Back to Journals » Infection and Drug Resistance » Volume 16
Analysis of Clinical and Microbiological Features of Listeria monocytogenes Infection
Authors Lu X, Yang H , Wang Y, Xie Y
Received 10 February 2023
Accepted for publication 20 April 2023
Published 8 May 2023 Volume 2023:16 Pages 2793—2803
DOI https://doi.org/10.2147/IDR.S408089
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Xingbing Lu,* Huan Yang,* Yanxi Wang, Yi Xie
Department of Laboratory Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi Xie, Email [email protected]
Introduction: To analyze the demographics and clinical features of 59 cases of Listeria monocytogenes, and determine the predisposing conditions for severe meningitis infections for reference.
Materials and methods: A total of 59 cases isolated L. monocytogenes from 2009 to 2020 were enrolled. Electronic medical record data were used to determine the epidemiological and clinical characteristics of L. monocytogenes infection. Univariate and multifactorial logistic regression analyses were performed to predict risk factors for Listeria meningitis.
Results: A total of 59 cases (median age of 52 years, 30 females and 29 males) were enrolled. Twenty-five patients (42.37%) developed a neuroinvasive infection. The indexes of interleukin-6 (IL-6), CD3+T, CD4+T, and CD8+T cells in the study group were higher than those in the control group (P< 0.05). In univariate analysis, the use of hormone drugs (odds ratio=3.21, P=0.000) and immunosuppressive agents (odds ratio=3.06, P=0.000) were relevant predictors of severe meningitis. 47 patients (79.66%) were treated with ampicillin (27.12%), carbapenems (18.64%), quinolones (11.86%), and β-lactamase inhibitors (11.86%) as the primary agents of antimicrobial therapy. Thirty-four patients (57.63%) showed clinical improvement, five patients (8.47%) had a poor prognosis, and two patients (3.39%) died.
Conclusion: Infection with Listeria changed the levels of IL-6, CD3+T, CD4+T, and CD8+T cells, and these analyzing items were significantly different between L. monocytogenes and other bacterial infections. Long-term use of immunosuppressants and hormones may be risk factors for severe adult forms of Listeria-related infections. Sensitive antibiotics, such as penicillins and carbapenems, should be added or replaced in the early empiric treatment of L. monocytogenes.
Keywords: Listeria monocytogenes, sepsis, meningitis, antimicrobials, clinical and laboratory characteristics
Introduction
L. monocytogenes is a short-gram-positive bacteria, facultative anaerobic, lacking a spore or capsule, and is a common zoonotic intracellular pathogen.1 It exists widely in the environment and food products, and can survive refrigeration temperatures, acidic environments, high salt concentrations, and other harsh conditions.2 Listeriosis is a preventable and treatable foodborne disease transmitted primarily through the ingestion of infected food (99% of cases).1 Although listeriosis is infrequent, invasive infections can cause some of the most severe syndromes such as self-limited febrile gastroenteritis, septicemia, stillbirth, spontaneous abortion, and neuroinvasive diseases,3,4 with high hospitalization and mortality rates approaching 50%.5 Liver disease, kidney disease, and gastrointestinal bleeding are the main predisposing conditions for L. monocytogenes infection. Furthermore, Pregnant women, newborns, patients with malignancy, the elderly, and immunosuppressed individuals are at high risk of severe infection.
Listeriosis is characterized by insidiousness, long incubation period, multiple susceptibility factors, severe symptoms, and persistent sequelae,6 which has a significant impact on public health worldwide. Diverse pathways could be used by L. monocytogenes to gain access to the central nervous system (CNS) and could include a retrograde neural route of invasion and crossing of the blood-brain barrier (BBB) by blood-borne bacteria.7 After infiltration of L. monocytogenes into the cerebrospinal fluid (CSF), it proliferates and releases a large amount of hemolysin and listerin,8 and the resulting subarachnoid inflammation and intracranial hypertension eventually lead to CNS damage. Neurological lesions caused by L. monocytogenes are the most severe clinical syndrome, which can manifest as meningeal encephalitis, rhomboencephalitis, or even brain abscess, usually with high mortality and lasting neurological sequelae.9
In China, L. monocytogenes remained an unreported disease, and sporadic cases in some provinces were not included in the national surveillance program until 2013.2 As a result, reports of Listeria monocytogenes listeriosis were mostly isolated cases and food contamination. In contrast, comprehensive clinical analysis on human infection with listeriosis was less frequent, and the incidence of the disease was increasing year by year. We collected information on 59 cases of Listeria monocytogenes infections admitted to West China Hospital of Sichuan University from January 2009 to April 2020, analyzed the population structure, clinical features, and laboratory data of the infections, summarized the treatment prognosis, and identified risk factors for severe neurological infections to provide a reference for the diagnosis and treatment of bacterial infections.
Methods
Recruitment Criteria
We conducted a retrospective case-control study of patients admitted to our hospital (West China Hospital, Sichuan University) between January 2009 and April 2020. The selection criteria were as follows: L. monocytogenes was isolated from a normally sterile site (eg blood or CSF) submitted to the laboratory at the time of the patient’s hospitalization.
Data Collection
Using electronic health records, we then collected reports of all demographic information, comorbidities, immunocompromised status, clinical symptoms and signs, antibiotic therapy, and clinical outcome data regarding infections caused by L. monocytogenes in our hospital. The study group consisted of 59 patients with L. monocytogenes cultured from blood, CSF, and other samples. The control group comprised 78 cases of meningitis and 55 cases of septicemia, with other pathogens from CSF and blood samples (Candida albicans, Escherichia coli, Klebsiella pneumoniae, Streptococcus pneumoniae, Staphylococcus aureus, Neisseria meningitidis, Enterococcus faecalis, Pseudomonas aeruginosa, Cryptococcus neoformans, Enterobacter cloacae complex and Serratia marcescens and other pathogens). This study was approved by the Ethics Committee of West China Hospital of Sichuan University (20,191,116), and patients have signed informed consents.
White blood cell (WBC), neutrophil ratio (N%), lymphocyte ratio (L%), and monocyte ratio (M%) were measured using Sysmex NX-2000. Procalcitonin (PCT) and IL-6 were measured using Roche E602. C-reactive protein (CRP), and immune indicators were measured using Beckman Image. The biochemical indices of CSF were measured using Roche E702. All of the above patients had evidence of an etiology examination. Drug sensitivity were conducted using a Biomerieux-Vitek-2 automatic microbial analyzer, and identification and mass spectrometry confirmation were performed using a Brooke mass spectrometer. Time to diagnosis was defined as the time from the onset of clinical symptoms of infection to the identification of the pathogen in the microbiology laboratory. Poor prognosis was defined as failure to achieve improvement in laboratory results or clinical symptoms at discharge; clinical improvement was defined as negative blood cultures or CSF reexamination and control of clinical symptoms.
Antimicrobial susceptibility testing of the L. monocytogenes isolates was performed using broth dilution method. The minimum inhibitory concentrations (MICs) of following 5 antimicrobials were tested: penicillin (PEN), meropenem (MEM), ampicillin (AMP), trimethoprim–sulfamethoxazole (SXT), and erythromycin (ERY) (Bio-Kont, Zhejiang Province, China). And the drug-sensitive quality control strain was Escherichia coli ATCC25922. Since resistance criteria for AMP, PEN, and SXT of L. monocytogenes existed in the Clinical and Laboratory Standard Institute (CLSI) international guidelines, and resistance criteria for ERY existed in European Committee on Antimicrobial Susceptibility Testing (EUCAST) international guidelines, the MICs of AMP, PEN, SXT were interpreted using CLSI international guidelines, and the MIC of ERY was interpreted according to EUCAST international guidelines.
Statistical Analysis
The clinical infection characteristics of the patients were analyzed and compared using SPSS 23.0. Counting data were represented by composition ratio (%) or rate (%), and compared by χ2 test and Fisher exact test when the data was limited. The measured data of normal distribution were expressed as X±S, and the t-test was used for comparison between groups. The non-normal distribution data were expressed by median and interquartile ranges (IQR), and the comparison between groups was performed using Mann–Whitney’s test. P<0.05 was used as an indication that the results were statistically different.
Results
Clinical Characteristics of L. monocytogenes Disease (LD) Infection
A total of 59 patients were included in our study. 30 patients (50.85%) were female and 2(3.39%) were pregnant. The mean age was (51.72±16.71) years (Figure 1). In the control group, 49(36.84%) of 133 cases were female, with a mean age of (50.00 ± 19.04) years. There were no significant differences in gender and age between the study and control groups (P>0.05). Immunosuppressive diseases (14[23.73%]) and pulmonary infections (14[23.73%]) were the most common basic diseases in the study group. Major symptoms included fever (19[32.20%]), headache (15[25.42%]), and neurological symptoms (15[25.24%]), and less common symptoms were gastrointestinal symptoms (6[10.17%]), change of consciousness (10[16.95%]), and body pain (7[11.86%]). In contrast, the common underlying diseases in the control group were immunosuppressive disorders (12[9.02%]) and surgery (16[10.53%]), and the main symptoms were fever (54[40.60%]), headache (48[36.09%]) and vomiting (43[32.33%]) (Table 1).
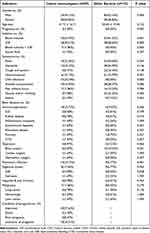 |
Table 1 Demographic and Clinical Data of Patients Infected with Listeria and Other Bacteria |
 |
Figure 1 Histogram (left) and density map (right) of the age distribution of patients with Listeria monocytogenes infection. |
The annual incidence of LD was the highest in 2019 (13[22.03%]) and the lowest in 2009 (2[3.38%]) (Figure 2). The seasonal distribution was the highest in summer (26[44.06%]) while the lowest in winter (9[15.25%]) (Figure 3). Among all L. monocytogenes isolates, 33 cases (55.93%) were derived from blood cultures, 18 cases (30.51%) from CSF cultures, and 7 cases (11.86%) were positive for both blood and CSF cultures. L. monocytogenes was detected in a sample of peritoneal dialysis solution culture in only one patient.
 |
Figure 2 Annual distribution of patients with Listeria monocytogenes infection. |
 |
Figure 3 Seasonal distribution of patients with Listeria monocytogenes infection. |
The indexes of IL-6, CD3+T, CD4+T and CD8+T cells were higher in the study group compared to other bacterial infections (P<0.05), but there were no significant differences in the results of WBC, Neutrophile/Lymphocyte ratio (NLR), Monocyte/Lymphocyte ratio (MLR), CRP, PCT, IgA, IgG, IgM, and IgE between the two groups (P>0.05) (Table 2).
 |
Table 2 Comparison of Infection Indices Between L. monocytogenes and Other Bacterial Infections |
Compared to the control group, the CSF protein, total nucleated cells (TNC), and multinucleated cell ratio increased while glucose, chlorine, and mononuclear cell ratio decreased in the study group. However, there was no significant difference between the two groups in any of the indicators (P>0.05) except TNC (P<0.05) (Table 2).
Treatment and Clinical Outcome of Listeria Meningitis
The susceptibility of bacterial strains of PEN, MEM, AMP, SXT, and ERY was 94.34%, 100.00%, 100.00%, 64.44%, and 87.23%, respectively, according to the drug sensitivity tests of L. monocytogenes isolated from clinical specimens (Table 3).
 |
Table 3 The Drug Sensitivity Results of L. monocytogenes |
After admission, 41(69.49%) patients were treated empirically, mainly with cephalosporins (28.81%), beta lactamase inhibitors (20.34%), and antiviral drugs (16.94%), 8(13.55%) patients received no antibiotic treatment, and 10(16.94%) patients did not receive treatment. After clinical samples were cultured for Listeria monocytogenes and drug sensitivity results were available, 47(79.66%) patients received anti-infective adjusted to ampicillin (27.12%), carbon penicillium alkene (eg, imipenem, meropenem) (18.64%), quinolone class (eg, levofloxacin) (11.86%), and β-amide inhibitor class (eg, cefoperazone/sulbactam, piperacillin sulbactam) (11.86%) treatment. After adequate and regular treatment, there were 34 cases (57.63%) with clinical improvement, 5 cases (8.47%) with poor prognosis, 2 cases (3.39%) with death, and 18 cases (30.51%) with unknown prognosis.
Two pregnant women received effective antibiotic treatment with ampicillin, piperacillin, and sulbactam during their hospitalization, and the infection was completely cured. They were followed up after discharge without recurrence of the disease. In addition, two patients with neurological syndrome were treated with ampicillin combined trimethoprim-sulfamethoxazole for three weeks, while patients with sepsis were treated with trimethoprim-sulfamethoxazole alone ten days. A summary of antibiotic treatment was shown in Table 4.
 |
Table 4 Treatment of Listeria monocytogenes Infection After Adjustment of Drug Susceptibility |
Logistic Regression Analysis of Predictors of Severe Nervous System Infection in Patients with Listeria monocytogenes
After sufficient treatment, there were 34 cases of clinical improvement (57.63%), 5 cases of poor prognosis (8.47%), and 2 deaths (3.39%). We performed univariate and multifactorial analyses of possible influences to identity relevant predictors of severe neurological infections. We found that the use of hormone drugs (odds ratio=3.21, P=0.000) and immunosuppressive agents (odds ratio=3.06, P=0.000) were associated predictors of severe meningitis in univariate analysis (Table 5).
 |
Table 5 Results of Univariate and Multifactorial Analysis of the Risk of Severe Central Nervous System Infection by Listeria monocytogenes Infection |
Discussion
Public Health England (2018) reported on average 166 cases of listeriosis per year in England and Wales (from 2008 to 2018).10 And 211 cases of listeriosis were reported in China during 2013−2017, with a fatality rate of 26.1%.11 In our study, a total of 59 cases positive for Listeria infection were included from 2009 to 2020, with a mortality rate of 3.39%. A few studies have reported the prevalence of L. monocytogenes,12–15 and the description of clinical L. monocytogenes was very limited. Therefore, to explore the clinical and microbiological characteristics of Listeria monocytogenes, 59 patients with Listeria infection were included for further study.
In this study, most of the patients were middle-aged and elderly (mean age 51.7±16.7 years), and the Listeria infection rate was higher in people over 40 years (72.88%) and over 60 years (33.90%), indicating that the infection rate increased with age and developed into serious diseases such as sepsis/meningitis.16 The main reason for this was the high risk of infection due to various underlying diseases (up to 90.70%) and the weak resistance of the elderly. Studies have reported that the impact of global climate change on the environment has significantly altered the prevalence of foodborne diseases such as listeriosis.17,18 In this study, 44.06% of listeriosis cases occurred in hot summer, while the lowest number of cases was in cold winter (15.25%), which was similar to the annual distribution reported by scholars in Taiwan, China,15 suggesting that the incidence was influenced by seasonal (temperature) factors, which may be related to the consumption of frozen and refrigerated food or unwashed seasonal fruits in summer.19 A significant microbial characteristic of Listeria was that it can grow and reproduce at 4°C.
In our study, we included fewer maternal patients since our hospital did not have an obstetrics and gynecology department, and they were early detection of the infection, treated promptly and had a good prognosis. Adequate treatment of maternal listeriosis prevented and treated fetal disease.20 However, studies have shown that invasive listeriosis in pregnant women can lead to intrauterine infections, preterm delivery, stillbirth, and neonatal infections.14,21 Neonatal listeriosis caused by vertical transmission can be fatal. Two healthy young patients were diagnosed with meningitis with an infection rate of only 3.38%, which was lower than 6% in relevant studies,22 and were treated and discharged according to guidelines. Generally, most cases of Listeria infection were self-limiting, but in patients who were immunocompromised, pregnant, or at an extreme age, it can cause invasive infections with a high associated mortality rate.
Severe Listeria infection can cause septicemia and meningitis, with fever, neurological syndrome, and gastroenteritis as the main symptoms. The infection rate of septicemia in this article was as high as 56%, which was lower than 69.5% in the United States6 and 84.9% in Spain,23 and slightly higher than 47% in Israel.24 The high infection rate in Europe and the United States may be related to local dietary habits and, on the other hand, patient comorbidity was a confounding factor, which required further study. The prevalence of meningitis infection accounted for 11.86%, higher than 7.3% in Taiwan,15 but lower than 15.11% in Spain.23 This may be related to the resident’s awareness of early medical treatment and improved medical detection levels. The infection rate of the CNS was 42.37%, much higher than the global rate of 30.7%.25 The main pathological features in patients with meningitis were ideological change (16.95%) and nervous system syndrome (25.24%). It has been reported that the mortality rate of meningitis was 30%, and neurological sequelae such as hydrocephalus, brainstem encephalitis, and cerebral hemorrhage can still occur in 23% of cured patients.26 The above findings were consistent with those of the scholar Feng, who retrospectively concluded that listeriosis in China was mainly septicemia (46%) and CNS infection (31%) from 1964 to 2010.27 Moreover, we concluded that long-term use of immunosuppressants and hormones may be risk factors for severe adult forms of Listeria-related infections, which were compatible with several studies that also found previous immunosuppressive therapy was the most important risk factor for Listeria meningitis.28–31 Patients with CNS infection, immunosuppression, and malignancy had a 3-fold higher mortality rate. Nervous system infection was a serious disease of listeriosis and a high risk factor for death.32
IL-6 was produced by myeloid phagocytes and epithelial cells within 0.5–3h after infection, stimulated CD38 expression, promoted the mature release of bone marrow neutrophils and recruitment to the infected site, and had pro- and anti-inflammatory effects, enhancing its phagocytosis.33,34 CD8+T cells secreted IFN and TNF to activate host macrophages to lyse infected host cells.35 CD4+T cells differentiated into Th17 cells and produced TNF, enhancing T cell expansion and contraction, and enhancing extracellular bacterial and fungal clearance.36 Compared with other bacterial infections, the levels of CD3+T, CD4+T, and CD8+T cells in the early stage of infection in the study group were significantly increased, which may be caused by basic immunological diseases such as chronic active hepatitis, connective tissue disease, and systemic lupus erythematosus (SLE). CD4+T/CD8+T, an essential immune status-monitoring indicator, was commonly seen in patients with immune system deficiency or tumors, indicating severe damage to the immune system and increased susceptibility.
The average diagnosis time here reported was 6.2 days, similar to 6.8 days in a transnational study from Turkish.37 Due to the low incidence and easy-to-miss diagnosis and misdiagnosis, Listeria can invade the nervous system. Therefore, before diagnosis, cephalosporin and antiviral drugs were often used for empirical treatment in the early clinical stage. According to The Sanford guide to antimicrobial therapy (43rd edition), the standard treatment regimen for LD was ampicillin and gentamicin, with methomycin/sulfamethoxazole or meropenem for penicillin allergy. The main choices were: quinolones such as moxifloxacin 0.4g daily intravenous drip, aminoglycosides such as amikacin 0.4g once daily intravenous drip, β-lactam combination such as piperacillin sulbactam 4.5g twice daily intravenous drip, ampicillin 2.0g four times daily intravenous drip, carbapenems such as panipenem 0.5g three times daily intravenously, with the specific course of treatment clinically adjusted according to the patient’s recovery to discontinue the drug. However, in 45.75% of cases, initial empirical antibiotic treatment was inappropriate and ineffective, and delayed treatment may increase the risk of mortality and neurological sequelae. At present, AMP alone or combined with gentamicin (GEN) was the first choice to treat Listeria infection. Patients allergic to PEN antibiotics can be given SXT or MEN, and there were also treatment regimens of MEN or AMP combined with SXT.38 Treatment should last at least two weeks for adults, 3–4 weeks for newborns, and 6 weeks for immunosuppressed patients.39
In this paper, according to the results of drug sensitivity and guidelines, AMP was adjusted to the target treatment with single or combined GEN and MEM, with a clinical improvement rate of 86.84%, which achieved good expected results. It has been reported that quinolones can accumulate intracellularly and attack Listeria.38 In this study, seven patients were adjusted to quinolones, and the prognosis was good, with an improvement rate of 85%. Furthermore, vancomycin was recommended for the treatment of invasive listeriosis in individuals allergic to PEN.40 Concerning the management of bacterial meningitis, the Infectious Diseases Society of America (IDSA), according to the 2004 Implementation Guidelines for the Management of Bacterial Meningitis, did not recommend treatment with vancomycin (VAN), which, although proven to be bactericidal in vitro, was potentially associated with clinical treatment failure.41 In this study, six patients were not treated with VAN alone, and five patients were treated with AMP, SXT, or MEN, and all showed improvement. In one case, cefoperazone /sulbactam resulted in death.
Therefore, for the elderly, tumor, immunodeficiency, and other high-risk patients, timely treatment and appropriate and reasonable empirical antibacterial therapy were needed to control disease progression and improve clinical outcomes. Pathogen culture and drug sensitivity tests were essential for the diagnosis and treatment of listeriosis. Clinicians should increase the frequency of specimen submission and culture. Microbiologists should also constantly improve their technology and efficiency, shorten the detection time, and provide a reliable basis for timely and effective clinical treatment.
Several limitations were associated with our study. On the one hand, it was a retrospective study based on medical records. Some drug sensitivity and treatment critical information was missing, and the statistical results were biased. In particular, malignancies and immunosuppression played an important role in the adverse consequences of Listeria-related infections. On the other hand, the sample size was relatively small. Although neurological diseases were a key factor for severe infection, we cannot draw conclusions based on the effects of immunosuppressive drugs or tumors and other immunocompromised factors. However, despite these limitations, our research still provided an important perspective on listeriosis. We also hope that our research can provide clinicians with a reference for the timely diagnosis and treatment of listeriosis.
Conclusion
In general, our results showed the clinical features of infections caused by L. monocytogenes. There were remarkable differences in laboratory routine analyzing items including IL-6, CD3+T, CD4+T, and CD8+T cells in LD patients. Listeria infection was rare but can lead to severe sepsis and neurological infections. In addition to pregnancy and foodborne factors, the main risk factors for adult L. monocytogenes infection were individuals with low immunity due to long-term use of immunosuppressive agents and hormones. Early empirical treatment of Listeria required the addition or replacement of penicillins and carbapenems as well as other sensitive antibiotics. Appropriate antibiotic therapy may reduce the mortality associated with L. monocytogenes infection.
Ethics Statement
The protocol was approved by the Ethics Review Committee of West China Hospital, Sichuan University (reference: 20191116). All subjects gave written informed consent in accordance with the Declaration of Helsinki. The subjects’ rights were adequately protected, and there was no potential risk to the subjects.
Acknowledgments
The authors would like to thank all the patients who participated in this research.
Author Contributions
Xingbing Lu and Huan Yang shared the first authorship. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
No funding to report.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Jeffs E, Williman J, Brunton C, et al. The epidemiology of listeriosis in pregnant women and children in New Zealand from 1997 to 2016: an observational study. BMC Public Health. 2020;20(1):116. doi:10.1186/s12889-020-8221-z
2. Li W, Bai L, Fu P, et al. The Epidemiology of Listeria monocytogenes in China. Foodborne Pathog Dis. 2018;15(8):459–466. doi:10.1089/fpd.2017.2409
3. Gerstein S, Gautam-Goyal P, Goyal S. A case of Listeria monocytogenes meningitis complicated by Hydrocephalus and Intraventricular hemorrhage: a review of treatment options and outcomes. IDCases. 2020;19:e00704. doi:10.1016/j.idcr.2020.e00704
4. Silk BJ, Date KA, Jackson KA, et al. Invasive listeriosis in the Foodborne Diseases Active Surveillance Network (FoodNet), 2004–2009: further targeted prevention needed for higher-risk groups. Clin Infect Dis. 2012;54(Suppl 5):S396–S404. doi:10.1093/cid/cis268
5. Schlech WF. Epidemiology and Clinical Manifestations of Listeria monocytogenes Infection. Microbiol Spectr. 2019;7(3). doi:10.1128/microbiolspec.GPP3-0014-2018
6. Zahedi Bialvaei A, Sheikhalizadeh V, Mojtahedi A, et al. Epidemiological burden of Listeria monocytogenes in Iran. Iran J Basic Med Sci. 2018;21(8):770–780. doi:10.22038/IJBMS.2018.28823.6969
7. Disson O, Lecuit M. Targeting of the central nervous system by Listeria monocytogenes. Virulence. 2012;3(2):213–221. doi:10.4161/viru.19586
8. Zhang X, Feng P, Meng P, et al. Case report: severe listeria encephalitis with complicated or secondary autoimmune encephalitis and CNS demyelinating diseases. Front Public Health. 2022;10:848868. doi:10.3389/fpubh.2022.848868
9. Levin SN, Lyons JL. Infections of the nervous system. Am J Med. 2018;131(1):25–32. doi:10.1016/j.amjmed.2017.08.020
10. Valenti M, Ranganathan N, Moore LS, et al. Listeria monocytogenes infections: presentation, diagnosis and treatment. Br J Hosp Med. 2021;82(10):1–6. doi:10.12968/hmed.2021.0107
11. Li W, Bai L, Ma X, et al. Sentinel listeriosis surveillance in selected hospitals, China, 2013–2017. Emerg Infect Dis. 2019;25(12):2274–2277. doi:10.3201/eid2512.180892
12. Lu B, Yang J, Gao C, et al. Listeriosis cases and genetic diversity of their L. monocytogenes isolates in China, 2008–2019. Front Cell Infect Microbiol. 2021;11:608352. doi:10.3389/fcimb.2021.608352
13. Zhang X, Liu Y, Zhang P, et al. Genomic characterization of clinical listeria monocytogenes isolates in Beijing, China. Front Microbiol. 2021;12:751003. doi:10.3389/fmicb.2021.751003
14. Zhang X, Niu Y, Liu Y, et al. Isolation and characterization of clinical listeria monocytogenes in Beijing, China, 2014–2016. Front Microbiol. 2019;10:981. doi:10.3389/fmicb.2019.00981
15. Huang SL, Chou YT, Hsieh YC, et al. Epidemiology and clinical characteristics of Listeria monocytogenes bacteremia in a Taiwanese medical center. J Microbiol Immunol Infect. 2010;43(6):485–490. doi:10.1016/S1684-1182(10)60075-8
16. Osek J, Wieczorek K. Listeria monocytogenes-how this pathogen uses its virulence mechanisms to infect the hosts. Pathogens. 2022;11(12):1491. doi:10.3390/pathogens11121491
17. Hellberg RS, Chu E. Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: a review. Crit Rev Microbiol. 2016;42(4):548–572. doi:10.3109/1040841X.2014.972335
18. Musengimana G, Mukinda FK, Machekano R, et al. Temperature variability and occurrence of diarrhoea in children under five-years-old in cape town metropolitan Sub-Districts. Int J Environ Res Public Health. 2016;13(9):859. doi:10.3390/ijerph13090859
19. Chersich MF, Scorgie F, Rees H, et al. How climate change can fuel listeriosis outbreaks in South Africa. S Afr Med J. 2018;108(6):453–454. doi:10.7196/SAMJ.2018.v108i6.13274
20. Madjunkov M, Chaudhry S, Ito S. Listeriosis during pregnancy. Arch Gynecol Obstet. 2017;296(2):143–152. doi:10.1007/s00404-017-4401-1
21. Kishnani PM, Kurkure NV, Barbuddhe SB, et al. Draft genome sequence of Listeria monocytogenes CIIMS-NV-3, a strain isolated from vaginal discharge of a woman from central India. Microbiol Resour Announc. 2019;8(6). doi:10.1128/MRA.01553-18
22. Allerberger F, Wagner M. Listeriosis: a resurgent foodborne infection. Clin Microbiol Infect. 2010;16(1):16–23. doi:10.1111/j.1469-0691.2009.03109.x
23. Muñoz-Gallego I, Candela Ganoza G, Chaves F, et al. Listeria monocytogenes bacteraemia over an 11-year period: clinical and epidemiologic characteristics in the south area of Madrid. J Infect. 2017;75(3):276–278. doi:10.1016/j.jinf.2017.05.004
24. Halbedel S, Prager R, Banerji S, et al. A Listeria monocytogenes ST2 clone lacking chitinase ChiB from an outbreak of non-invasive gastroenteritis. Emerg Microbes Infect. 2019;8(1):17–28. doi:10.1080/22221751.2018.1558960
25. De Noordhout CM, Devleesschauwer B, Angulo FJ, et al. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(11):1073–1082. doi:10.1016/S1473-3099(14)70870-9
26. Pizarro-Cerdá J, Cossart P. Listeria monocytogenes: cell biology of invasion and intracellular growth. Microbiol Spectr. 2018;6(6). doi:10.1128/microbiolspec.GPP3-0013-2018
27. Feng Y, Wu S, Varma JK, et al. Systematic review of human listeriosis in China, 1964–2010. Trop Med Int Health. 2013;18(10):1248–1256. doi:10.1111/tmi.12173
28. Lim S, Chung DR, Kim YS, et al. Predictive risk factors for Listeria monocytogenes meningitis compared to pneumococcal meningitis: a multicenter case-control study. Infection. 2017;45(1):67–74. doi:10.1007/s15010-016-0939-2
29. Huang YT, Kuo YW, Lee MR, et al. Clinical and molecular epidemiology of human listeriosis in Taiwan. Int J Infect Dis. 2021;104:718–724. doi:10.1016/j.ijid.2021.01.056
30. Scobie A, Kanagarajah S, Harris RJ, et al. Mortality risk factors for listeriosis - A 10 year review of non-pregnancy associated cases in England 2006–2015. J Infect. 2019;78(3):208–214. doi:10.1016/j.jinf.2018.11.007
31. Charlier C, Perrodeau É, Leclercq A, et al. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis. 2017;17(5):510–519.
32. Beamonte Vela BN, Garcia-Carretero R, Carrasco-Fernandez B, et al. Listeria monocytogenes infections: analysis of 41 patients. Med Clin. 2020;155(2):57–62.
33. Lücke K, Yan I, Krohn S, et al. Control of Listeria monocytogenes infection requires classical IL-6 signaling in myeloid cells. PLoS One. 2018;13(8):e0203395.
34. Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888.
35. Ghosh P, Zhou Y, Richardson Q, et al. Characterization of the pathogenesis and immune response to Listeria monocytogenes strains isolated from a sustained national outbreak. Sci Rep. 2019;9(1):19587.
36. D’orazio SEF. Innate and adaptive immune responses during Listeria monocytogenes infection. Microbiol Spectr. 2019;7(3):7–3.
37. Arslan F, Meynet E, Sunbul M, et al. The clinical features, diagnosis, treatment, and prognosis of neuroinvasive listeriosis: a multinational study. Eur J Clin Microbiol Infect Dis. 2015;34(6):1213–1221.
38. Van Ettekoven CN, Van De Beek D, Brouwer MC. Update on community-acquired bacterial meningitis: guidance and challenges. Clin Microbiol Infect. 2017;23(9):601–606.
39. Roed C, Engsig FN, Omland LH, et al. Long-term mortality in patients diagnosed with Listeria monocytogenes meningitis: a Danish nationwide cohort study. J Infect. 2012;64(1):34–40.
40. Abdollahzadeh E, Ojagh SM, Hosseini H, et al. Antimicrobial resistance of Listeria monocytogenes isolated from seafood and humans in Iran. Microb Pathog. 2016;100:70–74.
41. Brouwer MC, Tunkel AR, Van De Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23(3):467–492.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
