood morbidity and mortality.3
Approximately 25,000 to 32,000 children develop rifampicin-resistant TB (RR-TB) and even multidrug-resistant TB (MDR-TB, defined as TB strains resistant to both rifampicin and isoniazid) each year.4 In 2018, 3398 children started second-line anti-TB drugs for treating MDR/RR-TB and the number of children on second-line TB drugs increased to 5586 in 2019 but dropped to 3234 in 2020, probably due to the COVID-19 pandemic.2 Diagnosis of childhood TB is very difficult, and the reporting and treatment of childhood TB are not easy.5 Difficulty in collecting samples, paucity in the bacteria of pediatric TB patients, and lack of sensitive pathogenic diagnostic techniques all contribute to the difficulties in the diagnosis and drug sensitivity testing (DST) of pediatric TB.6,7
Investigation and analysis of the status and epidemiology of DR-TB in children are very helpful in developing timely and appropriate treatment regimens for children with DR-TB. In general, first-line anti-tuberculosis drugs were used for children with TB.6 If the pediatric TB patients are not responding to a drug-susceptible TB regimen, or the infection source is known as a drug-resistant case, the children are then presumptively diagnosed as DR-TB. Treating pediatric DR-TB is complicated, especially for RR-TB cases. Most guidelines group MDR-TB and RR-TB as MDR/RR-TB and the patients who fall into MDR/RR-TB group should be managed with an MDR-TB regimen.2 Appropriate drug-resistant anti-TB regimens initiation relies on the DST results of multiple respiratory and extrapulmonary samples in children. To improve diagnostic and therapeutic targeting, WHO recommended Xpert MTB/RIF in 2010 for diagnosing pulmonary TB and RR-TB in adults, and in 2013 this recommendation further included the screening of children with suspected TB.8,9 This technology takes a shorter time (approximately 2 hours), compared with the gold standard, the culture method (2–6 weeks).10,11
China is one of high DR-TB burden countries, but the epidemiological data on drug resistance in childhood TB is still lacking. A study showed that among 784 new pediatric TB cases ≤18 years in China, drug resistance was highest to streptomycin at 14.3%, followed by isoniazid at 12.1%, rifampicin at 8.3% and ethambutol at 5.5%, including 54 (6.9%) children with MDR-TB.12 However, the DR-TB situation among children in different regions of the country may vary due to many factors such as environment, economy, medical level, and ethnicity. We aimed to describe the epidemiology of the drug resistance of the childhood TB patients confirmed by BACTEC MGIT 960 culture and/or Xpert MTB/RIF in Shenyang Tenth People’s Hospital from 2017–2022 in Shenyang and describe the 5-year trends in DR-TB among them and investigated the possible risk factors in association with childhood drug resistance. Some previous studies have defined children as <15 years of age,13 others as ≤18 years of age.12 In our retrospective study, children were defined as ≤18 years of age.
Methods and Materials
Study Population and Data Collection
This retrospective study was carried out using clinical records and lab results obtained between January 1, 2017, and December 31, 2021, in Shenyang Tenth People’s Hospital, also known as Shenyang Chest Hospital (the only designated TB hospital in Shenyang. All children ≤18 years of age with TB in Shenyang are registered and management in this hospital). The inclusion criteria included those children (≤18 years) clinically diagnosed as TB patients during the study period in our hospital. The exclusion criteria included those with contaminated cultures or indeterminate Xpert MTB/RIF results. The second or third specimens results from the same individual were excluded, except for extrapulmonary specimens, which were kept as a priority, be it the second or third specimen.
Data on patient demographics and drug sensitivity profile of Mycobacterium tuberculosis (MTB) isolates were collected and recorded. MTB was identified by BACTEC MGIT 960 culture and/or Xpert MTB/RIF; susceptibility to isoniazid, rifampin, ethambutol, streptomycin, levofloxacin and amikacin was identified by DST. Descriptive epidemiology of rifampicin-susceptible DR-TB and RR-TB was performed. Trends of the changes of DR-TB incidence over time was analyzed, and the Kappa value was calculated to assess the agreement between MGIT 960 DST and Xpert MTB/RIF for detecting rifampicin resistance. Logistic regression used to identify the risk factors for DR-TB in pediatric patients.
Definitions
Clinical pediatric TB patients are defined as children who exhibit clinical symptoms suggestive of TB, radiographic findings consistent with the disease, and meet at least one of the following criteria: positive tuberculin skin test or interferon release assay results or confirmed exposure to TB.
Extrapulmonary TB (EPTB) is defined as any bacteriologically confirmed or clinically diagnosed TB case that involves organs other than the lungs (eg, pleura, peripheral lymph nodes, abdomen, genitourinary tract, skin, joints and bones, meninges).
Sensitive strains in this study are defined as those susceptible to all six anti-TB drugs (isoniazid, rifampicin, ethambutol, streptomycin, amikacin, and levofloxacin) tested by the MGIT 960 DST or MTB identified by Xpert MTB/RIF without RR-TB related rpoB gene mutation.
When an MDR/RR-TB case is also found with drug resistance to any fluoroquinolone antibiotics, this person is defined as having pre-extensively drug-resistant TB (Pre-XDR-TB).14
TB contact is defined as any person exposed to a TB case.
TB treatment history refers to individuals who have previously undergone treatment for TB for a minimum of one month. These individuals may have received a first-line regimen for drug-susceptible TB or a second-line regimen for drug-resistant forms of the disease.2
Malnutrition in this study refers to severe acute malnutrition, which is diagnosed if the child falls into one of the three groups: 1. both feet with edema, 2. any mid-upper arm circumference (MUAC) falls below 115 mm, 3. weight-for-height/length less than-3 Z-scores.15
Tobacco smoking is defined as a person smoking ten cigarettes or more in a month.16
Statistical Analysis
SPSS 23.0 was used for the statistical analysis. We analyzed the changes in the number and proportion of DR-TB incidence over time using the chi-square test, and the correlated factors were analyzed using an unconditional logistic regression model for univariate and multivariate analysis. P <0.05 was considered a statistically significant difference.
Ethical Considerations
The study was approved by the Medical Ethics Committee of Shenyang Tenth People’s Hospital with the reference number KYXM-2020-004-02. Written informed consent was obtained from all participants and/or their guardians. (In our hospital, patients who are admitted to the hospital were invited to give a pan-informed consent and signed written consent for the use of clinical information, laboratory data, and anti-tuberculosis treatment results for research purposes.) This study was conducted in accordance with the Declaration of Helsinki.
Results
Characteristics of the Pediatric TB Patients
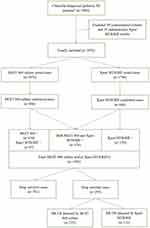
A total of 3084 clinically diagnosed pediatric TB patients were registered during the study period in our hospital. All the repeated specimens’ results of these 3084 patients were excluded, as described in the methods. Another 108 patients were excluded as they only had contaminated liquid cultures (89 samples) or indeterminate Xpert MTB/RIF results (19 samples). Finally, 2976 clinically diagnosed pediatric TB patients’ data were included in this study.
As shown in Figure 1, out of the 2976 clinically-diagnosed TB patients (≤18 years) during the study period, 1076 TB cases were diagnosed by MGIT 960 culture (806/1076) and/or Xpert MTB/RIF (646/729). The two methods together determined 781 cases as drug-sensitive TB and 295 cases as DR-TB. Of the 295 DR-TB patients, 232 were detected by MGIT 960 DST, 122 by Xpert MTB/RIF, and 69 RR-TB cases were identified by Xpert MTB/RIF only. These lab results were based on the following samples, for pulmonary TB patients, 598 sputum samples, 198 bronchoalveolar lavage fluid, 8 feces, and 2 gastric fluids; for EPTB cases, 97 extrapulmonary tissue samples, 39 bone and joint puncture fluid, 48 lymph node pus, 55 pleural effusions, 18 cerebrospinal fluid, 5 urine, 4 feces, 3 peritoneal effusions, and 1 pericardial effusion. We further followed up the clinical information of 108 patients excluded from this study, out of which 35 had solid culture results, 7 (20.00%) were positive and 2 were suggestive of either resistance (28.57%), the rate of resistance was not significantly different from our study population of 27.42% (295/1076).
As shown in Figure 2, all of the 2976 TB patients had done MGIT 960 test and the positive rate of MGIT 960 culture was 27.08% (806/2976). Xpert MTB/RIF testing was performed in 1786 of the 2976 TB patients, out of which, 646 were MTB positive (36.17%). Of the 729 confirmed patients who underwent both testing methods, the results were inconsistent in 353 cases (Xpert MTB/RIF positive alone in 270 and MGIT 960 culture positive alone in 83). The participants’ characteristics including sex, age, treatment history, and MGIT 960 DST results of six anti-TB drugs were included. The six drugs were isoniazid, rifampin, ethambutol, streptomycin, amikacin, and levofloxacin. Xpert MTB/RIF were used for suspected pulmonary TB patients, including 310 sputum samples, 103 bronchoalveolar lavage fluid, 6 feces, and 2 gastric fluids; for suspected EPTB cases, 91 extrapulmonary tissue samples, 39 bone and joint puncture fluid, 40 lymph node pus, 31 pleural effusions, 15 cerebrospinal fluid, 5 urine, 3 feces, and one peritoneal effusion.
 |
Figure 2 Venn diagram shows the numbers of MGIT 960 culture and Xpert MTB/RIF tested and confirmed cases (n=2976). |
As shown in Table 1, the mean age of the 1076 TB patients confirmed by MGIT 960 culture and/or Xpert MTB/RIF was 14.95 ± 2.83 years. Of these, less than half of the participants were children younger than 15 years (395, 36.71%), with few <5 years (14, 1.30%), 570 were boys (52.95%), and 117 had an anti-TB treatment history (10.87%). All of the 1076 participants had BCG vaccination. The majority of the patients had pulmonary TB (806/1076,74.91%), of which 214 (26.55%) had pulmonary cavities and 324 (40.20%) had bilateral pulmonary lesions. Approximately 25% were EPTB (270/1076,25.09%). Among these EPTB patients, 81 also had pulmonary TB and their extrapulmonary specimens’ results were kept as a priority. Around 10% of the patients had a TB exposure history (122/1076,11.34%). Among these 1076 pediatric TB patients, 295 (27.4%) developed DR-TB, including 190 (17.7%) developed RR-TB (including 69 rifampicin resistance cases detected by Xpert MTB/RIF only). The characteristics of the participants are presented in Table 1.
 |
Table 1 Characteristics of the Pediatric TB Patients from 2017–2021 Included in This Study |
Drug Resistance in Pediatric Tuberculosis Patients, Based on MGIT 960 DST Results, 2017–2021, Shenyang
Among the 806 patients identified positive by MGIT 960 culture, 574 cases (71.22%) were sensitive to all six anti-TB drugs and 232 cases (28.78%) were resistant to one or more anti-TB drugs. Resistance to the six drugs was in the following order: streptomycin 21.09% (170/806), isoniazid 19.35% (156/806), rifampicin 15.01% (121/806), levofloxacin 6.20% (50/806), ethambutol 4.22% (34/806) and amikacin 3.23% (26/806). Alarmingly, 12.90% were MDR-TB (104/806), and 3.47% were Pre-XDR-TB (28/806). As rifampicin is a critical TB drug, we further divided the DR-TB samples into rifampicin-susceptible DR-TB and RR-TB.
Drug Resistance in Rifampicin-Susceptible DR-TB Patients, Based on MGIT 960 DST Results
Details of drug resistance profiles are shown in Figure 3 and Table 2. To sum up, among the 232 patients with MGIT 960 DST results indicating drug resistance, 121 (52.16%) were RR-TB, and 111 (47.84%) were rifampicin-susceptible DR-TB. Among the rifampicin-susceptible DR-TB cases, mono-resistant TB was most frequent with 73 cases (65.77%) and streptomycin-resistant TB was the most frequent mono-resistant TB with 46 cases (41.44%). No case was resistant to Amikacin alone in the mono-resistant TB cases. Resistant to two drugs were identified in 30 patients (27.03%) and the most frequent combination was isoniazid and streptomycin, which was found in 24 cases (21.62%).
 |
Table 2 Anti-Tuberculosis Drug Resistance Profiles in the Drug-Resistant Strains That Were Rifampicin-Susceptible, Shenyang, China, 2017–2021 |
Drug Resistance in RR-TB Patients, Based on MGIT 960 DST Results
As shown in Figure 4 and Table 3, among the 232 patients with MGIT 960 DST results indicating drug resistance, 121 cases (52.16%) were RR-TB, and 104 were MDR-TB (44.83%). Alarmingly, most of these patients were resistant to three or more drugs (95/121,78.51%). The most frequent type of resistance was: isoniazid + rifampicin + streptomycin in 40 cases (33.06%). About 84% of the MDR-TB cases were identified as streptomycin-resistant (87/104, 83.65%).
 |
Table 3 Anti-Tuberculosis Drug Resistance Profile in the Rifampicin-Resistant Strains, Shenyang, China, 2017–2021 |
The 5-Year Trend of DR-TB in Children Based on MGIT 960 DST Results, Shenyang, 2017–2021
Among the 806 patients identified positive by MGIT 960 culture, the trends in the number and proportion of the patients with DR, RR, MDR, MDR+AMK, and Pre-XDR between 2017–2021 are shown in Figure 5. DR-TB decreased from 27.27% to 24.67%, or 0.52% annually (P>0.05); RR-TB decreased from 18.18% to 10.67%, or 1.50% annually (P>0.05); MDR-TB decreased from 16.58% to 10.67%, or 1.18% annually (P>0.05); MDR+AMK TB decreased from 3.74% to 0.67% with an annual decline rate of 0.61% (P>0.05); Pre-XDR TB decreased from 3.21% to 2.00% with an annual decline rate of 0.24% (P>0.05).
Analysis of DR-TB Identified by Xpert MTB/RIF
In 2017–2021, Xpert MTB/RIF has gradually become an important tool for RR-TB diagnosis. In 2017, only 27.56% of the childhood clinical TB (191/693) was sent for Xpert MTB/RIF test. The proportion sent for Xpert MTB/RIF test increased to 48.19% (279/579) in 2018, and further to 63.70% (393/617) in 2019, 75.99% (424/558) in 2020 and 94.33% (499/529) in 2021, with an average annual growth rate of 13.35% (P<0.01). In Figure 6, we could observe the advantage of Xpert MTB/RIF for RR-TB diagnosis was especially seen in the RR-TB (Xpert MTB/RIF only) cases, or the cases undiagnosed by MGIT 960 DST, which increased from 6 (6/40,15.00%,) in 2017 to 19 (19/35,54.29%) in 2021.
Meanwhile, as shown in Table 4, among the 376 patients who were positive for both MGIT 960 culture and Xpert MTB/RIF, using the results of MGIT 960 DST as the reference, the sensitivity of Xpert MTB/RIF for identifying RR-TB was 91.38% (53/58) and the specificity was 94.65% (301/318). Among these patients, 4.52% (17/376) were identified as RR-TB by Xpert MTB/RIF, but not identified as RR-TB by MGIT 960 DST results. The sensitivity of Xpert MTB/RIF and MGIT 960 DST for Kappa concordance comparison, Kappa=0.793, demonstrated good concordance.
 |
Table 4 Agreement Analysis of Xpert MTB/RIF with DST for Rifampicin Resistance Detection |
Risk Factors Affecting Drug Resistance Status
As indicated in Table 5, among the 295 DR-TB patients and 781 sensitive cases, significant differences in the following variables were identified: age, smoking history, malnutrition status, history of previous anti-TB treatment, and pulmonary TB status (P<0.05). Among the DR-TB cases, there were more patients aged <15 years than among sensitive cases (46.1% vs 32.9%, P<0.001); 15.9% of drug-resistant cases had a smoking history, which was higher than among sensitive cases (15.9% vs 7.9%, P<0.001) (all 109 cases with a smoking history were patients over 12 years of age); 15.6% of drug-resistant cases had malnutrition, while only 5.0% of sensitive cases had malnutrition (15.6% vs 5.0%, P<0.001). Compared with the drug-sensitive TB cases, a higher proportion of TB patients in the DR-TB cases was found with a previous anti-TB treatment history (21.0%) (P<0.001). Among the patients with an anti-TB treatment history, the risk of drug resistance was higher among those with a treatment interruption history and the OR was 4.05 (95% CI: 1.04–19.47) compared to those without interruption. Among DR-TB cases, pulmonary tuberculosis cases were predominated, accounting for 79.7% of all drug-resistant cases, which was higher than the sensitive group (79.7% vs 73.1%, P<0.05). Among the pulmonary tuberculosis patients, the risk of drug resistance was higher in those with cavitation (34.5%), compared with those without cavitation, with an OR=1.73 (95% CI: 1.24–2.41).
 |
Table 5 Univariate Analysis of Factors Related to DR-TB |
As shown in Table 6, age<15(OR=1.84, 95% CI: 1.38–2.45), history of smoking (OR=2.39 95% CI: 1.54–3.69, P<0.001), malnutrition status (OR=2.62,95% CI: 1.62–4.23), history of anti-TB treatment (OR= 3.11, 95% CI: 2.06–4.71), and pulmonary TB (OR = 1.53, 95% CI: 1.09–2.16) were independent risk factors for DR-TB in pediatric patients (P< 0.05).
 |
Table 6 Unconditional Multivariate Analysis of Factors Related to DR-TB |
Discussion
Drug-resistant TB in children is a serious public health problem, especially in countries with a high TB prevalence. Definitive bacteriological diagnosis as well as the determination of antibiotic susceptibility are important for treatment, particularly for children. There are very limited data on pediatric DR-TB patients’ epidemiology in China. We summarized the profile of the pediatric patients with DR-TB in Shenyang between 2017 and 2021 and assessed the trends and risk factors of DR-TB, which could inform TB control programs. This study indicates that the incidence of DR-TB in children remained almost unchanged in Shenyang during the study period. The positive rate of MGIT 960 culture was 27.08% (806/2976), out of which, 232 (28.78%) were resistant to one or more antibiotics. The proportion was similar to previous studies in China in recent years. A retrospective study in Beijing Children’s Hospital from 2010–2016 by Wang et al in 2018 found that the rate of pediatric DR-TB among new patients was 25%.17 A retrospective study by Xu et al using data between 2015 and 2018 in Chengdu, Sichuan Province, found a drug resistance rate of 24.3% for pediatric TB.18 A meta-analysis by DiNardo which included 9 studies showed that a total of 939 children with suspected TB had a positive MGIT 960 culture detection rate of 24.4% (229/939), including 22.03% (154/699, median 21.1%, IQR 14.5–38.4%) in children under 5 years and 35.57% in children 5–15 years (69/194, median 31.5%, IQR 23.8–43.4%).19 Another multicenter study of EPTB in children in mainland China showed a bacteriological positivity rate of 29.88% (401/1342).20 All data showed that the status of childhood DR-TB in China is not optimistic. Xpert MTB/RIF can help to identify TB in children and is an important tool to detect RR-TB that should be utilized. The positive rate of Xpert MTB/RIF was 36.17% (646/1786) in our study, which was higher than the positive detection rate of MGIT 960 culture at 27.08% (806/2976). Pang et al showed that the positive rate of Xpert MTB/RIF was 32.70% (69/211), which is similar to our result.21 Nhu et al found the positive rate of Xpert MTB/RIF was 39.73% (29/73).22 In our study, of the 646 TB patients identified by Xpert MTB/RIF, 122 (18.89%) were diagnosed as RR-TB (including 69 RR-TB cases identified by Xpert MTB/RIF only). With the gradual increase in the samples sent for Xpert MTB/RIF, the positive rate of childhood TB diagnosis as well as the rate of RR-TB detection in Shenyang has been increasing. This is in line with the WHO recommendation to prioritize using Xpert MTB/RIF for diagnosing children with suspected TB because of its low biosafety requirements and rapid reporting time.9
WHO recommends stool and gastric fluid samples combined with Xpert or Ultra for the diagnosis of TB in younger children, and studies have confirmed that stool samples provide a non-invasive alternative for pediatric TB patients.23,24 We have also evaluated in a multicenter study in China that in children, stools can be used as alternative samples to gastric fluid for Ultra tests.25 In our retrospective study, only 8 fecal samples were used for TB diagnosis, which is inadequate to make any conclusion. Similar to other countries and regions, there is a gap between the identification of childhood TB and the estimated number of childhood TB cases, especially among those under 5 years in China. In other words, passive case finding severely underestimates TB in children, only about a quarter of these children under 5 (27.5%) with TB were notified to the national TB programs.2 Therefore, it is important to enhance screening and drug resistance in children under 5, suitable alternative samples such as stool and more child-friendly diagnosis is needed.
We included about 400 children younger than 15 years (395, 36.71%), and the low proportion of patients younger than 15 years could be related to the high BCG vaccination rate in China. A randomized trial with 250,000 participants in Chengalpattu, India, found that the effect of BCG vaccination in infants was reduced at 12.5 years, thus those children at older age could be more susceptible.26 In this study, all patients were vaccinated with BCG, so the number of patients younger than 15 years who were better protected by the BCG vaccination was significantly smaller than the number of patients aged 15–18 years.
Only 10.87% of the confirmed by MGIT 960 culture and/or Xpert MTB/RIF TB cases had anti-TB treatment history (117/1076), and most DR-TB in children were new cases, which was different from adult TB cases. This might indicate that most of the DR-TB in children were infected by DR-TB strains, therefore once a TB case is identified, the screening of children contacts of him or her at home or in schools needs to be strengthened. We have identified about 25% (270/1076,25.09%) of the TB cases as EPTB cases, which was similar to the data from Li et al. They did a national cross-sectional study in China with a multi-stage, a stratified whole-group random sample that showed that of the 6843 TB cases included, 24.6% were EPTB patients.27 This is in line with our data, both higher than the world average (16%) and about to reach the highest rate of 24% in the Eastern Mediterranean region,28 suggesting that China is also a high burden country for EPTB and that EPTB should not be neglected in TB control. The contribution of EPTB to transmission is usually considered lower than that of PTB, but given the higher mortality, economic burden, and catastrophic expenditure of pediatric EPTB, pediatric EPTB, especially the drug-resistant type, should be addressed.20
Our study shows that among 806 MGIT 960 culture-confirmed pediatric TB patients, resistance to streptomycin 21.09% (170/806), and isoniazid 19.35% (156/806) were the most frequent mono-resistance. Tao et al found that among isolates of Lowenstein-Jensen culture from 784 new TB case-patients <18 years of age, the highest proportion of resistance was found for streptomycin (14.3%), followed by isoniazid (12.1%), rifampin (8.3%), and ethambutol (5.5%), which is similar to our study.12 The situation of streptomycin and isoniazid resistance is very critical. Although the more ototoxic streptomycin is rarely used to treat TB in children, its resistance rate is influenced by the high resistance rates in adults with TB in the region. This result is in line with the current WHO treatment which no longer recommended streptomycin for multidrug-resistant TB treatment.29 The high rate of isoniazid resistance in children was considered to be related to the widespread use of isoniazid as a first-line anti-TB drug in the treatment of active tuberculosis and the preventive therapy in child contacts. Besides, our study showed that the problem of levofloxacin resistance requires attention in 15–18 years. In China, fluoroquinolones such as levofloxacin were widely used for infections including respiratory infection and later for TB patients, which may have led to a high resistance rate to fluoroquinolones in general including TB patients.30 The clinical features of childhood TB in older children are very similar to those of adults and are prone to social transmission and the high resistance to fluoroquinolones in this age group needs to be urgently addressed.
MDR-TB, RR-TB, Pre-XDR-TB, and resistance to second-line injectable anti-TB antibiotics have become a huge global health challenge. The severity and harms of the childhood RR-TB burden deserve urgent attention. Our study shows that of the 232 patients with MGIT 960 DST results indicating drug resistance, 121 cases (52.16%) of RR-TB were identified. Alarmingly, most of these RR-TB patients were resistant to three or more drugs (95/121,78.51%). Among the 806 patients’ samples identified positive by MGIT 960 culture, the trends in the number and proportion of TB patients with DR, RR, MDR, MDR+AMK, and Pre-XDR between 2017–2021 remained almost unchanged. This may indicate that DR-TB is still not effectively controlled in children in the region in recent years.
Following the WHO recommendation, Xpert MTB/RIF has gradually become an important tool for RR-TB diagnosis, and its proportion in RR-TB detection has been increasing with the increase of the number of tests sent every year. Zhou et al found that among the TB cases with DST results, the use of Xpert MTB/RIF was as low as 10.9% (812/7470) in 2014–2017, but increased to 69.0% (7557/10,946) in 2018–2020.31,32 This rapid diagnosis is welcome by both hospitals and TB patients. A meta-analysis by Wang et al (including 11 studies, 3801 children), found that Xpert MTB/RIF’s sensitivity was 65% (95% CI: 61% to 69%) and specificity of 99% (95% CI:98% to 99%) against culture.33 Detjen et al reported that Xpert MTB/RIF’s sensitivity and specificity were 62% (95% CI: 51% to 73%) and 98% (95% CI: 97% to 99%), using children’s sputum samples.34 Lavu et al showed high similarity in the identification of RR-TB cases by DST and Xpert MTB/RIF.35 Currently, WHO recommends using Xpert MTB/RIF to diagnose childhood TB in the following situations (1. diagnosing all suspected childhood TB, 2. diagnosing all suspected MDR-TB/HIV-associated TB in children, 3. alternative tests for children suspected of EPTB, 4. diagnosing children suspected with TB meningitis where cerebrospinal fluid is recommended).36 In our study, of the 190 RR-TB patients, 69 were identified by Xpert MTB/RIF alone. Xpert MTB/RIF diagnosed a significantly higher number of RR-TB than DST (P=0.017). The advantage of Xpert MTB/RIF or RR-TB diagnosis was especially seen in the undiagnosed cases by MGIT 960 DST, which increased from 6 (15.00%) in 2017 to 19 (54.29%) in 2021. We recommend that accurate molecular drug-sensitive assays be widely implemented in children for early identification and accurate treatment of RR, MDR, and Pre-XDR to eliminate childhood TB as soon as possible. Timely initiation of effective treatment regimens for RR-TB is crucial to avoid the further spread and progression of this serious drug-resistant type.
Our study showed the issue of drug resistance needs to be addressed in children with pulmonary TB who are <15 years old, smokers, malnourished, and have an anti-TB treatment history. Health education and medication supervision for children and parents should also be enhanced to improve patient compliance and increase the proportion of successful treatment outcomes. Previous papers have suggested that smoking history is a clear risk factor for drug-resistant TB.37 Compared to TB patients who are nonsmokers, those who smoke had 3.44 times higher chance to develop multidrug-resistant TB (OR 3.44, 95% CI 1.68–7.03). During anti-TB treatment, smoking has a detrimental effect on culture conversion, which prolongs the treatment period.38
The Global TB Report 2022 states that malnutrition is the most important risk factor for people with latent MTB infection to become active TB cases, and in a broader attribution analysis, there are 2.2 million cases of tuberculosis attributed to malnutrition worldwide.1 The risk of DR-TB in patients with malnutrition was 2.62 times higher than in those without malnutrition as found in our study. Drug-resistant TB can cause malnutrition, and malnutrition can lead to the worsening of the disease. The WHO recommends nutritional support for the treatment of tuberculosis when malnutrition is a comorbidity.39 As a result, it is necessary to give nutritional support to patients with DR-TB.40 Patients exposed to anti-TB treatment before were more likely to be found with DR-TB. This finding is consistent with that of Ullah, who reported that the number of drug-resistant TB patients was five times higher in the suspected cases with previous anti-TB treatment than in newly diagnosed cases.41
Among the cases with anti-TB treatment history, the risk of drug resistance was higher in those with interrupted treatment, with an OR of 4.5 (95% CI: 1.04–19.47) compared to those without interrupted treatment, therefore, standardized anti-TB drug therapy should be intensified and the treatment course should be monitored closely to avoid unnecessary treatment interruptions. The risk of drug resistance was higher in patients with cavities compared to patients without cavities, OR=1.73 (95% CI: 1.24–2.41). Mulu et al showed that the more cavities in the lungs and the more extensive the lesions, the greater the risk of multidrug resistance (OR=1.90, 95% CI: 1.10–3.38).42 The reason for this is that untreated tuberculosis with caseous necrosis is prone to cavity formation, and inappropriate treatment leads to long-term cavity with cavity wall thickening, and fibrosis of the lesion, resulting in the development of a slow, fibrous cavity type of tuberculosis, where oral and intravenous routes of drug administration are difficult to achieve effective blood levels in the cavity and are prone to drug resistance.
In the Global tuberculosis report 2022, WHO estimated the number of children with drug-resistant TB from the study using a risk assessment model and reported that the global incidence of MDR-TB in children was about 25,000–32,000 per year. Our study provides a comprehensive analysis of the epidemiology of drug resistance TB among children ≤18 years in Shenyang city from 2017 to 2021 and examines the trends. Efforts to reduce the burden of DR-TB in children should be made. Policymakers can utilize the study’s findings to implement interventions and allocate healthcare resources more effectively.
This study is subject to certain limitations. Firstly, this research was limited by the lower pathogenic detection rate in younger children, especially <5 years children as qualified samples are difficult to be obtained from them, which may introduce selection bias, leading to an underestimation of the burden of DR-TB among young children. Older children usually present with TB similar to that of adults, such as cavities on chest X-ray (CXR) and easier to be bacteriologically confirmed.43 In the given the retrospective nature of the study, which required analysis of resistance based on obtaining positive MGIT 960 culture and/or Xpert MTB/RIF results, it was difficult to identify bacteriologically positive cases in younger children. This was in line with the fact that the microbiological confirmation rate for younger children is relatively low.44,45 We believe that difficulty in obtaining samples, and limited sensitivity of available assays may be partly responsible for the low proportion of younger children. Secondly, this study adopted a retrospective design in only one hospital, and more data from prospective studies to address the regional, and national burden and trends are needed to enhance the validity of these findings. However, Shenyang Tenth People’s Hospital is one of the few specialized medical institutions in China that specializes in pediatric TB cases and has specialized in drug-resistant TB treatment in children. As a result, pediatric TB patients also come from neighboring cities outside of Shenyang, reflecting the status of drug-resistant childhood TB in a broader geographic area. Having said that, this study shed light on the status and enlighten the control of drug-resistant pediatric TB in China.
Conclusion
Between 2017 and 2021, the incidence of childhood DR-TB in Shenyang remained unchanged. RR-TB, MDR-TB, and Pre-XDR-TB require attention in children. Xpert MTB/RIF demonstrated advantage in the diagnosis of pediatric TB and should be widely used as an important complementary tool to culture drug-sensitivity testing to detect rifampicin-resistant TB in children.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
Ethics Statement
The study protocol was approved by the Ethics Committee of Shenyang Tenth People’s Hospital (No. KYXM-2020-004-02).
Acknowledgments
Jiao Sun, Lichao Fan, and Yanping Zhao are co-first authors for this study. We would like to thank the staff and patients of Shenyang Tenth People’s Hospital for their hard work and the data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; have drafted, revised, or critically reviewed the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no commercial/financial or other conflicts of interest involved.
References
1. World Health Organization. Global Tuberculosis Report 2022. Geneva: World Health Organization; 2022.
2. World Health Organization. WHO Consolidated Guidelines on Tuberculosis Module 5: Management of Tuberculosis in Children and Adolescents. Geneva: World Health Organization; 2022.
3. World Health Organization. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children.
4. Jenkins HE, Yuen CM. The burden of multidrug-resistant tuberculosis in children. Int J Tuberc Lung Dis. 2018;22(5):3–6. doi:10.5588/ijtld.17.0357
5. Dodd PJ, Gardiner E, Coghlan R, Seddon JA. The burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2(8):e453–459. doi:10.1016/S2214-109X(14)70245-1
6. Seddon JA, Hesseling AC, Willemse M, et al. Culture-confirmed multidrug-resistant tuberculosis in children: clinical features, treatment, and outcome. Clin Infect Dis. 2012;54(2):157–166. doi:10.1093/cid/cir772
7. Zignol M, Sismanidis C, Falzon D, et al. Multidrug-resistant tuberculosis in children: evidence from global surveillance. Eur Respir J. 2013;42(3):701–707. doi:10.1183/09031936.00175812
8. World Health Organization. Rapid Implementation of the Xpert MTB/RIF Diagnostic Test: Technical and Operational’how-To’; Practical Considerations. Geneva: World Health Organization; 2011.
9. World Health Organization. Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update. Geneva: World Health Organization; 2013.
10. Helb D, Jones M, Story E, et al. Rapid detection of mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48(1):229–237. doi:10.1128/JCM.01463-09
11. Saeed M, Ahmad M, Iram S, Riaz S, Akhtar M, Aslam M. GeneXpert technology: a breakthrough for the diagnosis of tuberculous pericarditis and pleuritis in less than 2 hours. Saudi Med J. 2017;38(7):699–705. doi:10.15537/smj.2017.7.17694
12. Tao NN, He XC, Zhang XX, Liu Y, Yu CB, Li HC. Drug-resistant tuberculosis among children, China, 2006–2015. Emerg Infect Dis. 2017;23(11):800–1805. doi:10.3201/eid2311.170234
13. Sun L, Qi X, Liu F, et al. A test for more accurate diagnosis of pulmonary tuberculosis. Pediatrics. 2019;144(5):e20190262. doi:10.1542/peds.2019-0262
14. World Health Organization. WHO Consolidated Guidelines on Tuberculosis Module 4: Treatment-Drug-Resistant Tuberculosis Treatment. Geneva: World Health Organization; 2020.
15. World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses. Geneva: World Health Organization; 2013.
16. Carter BB, Zhang Y, Zou H, et al. Survival analysis of patients with tuberculosis and risk factors for multidrug-resistant tuberculosis in Monrovia, Liberia. PLoS One. 2021;16(4):e0249474. doi:10.1371/journal.pone.0249474
17. Wang T, Dong F, Li QJ, et al. Clinical and drug resistance characteristics of new pediatric tuberculosis cases in Northern China. Microb Drug Resist. 2018;24(9):1397–1403. doi:10.1089/mdr.2017.0382
18. Xu Y, Li Q, Zhu M, et al. The epidemiological characteristics and profile of drug-resistant tuberculosis among children with tuberculosis in Sichuan, China, 2015–2018: a retrospective study. Medicine. 2020;99(43):e22608. doi:10.1097/MD.0000000000022608
19. DiNardo AR, Detjen A, Ustero P, et al. Culture is an imperfect and heterogeneous reference standard in pediatric tuberculosis. Tuberculosis. 2016;101S:S105–S108. doi:10.1016/j.tube.2016.09.021
20. Chu P, Chang Y, Zhang X, et al. Epidemiology of extrapulmonary tuberculosis among pediatric inpatients in mainland China: a descriptive, multicenter study. Emerg Microbes Infect. 2022;11(1):1090–1102. doi:10.1080/22221751.2022.2054367
21. Pang Y, Wang Y, Zhao S, Liu J, Zhao Y, Li H. Evaluation of the Xpert MTB/RIF assay in gastric lavage aspirates for diagnosis of smear-negative childhood pulmonary tuberculosis. Pediatr Infect Dis J. 2014;33(10):1047–1051. doi:10.1097/INF.0000000000000403
22. Nhu NT, Ha DT, Anh ND, et al. Evaluation of Xpert MTB/RIF and MODS assay for the diagnosis of pediatric tuberculosis. BMC Infect Dis. 2013;13(1):31. doi:10.1186/1471-2334-13-31
23. Ness TE, Meiwes L, Kay A, et al. Optimizing DNA extraction from pediatric stool for diagnosis of tuberculosis and use in next-generation sequencing applications. Microbiol Spectr. 2023;11(1):e0226922. doi:10.1128/spectrum.02269-22
24. De Haas P, Nhung NV, Hng NT, et al. Introduction of the simple one-step stool Xpert ultra method to detect TB in children and adults. Int J Tuberc Lung Dis. 2023;27(1):19–27. doi:10.5588/ijtld.22.0161
25. Sun L, Liu Y, Fang M, et al. Use of Xpert MTB/RIF Ultra assay on stool and gastric aspirate samples to diagnose pulmonary tuberculosis in children in a high-tuberculosis-burden but resource-limited area of China. Int J Infect Dis. 2022;114:236–243. doi:10.1016/j.ijid.2021.11.012
26. Hart PD, Sutherland I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br Med J. 1977;2(6082):293–295. doi:10.1136/bmj.2.6082.293
27. Li T, Yan X, Du X, et al. Extrapulmonary tuberculosis in China: a national survey. Int J Infect Dis. 2023;128:69–77. doi:10.1016/j.ijid.2022.12.005
28. World Health Organization. Global Tuberculosis Report 2020. Geneva: World Health Organization; 2020.
29. World Health Organization. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: 2011 Update. Geneva: World Health Organization; 2011.
30. Zhang X, Yin J, Li H, et al. Diagnostic and treatment delays of multidrug-resistant tuberculosis before initiating treatment: a cross-sectional study. Trop Med Int Health. 2015;20(11):1431–1437. doi:10.1111/tmi.12566
31. Zhou M, Liu S, Li Q, et al. Drug resistance characteristics of Mycobacterium tuberculosis isolates between 2014 and 2017 in Sichuan, China: a retrospective study. PLoS One. 2018;13(12):e0209902. doi:10.1371/journal.pone.0209902
32. Zhou R, Zheng T, Luo D, et al. Drug resistance characteristics of Mycobacterium tuberculosis isolates obtained between 2018 and 2020 in Sichuan, China. Epidemiol Infect. 2022;150:e27. doi:10.1017/S0950268822000127
33. Wang XW, Pappoe F, Huang Y, et al. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in children: a meta-analysis. Clin Lab. 2015;61(11/2015):1775–1785. doi:10.7754/clin.lab.2015.150509
34. Detjen AK, Dinardo AR, Leyden J, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:451–461. doi:10.1016/S2213-2600(15)00095-8
35. Lavu EK, Johnson K, Banamu J, et al. Drug-resistant tuberculosis diagnosis since Xpert ® MTB/RIF introduction in Papua New Guinea, 2012–2017. Public Health Action. 2019;9(1):S12–S18. doi:10.5588/pha.19.0005
36. World Health Organization. Xpert MTB/RIF Implementation Manual: Technical and Operational ‘How-To’; Practical Considerations. Geneva: World Health Organization; 2014.
37. Tao NN, Li YF, Song WM, et al. Risk factors for drug-resistant tuberculosis, the association between comorbidity status and drug-resistant patterns: a retrospective study of previously treated pulmonary tuberculosis in Shandong, China, during 2004–2019. BMJ Open. 2021;11(6):e044349. doi:10.1136/bmjopen-2020-044349
38. Maciel EL, Brioschi AP, Peres RL, et al. Smoking and 2-month culture conversion during anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2013;17(2):225–228. doi:10.5588/ijtld.12.0426
39. World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. Nutritional Care and Support for Patients with Tuberculosis. Geneva: World Health Organization; 2013.
40. Magassouba AS, Touré AA, Diallo BD, et al. Malnutrition prevalence and associated biochemical factors among drug-resistance tuberculosis (DR-TB) patients at key treatment sites in Conakry City, Republic of Guinea. Pan Afr Med J. 2021;38:279. doi:10.11604/pamj.2021.38.279.27270
41. Ullah I, Javaid A, Tahir Z, et al. Pattern of drug resistance and risk factors associated with development of drug resistant mycobacterium tuberculosis in Pakistan. PLoS One. 2016;11(1):e0147529. doi:10.1371/journal.pone.0147529
42. Mulu W, Mekonnen D, Yimer M, et al. Risk factors for multidrug resistant tuberculosis patients in Amhara National Regional State. Afr Health Sci. 2015;15(2):368–377. doi:10.4314/ahs.v15i2.9
43. World Health Organization. Roadmap Towards Ending TB in Children and Adolescents. Geneva: World Health Organization; 2018.
44. Marais BJ, Schaaf HS. Childhood tuberculosis: an emerging and previously neglected problem. Infect Dis Clin North Am. 2010;24(3):727–749. doi:10.1016/j.idc.2010.04.004
45. Kunkel A, Abel ZWP, Nathavitharana RR, et al. Smear positivity in paediatric and adult tuberculosis: systematic review and meta-analysis. BMC Infect Dis. 2016;16(1):282. doi:10.1186/s12879-016-1617-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.