Back to Journals » Infection and Drug Resistance » Volume 16
Analysis of Characteristics, Pathogens and Drug Resistance of Urinary Tract Infection Associated with Long-Term Indwelling Double-J Stent
Authors Li Y, Jiang L, Luo S, Hu D, Zhao X, Zhao G, Tang W, Guo Y
Received 10 October 2022
Accepted for publication 21 February 2023
Published 8 April 2023 Volume 2023:16 Pages 2089—2096
DOI https://doi.org/10.2147/IDR.S392857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Professor Suresh Antony
Yuehua Li,1 Li Jiang,2 Shengjun Luo,2 Daixing Hu,2 Xin Zhao,2 Guozhi Zhao,2 Wei Tang,2 Yu Guo3
1Department of Urology, University-Town Hospital of Chongqing Medical University, Chongqing, 400000, People’s Republic of China; 2Department of Urology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400000, People’s Republic of China; 3Department of Urology, Chongqing Traditional Chinese Medicine Hospital, Chongqing, 400000, People’s Republic of China
Correspondence: Yu Guo, Department of Urology, Chongqing Traditional Chinese Medicine Hospital, Chongqing, 400000, People’s Republic of China, Tel/Fax +8623 67665886, Email [email protected]
Objective: To investigate the characteristics, pathogens and drug resistance of urinary tract infection (UTI) associated with long-term indwelling double-J stent.
Methods: The clinical data of 102 patients with urinary tract infection associated with long-term indwelling double-J stent in University-Town Hospital of Chongqing Medical University and Chongqing Traditional Chinese Medicine Hospital from September 2010 to July 2022 were collected retrospectively, and the difference between etiological characteristics were analyzed. Urine and double-J stent samples of patients were collected for pathogen identification and drug sensitivity test.
Results: A total of 102 patients, 39 (38.23%) males and 63 (61.77%) females, aged 24– 72 years, with a median age of 48 years, were included in this study. Urinary calculi (40.20%) and ureteral stricture (24.50%) were the main causes of urinary tract infection associated with long-term indwelling double-J stent. Among the patients with urinary tract infection caused by double-J stent, female patients were higher than male patients (61.77% vs 38.23%). In terms of positive rate of pathogenic bacteria culture, the rate of double-J stent was higher than that of urine (67.65% vs 35.29%). The main pathogenic bacteria in urine were Escherichia coli (30.55%) of Gram negative bacteria, while the main pathogenic bacteria in double-J stent were enterococcus faecalis (27.53%) of Gram positive bacteria. The resistance rate of Gram positive bacteria in double-J stent to vancomycin, ciprofloxacin, meropenem and piperacillin/tazobactam was significantly higher than that in urine (P< 0.05). The resistance rate of Gram negative bacteria in double-J stent to imipenem, cefepime, piperacillin/tazobactam, meropenem and cefoperazone/sulbactam was significantly higher than that in urine (P< 0.05).
Conclusion: Double-J stent associated urinary tract infection is more common in women than in men. Escherichia coli and Enterococcus faecalis are the main pathogens, and the pathogens show strong drug resistance.
Keywords: double-J stent, biofilm, pathogens, urinary tract infection, drug resistance, drug resistant bacteria, infection
Introduction
Nowadays, healthcare-associated infection is one of the most important public health problems in the world, and the use of double-J tube increases the risk of urinary tract infection.1 In the treatment of urological diseases, double-J stent has dual functions of support and drainage.2–4 Therefore, it is widely used in urological surgery, such as kidney stone, ureteral stone, hydronephrosis, kidney transplantation, ureteral stenosis, etc.1–4 However, in actual clinical work, double-J stent indwelling can cause pain, bladder irritation, hematuria, fever and other conditions.4–7 In severe cases, it can induce retrograde systemic inflammatory response syndrome and urinary sepsis, endangering the life safety of patients.4–7 Therefore, exploring the clinical characteristics of double-J stent associated infection and the characteristics of pathogenic bacteria is the core of treating double J stent associated infection.
Current research suggests that the repeated urinary tract infection caused by indwelling double-J stent is mainly caused by bacterial biofilm on the surface of double-J stent.8–10 Bacteria can produce polysaccharide protein complexes, such as fibrin, lipoprotein and polysaccharide matrix on the surface of double-J stent to form a biofilm, which is called bacterial biofilm. The formation of bacterial biofilm is a defense reaction for bacteria to adapt to the external environment.8–10 Bacterial biofilm can prevent bacteria from killing by antibacterial agents and promote bacteria to develop drug resistance.8–10 Bacterial biofilm and bacterial colony formation are important factors for the recurrence and treatment difficulty of double-J stent associated infection.8–11 In view of the refractory and high recurrence of double-J stent-associated infection, it is important to explore the clinical characteristics and pathogenic characteristics of double-J stent-associated infection for the treatment of double-J stent-associated infection. Accordingly, this study analyzed the characteristics of urinary tract infection caused by long-term indwelling double-J stent, and explored the bacterial species and sensitive drugs related to double-J stent infection, so as to provide reference for clinical intervention and treatment.
Materials and Methods
Data Collection
The clinical data of 102 patients with urinary tract infection associated with long-term indwelling double-J stent in University-Town Hospital of Chongqing Medical University and Chongqing Traditional Chinese Medicine Hospital from September 2010 to July 2022 were collected retrospectively, and the etiology and characteristics of urinary tract infection were analyzed. Double-J stent is made by American Bud Company, and the size is 4.7F. Double-J stent is placed during operation. The retention time of double-J stent is 1–3 months. The position of the support tube before taking out the support tube is normal, without double J tube displacement. As previously reported, the diagnostic criteria for double-J stent associated urinary tract infection are: (1) the patient’s fever is higher than 37.5°C; (2) The number of white blood cells in urine is greater than 5/HPF; (3) The number of bacteria cultured in the middle urine is more than 105/mL, and there are obvious urinary tract irritation symptoms, such as frequent urination and discomfort.12,13 Urine and double-J stent bladder samples were collected for bacterial culture, identification and drug sensitivity test. This study was conducted with the approval of the ethics committee of the University-Town Hospital of Chongqing Medical University and Chongqing Traditional Chinese Medicine Hospital. All participants gave written informed consent. All research studies on humans (individuals, samples or data) have been performed in accordance with the principles stated in the Declaration of Helsinki.
Urine and Double J Tube Sample Acquisition
After cleaning the vulva, the urine was collected, and the middle part of the clean urine was left in the designated sterile container, and sent it to the laboratory for analysis within 30 minutes. The patient pulled out the double-J stent under the ureteroscope in hospital, and the double-J stent bladder segment was selected on the aseptic operation platform and placed in the aseptic inoculation bottle, and was quickly sent to the laboratory for analysis.
Bacterial Culture, Identification and Drug Sensitivity Test
Bacterial culture, identification and drug sensitivity tests were completed with the assistance of the Laboratory Department of Chongqing Traditional Chinese Medicine Hospital. The full automatic bacterial identification system (France BioMerier Company) was used to isolate and identify the strains.14–16 The K-B disk diffusion method was used to conduct drug sensitivity test on the obtained pathogenic bacteria, and the results were judged according to the relevant standards of the Clinical and Laboratory Standards Institute (CLSI) in the United States.14–16 The drug sensitivity of Gram positive bacteria was tested on MH medium with Tekoplanin, minocycline, cefoperazone/sulbactam, erythromycin, gentamicin, vancomycin, linezolidine, ciprofloxacin, compound sulfamethoxazole, imipenem, meropenem and piperacillin/tazobactam. The drug sensitivity of Gram negative bacteria was tested on MH medium with gentamicin, ampicillin/sulbactam, ciprofloxacin, compound sulfamethoxazole, ceftazidime, cefepime, levofloxacin, aztreonam, Piperacillin/tazobactam, Cefazolin, Cefotaxime, Amikacin, Meropenem, imipenem and Cefoperazone/sulbactam.
Statistical Analysis
SPSS 22.0 software (IBM Corp, USA) was used for data analysis. The measurement data conforming to the normal distribution is expressed by mean ± SD. The comparison between the two groups of independent, normal and homogeneous data is performed by t test. The nonparametric numerical data were expressed as median (range) and analyzed using Kruskal–Wallis test. Counting data are expressed by number of cases or percentages, and categorical data were analyzed by Pearson’s chi-squared test and Fisher’s test. P<0.05 means the difference is statistically significant.
Results
Etiological Analysis of Urinary Tract Infection Associated with Long-Term Indwelling Double-J Stent
As shown in Table 1, a total of 102 patients, aged 24–72 years, with a median age of 48 years, were included in this study. The main causes of urinary tract infection associated with long-term indwelling double-J stent were urinary calculi (40.20%) and ureteral stricture (24.50%). Among the patients with urinary tract infection associated with long-term indwelling double-J stent, female patients were higher than male patients (61.77% vs 38.23%). In terms of positive rate of pathogenic bacteria culture, the rate of double-J stent was higher than that of urine (67.65% vs 35.29%).
 |
Table 1 Etiological Analysis of Urinary Tract Infection Associated with Long-Term Indwelling Double-J Stent |
Distribution of Pathogens in Urine and Double-J Stent
As shown in Table 2, the main Gram negative pathogens in the urine of patients with urinary tract infection related to long-term double-J stent indwelling are Escherichia coli (30.55%), Klebsiella pneumoniae (16.66%) Staphylococcus aureus and Pseudomonas aeruginosa (11.11%), while the Gram positive pathogens are Enterococcus faecalis (13.89%) and Staphylococcus epidermidis (8.33%). The main Gram negative pathogens in double-J stent associated with urinary tract infection were Enterococcus faecalis (18.84%), Klebsiella pneumoniae (8.69%) and Pseudomonas aeruginosa (7.24%), while the Gram positive pathogens were Enterococcus faecalis (27.53%) and Staphylococcus epidermidis (11.59%). According to the research results, the main pathogenic bacteria in the urine is Escherichia coli, while the main pathogenic bacteria in the double-J stent is Enterococcus faecalis.
 |
Table 2 Distribution of Pathogenic Bacteria in Urine and Double-J Stent |
Analysis of Drug Resistance of Pathogenic Bacteria in Urine and Double-J Stent
As shown in Table 3, Gram positive bacteria in urine and double-J stent showed strong resistance to minocycline, erythromycin, gentamicin and compound sulfamethoxazole, and relatively low resistance to teicoplanin, vancomycin, linezolidine, imipenem, meropenem and piperacillin/tazobactam. However, the resistance rate of Gram positive bacteria in double-J stent to vancomycin, ciprofloxacin, meropenem and piperacillin/tazobactam was significantly higher than that in urine (P<0.05). As shown in Table 4, Gram negative bacteria in urine and double-J stent showed strong resistance to gentamicin, ampicillin/sulbactam, ciprofloxacin, compound sulfamethoxazole, cefepime, levofloxacin, aztreonam, cefazolin and cefotaxime, and relatively low resistance to imipenem, piperacillin/tazobactam, amikacin, meropenem, tegacycline and cefoperazone/sulbactam. However, the drug resistance rate of Gram negative bacteria in double-J stent to imipenem, cefepime, piperacillin/tazobactam, meropenem and cefoperazone/sulbactam was significantly higher than that of Gram negative bacteria in urine (P<0.05).
 |
Table 3 Drug Sensitivity Analysis of Gram Positive Bacteria |
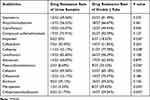 |
Table 4 Drug Sensitivity Analysis of Gram Negative Bacteria |
Discussion
Double-J stent is the most commonly used medical implant in urological surgery. It is widely used in clinic because it can relieve urinary obstruction, drain urine and protect renal function.17,18 In recent years, the rapid development of endourological technology has greatly increased the indication and application range of double-J stent implantation.17–20 Clinically, the duration of double-J stent indwelling is generally 2 to 6 weeks, but due to the complexity of patients’ conditions, the duration of double-J stent indwelling in some patients can be as long as half a year to one year. However, prolonged double-J stent indwelling will cause patients pain, bladder irritation, hematuria, fever, etc. Among them, double-J stent-related infection is a common complication of double-J stent indwelling, which can lead to retrograde urogenic sepsis and endanger the life safety of patients in severe cases.17–20
The colonization of bacteria on ureteral stent is an important problem. Bacteria can interact and adhere to the surface of bare double-J tube. After bacteria adhere to the surface of double-J tube, they can produce polysaccharide protein complex such as fibrin, lipoprotein, polysaccharide matrix on the surface of double-J tube to form a biofilm, which is called bacterial biofilm.8–10 The bacterial biofilm on the double-J tube has extremely strong drug resistance and immune evasion, which is the main reason for repeated and refractory clinical infection.8–10 Previous studies have shown that long-term indwelling of double-J stent is an important risk factor for recurrent urinary tract infection.21 In addition, the increase of double-J stent retention time significantly increases the risk of gross hematuria, pain, bladder irritation, urinary tract infection and fever.22 However, there are few reports on the etiology of urinary tract infection caused by long-term (1–3 months) double-J stent indwelling, as well as the characteristics of pathogenic bacteria and drug resistance in urine and double-J stent. Therefore, this study explored the etiological characteristics of 102 cases of urinary tract infection associated with long-term indwelling of double-J stent, and analyzed the characteristics of pathogenic bacteria and drug resistance in urine and double-J stent. In this study, we found that the most common causes of urinary tract infection associated with long-term indwelling double-J stent were urinary calculi (40.20%) and ureteral stenosis (24.50%). In infected patients, we found that female patients were significantly higher than male patients (61.77% vs 38.23%). Studies have shown that women are more than men in patients with urinary sepsis, and perimenopausal women are more likely to develop urinary sepsis than women of other ages.23–25 The female urethra is shorter than the male urethra. At the same time, the external orifice of the urethra is adjacent to the rectum and vagina, and the mucosa is more vulnerable to the influence of venous circulation, so female is prone to infection. Animal experiments show that the immune function and organ response ability of ovariectomized and aging animals will be inhibited under stress.26 The integrity of female urinary system needs estrogen to maintain.27 The decline of estrogen will cause atrophy of urinary system mucosa, increase the susceptibility of urethral epithelium and mucosa to local pathogens, and thus cause urinary tract infection.27 These factors may be the reason why the urinary tract infection caused by double-J stent is higher in women than in men.
This study showed that the culture rates of pathogenic bacteria in bladder, ureter, pelvis and urine of double-J stent were 85.0%, 42.9%, 67.3% and 24.3% respectively, and the positive culture rate of double-J stent bladder was high.10 Therefore, we chose the bladder segment of double-J stent as the object of bacterial culture and detection. Previous studies have suggested that the pathogens of double-J stent-related infections mainly include Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis and Klebsiella pneumoniae.12,21,28 In this study, we found that under the condition of double-J stent associated urinary tract infection, the pathogenic bacteria culture rate of double-J stent was significantly higher than that of urine (67.65% vs 35.29%). In addition, we also found that the main pathogenic bacteria in the urine were Escherichia coli (30.55%) from Gram negative bacteria, while the main pathogenic bacteria in double-J stent were Enterococcus faecalis from Gram positive bacteria (27.53%). The Gram positive bacteria in urine and double-J stent showed strong resistance to minocycline, erythromycin, gentamicin and compound sulfamethoxazole, and the resistance rate of Gram positive bacteria in double-J stent to vancomycin, ciprofloxacin, meropenem and piperacillin/tazobactam was significantly higher than that of Gram positive bacteria in urine (P<0.05). Gram negative bacteria in urine and double-J stent showed strong resistance to gentamicin, ampicillin/sulbactam, ciprofloxacin, compound sulfamethoxazole, cefepime, levofloxacin, aztreonam, cefazolin and cefotaxime. In addition, the drug resistance rate of Gram negative bacteria in double-J stent to imipenem, cefepime, piperacillin/tazobactam, meropenem and cefoperazone/sulbactam was significantly higher than that of Gram negative bacteria in urine (P<0.05). The results of this study are similar to those of other scholars. It is found that Escherichia coli is the main pathogenic bacteria, and the pathogenic bacteria have strong drug resistance.29,30 These results suggest that the drug resistance of Gram negative and Gram positive bacteria in double-J stent is significantly higher than that in urine. The increase of bacterial resistance in the double-J stent may be related to the biofilm formed by bacteria gathering on the surface of the double-J stent.8–10 Previous studies suggest that bacteria colonized on the double-J stent can form microbiota by secreting polysaccharide, fibrin, lipopolysaccharide and other polysaccharide compounds, and further differentiate and mature to form a biofilm on the surface of the double-J stent.31,32 Under the protection of biofilm, bacteria can escape the body’s phagocytosis and the killing of antibacterial drugs, and the resistance of biofilm bacteria to antibacterial drugs can be increased by 10 to 1000 times, which is difficult to be completely eliminated by drugs. With the passage of time, these bacteria have formed strong resistance.31–34 The formation of biofilm causes repeated infection of the patient’s urinary system and the whole body, which ultimately leads to the forced removal of the double J tube, which cannot play its due therapeutic role.31–34 Based on the findings of this study, we suggest that the urinary tract infection associated with double J tube should be conducted with simultaneous bacterial culture and drug sensitivity test on the urine and bladder segment of double-J stent. At the same time, considering the strong drug resistance of urinary tract infection bacteria associated with double J tubes, we suggest that before drug sensitivity test, empirical antibiotics, such as piperacillin/tazobactam, meropenem, tegacyclin, cefoperazone/sulbactam, teicoplanin, imipenem and vancomycin with relatively low drug resistance rates can be used.
Limitation of the Study
Although there are some findings in this study, this study still has the following limitations: First, the sample size of this study is small, and the research results have certain limitations. Second, this study is a single center, retrospective study, which has some limitations. Third, due to the differences in testing methods and equipment, the results of this study have certain limitations. Fourth, because of the differences in age, race and living environment, the research results only reflect the characteristics of the population in the center. Fifth, in this study, the phenotype of bacteria and molecular phenotype of bacteria has not been described in detail, and further research and exploration are needed in the future.
Conclusion
In this study, we found that double-J stent associated urinary tract infection is more common in women than in men. Escherichia coli and Enterococcus faecalis are the main pathogens, and the pathogens show strong drug resistance, in which the resistance of double-J tube pathogenic bacteria is significantly stronger than that of urine pathogenic bacteria.
Data Sharing Statement
The datasets are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with the approval of the ethics committee of the University-Town Hospital of Chongqing Medical University and Chongqing Traditional Chinese Medicine Hospital. All participants gave written informed consent. All research studies on humans (individuals, samples or data) have been performed in accordance with the principles stated in the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau), Fund No. 2023MSXM158.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Ripabelli G, Salzo A, Mariano A, et al. Healthcare-associated infections point prevalence survey and antimicrobials use in acute care hospitals (PPS 2016–2017) and long-term care facilities (HALT-3): a comprehensive report of the first experience in Molise Region, Central Italy, and targeted intervention strategies. J Infect Public Health. 2019;12(4):509–515. doi:10.1016/j.jiph.2019.01.060
2. Gauhar V, Pirola GM, Scarcella S, et al. Nephrostomy tube versus double J ureteral stent in patients with malignant ureteric obstruction. A systematic review and meta-analysis of comparative studies. Int Braz J Urol. 2022;48(6):903–914. doi:10.1590/S1677-5538.IBJU.2022.0225
3. Lin C, Liang A, Liang S, Wang X, Meng L, Chen M. Laparoscopic surgery combined with the double-J tube implantation for the rare cystic-solid schwannoma of seminal vesicle: a Case Report and Literature Review. Medicine. 2022;101(28):e29352. doi:10.1097/MD.0000000000029352
4. Ozturk H. Tubeless versus standard PCNL in geriatric population. Actas Urol Esp. 2015;39(8):494–501. doi:10.1016/j.acuro.2015.02.010
5. Al-Hajjaj M, Sabbagh AJ, Al-Hadid I, et al. Comparison complications rate between double-J ureteral stent and percutaneous nephrostomy in obstructive uropathy due to stone disease: a randomized controlled trial. Ann Med Surg. 2022;81:104474. doi:10.1016/j.amsu.2022.104474
6. Lara-Isla A, Medina-Polo J, Alonso-Isa M, et al. Urinary infections in patients with catheters in the upper urinary tract: microbiological study. Urol Int. 2017;98(4):442–448. doi:10.1159/000467398
7. Betschart P, Zumstein V, Buhmann MT, et al. Symptoms associated with long-term double-J ureteral stenting and influence of biofilms. Urology. 2019;134:72–78. doi:10.1016/j.urology.2019.08.028
8. Zeng K, Zhang JM, Li XB, et al. Analysis of bacterial biofilm formation in patients with malignancy undergoing double J stent indwelling and its influencing factors. Infect Drug Resist. 2021;14:5209–5217. doi:10.2147/IDR.S333421
9. Minardi D, Montanari MP, Tili E, et al. Effects of fluoroquinolones on bacterial adhesion and on preformed biofilm of strains isolated from urinary double J stents. J Chemother. 2008;20(2):195–201. doi:10.1179/joc.2008.20.2.195
10. Zhang JM, Liu J, Wang K, Zhang X, Zhao T, Luo HM. Observations of bacterial biofilm on ureteral stent and studies on the distribution of pathogenic bacteria and drug resistance. Urol Int. 2018;101(3):320–326. doi:10.1159/000490621
11. Reid G, Habash M, Vachon D, Denstedt J, Riddell J, Beheshti M. Oral fluoroquinolone therapy results in drug adsorption on ureteral stents and prevention of biofilm formation. Int J Antimicrob Agents. 2001;17(4):317–320. doi:10.1016/S0924-8579(00)00353-8
12. Scotland KB, Lo J, Grgic T, Lange D. Ureteral stent-associated infection and sepsis: pathogenesis and prevention: a review. Biofouling. 2019;35(1):117–127. doi:10.1080/08927014.2018.1562549
13. Souza JC, Henriques M, Oliveira R, Teughels W, Celis JP, Rocha LA. Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling. 2010;26(4):471–478. doi:10.1080/08927011003767985
14. Ya-Ping J, Wei-liang C, Rong Z, Bai-rong Z. The biological activity of antibacterial substance produced by Enterobacter cloacae B8. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi. 2003;13(2):115–120.
15. Weinstein MP, Lewis JS, Kraft CS. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J Clin Microbiol. 2020;58(3):e01864–19. doi:10.1128/JCM.01864-19
16. de Sousa ESO, Cortez ACA, de Souza Carvalho Melhem M, et al. Factors influencing susceptibility testing of antifungal drugs: a critical review of document M27-A4 from the Clinical and Laboratory Standards Institute (CLSI). Braz J Microbiol. 2020;51(4):1791–1800. doi:10.1007/s42770-020-00354-6
17. Tomer N, Garden E, Small A, Palese M. Ureteral stent encrustation: epidemiology, pathophysiology, management and current technology. J Urol. 2021;205(1):68–77. doi:10.1097/JU.0000000000001343
18. Geavlete P, Georgescu D, Mulțescu R, Stanescu F, Cozma C, Geavlete B. Ureteral stent complications -– experience on 50,000 procedures. J Med Life. 2021;14(6):769–775. doi:10.25122/jml-2021-0352
19. Ogreden E, Oguz U, Demirelli E, et al. The impact of ureteral Double-J stent insertion following ureterorenoscopy in patients with ureteral stones accompanied by perirenal fat stranding. Arch Ital Urol Androl. 2018;90(1):15–19. doi:10.4081/aiua.2018.1.15
20. Demirelli E, Öğreden E, Bayraktar C, et al. The effect of perirenal fat stranding on infectious complications after ureterorenoscopy in patients with ureteral calculi. Asian J Urol. 2022;9(3):307–312. doi:10.1016/j.ajur.2021.11.006
21. Kar M, Dubey A, Patel SS, et al. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent and percutaneous nephrostomy tube. J Glob Infect Dis. 2022;14(2):75–80. doi:10.4103/jgid.jgid_276_21
22. Zhou HY, Deng JP, Zhang H. Investigation on the different indwelling time and removal methods for double-pigtail stent after PCNL. J Third Mil Med Univ. 2012;34(06):564–566.
23. Corrales M, Sierra A, Doizi S, Traxer O. Risk of sepsis in retrograde intrarenal surgery: a systematic review of the literature. Eur Urol Open Sci. 2022;44:84–91. doi:10.1016/j.euros.2022.08.008
24. Kaur R, Kaur R. Symptoms, risk factors, diagnosis and treatment of urinary tract infections. Postgrad Med J. 2021;97(1154):803–812. doi:10.1136/postgradmedj-2020-139090
25. Chugh S, Pietropaolo A, Montanari E, Sarica K, Somani BK. Predictors of urinary infections and urosepsis after ureteroscopy for stone disease: a systematic review from EAU Section of Urolithiasis (EULIS). Curr Urol Rep. 2020;21(4):16. doi:10.1007/s11934-020-0969-2
26. Kawasaki T, Chaudry IH. The effects of estrogen on various organs: therapeutic approach for sepsis, trauma, and reperfusion injury. Part 2: liver, intestine, spleen, and kidney. J Anesth. 2012;26(6):892–899. doi:10.1007/s00540-012-1426-2
27. Ferrante KL, Wasenda EJ, Jung CE, Adams-Piper ER, Lukacz ES. Vaginal estrogen for the prevention of recurrent urinary tract infection in postmenopausal women: a randomized clinical trial. Female Pelvic Med Reconstr Surg. 2021;27(2):112–117. doi:10.1097/SPV.0000000000000749
28. Zeng K, Huang J, Li X, Ye J, Yang J, Zhang S. Distribution and drug resistance of pathogenic bacteria in diabetic patients with double J-stent associated infections. Infect Drug Resist. 2022;15:2671–2678. doi:10.2147/IDR.S360086
29. Sokhn ES, Salami A, El Roz A, et al. Antimicrobial susceptibilities and laboratory profiles of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates as agents of urinary tract infection in Lebanon: paving the way for better diagnostics. Med Sci. 2020;8(3):32.
30. Ripabelli G, Sammarco ML, Scutellà M, et al. Carbapenem-resistant KPC- and TEM-producing Escherichia coli ST131 isolated from a hospitalized patient with urinary tract infection: first isolation in Molise Region, Central Italy, July 2018. Microb Drug Resist. 2020;26(1):38–45. doi:10.1089/mdr.2019.0085
31. Roy R, Tiwari M, Donelli G, Tiwari V. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9(1):522–554. doi:10.1080/21505594.2017.1313372
32. Venkatesan N, Perumal G, Doble M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015;10(11):1743–1750. doi:10.2217/fmb.15.69
33. Assefa M, Amare A. Biofilm-associated multi-drug resistance in hospital-acquired infections: a review. Infect Drug Resist. 2022;15:5061–5068. doi:10.2147/IDR.S379502
34. Grande R, Puca V, Muraro R. Antibiotic resistance and bacterial biofilm. Expert Opin Ther Pat. 2020;30(12):897–900. doi:10.1080/13543776.2020.1830060
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
