Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Analyses of Factors Associated with Acute Exacerbations of Chronic Obstructive Pulmonary Disease: A Review
Authors Qian Y, Cai C, Sun M, Lv D, Zhao Y
Received 27 August 2023
Accepted for publication 9 November 2023
Published 24 November 2023 Volume 2023:18 Pages 2707—2723
DOI https://doi.org/10.2147/COPD.S433183
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Yang Qian, Chenting Cai, Mengqing Sun, Dan Lv, Yun Zhao
The First Affiliated Hospital of Ningbo University, Ningbo, People’s Republic of China
Correspondence: Dan Lv, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang Province, People’s Republic of China, Email [email protected]
Abstract: Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) is the exacerbation of a range of respiratory symptoms during the stable phase of chronic obstructive pulmonary disease (COPD). AECOPD is thus a dangerous stage and key event in the course of COPD, as its deterioration and frequency seriously affects the quality of life of patients and shortens their survival. Acute exacerbations occur and develop due to many factors such as infection, tobacco smoke inhalation, air pollution, comorbidities, airflow limitation, various biomarkers, history of previous deterioration, natural killer cell abnormalities, immunoglobulin G deficiency, genetics, abnormal muscle and nutritional status, negative psychology, and seasonal temperature changes. There is relatively limited research on the impact of the role of standardized management on the alleviation of AECOPD. However, with the establishment of relevant prevention and management systems and the promotion of artificial intelligence technology and Internet medical approaches, long-term effective and standardized management of COPD patients may help to achieve the quality of life and disease prognosis in COPD patients and reduce the risk of AE.
Keywords: chronic obstructive pulmonary disease, acute exacerbation, related risk factors
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic respiratory disease characterized by high prevalence, disability, and mortality. Acute exacerbation (AE) of chronic obstructive pulmonary disease (AECOPD) is defined as the worsening of a series of respiratory symptoms, such as dyspnea, increased cough, and sputum, in the presence of stable COPD within 14 days of medication adjustments.1 COPD is one of the four most common diseases globally, with an annual incidence of approximately 12% and a global prevalence of 9%-10% in people ≥40 years of age.2,3 It became the top three causes of global death in 2016 and was ranked as the fifth leading cause of death in China in the same year. A large epidemiologic survey in 2017 showed that COPD numbered about 300 million people globally, with a prevalence rate of 3.9% in the whole population and became the fourth leading cause of death in America, accounting for 6% of all-cause deaths globally.4,5 Further, the disease burden is expected to increase in the coming decades.6,7 Additionally, COPD showed a prevalence of 8.6% and 13.7% in adults over 20 and 40 years old, respectively, based on a 2018 study by the China Pulmonary Health (CPH). The number of individuals with COPD in China is estimated at approximately 100 million.7 As the population ages and exposure to related risk factors gradually increases, the prevalence of COPD will continue to increase and the age of onset will gradually decrease, causing a major health burden and economic pressure on society and families, rendering COPD one of the major public health problems globally.
The literature suggests that approximately 25% of patients with COPD exhibit declined lung function, attributable to AE.8 Patients with COPD experience AEs 0.5–3.5 times per year.9 As a dangerous stage and key event in the course of COPD, AE can worsen disease progression and comorbidities, seriously affecting the patients’ quality of life and shortening their survival. Patients with frequent AEs (≥2 times/year) experience a more rapid decline in lung function and have a worse prognosis, and an increased risk of death than those with infrequent AEs (<2 times/year).10 It has been shown that patients with COPD hospitalized for AEs have a mortality rate of 25% and 65% at one year and five years, respectively.11 Approximately 2%-19% of hospitalized AECOPD patients are ultimately transferred to the Intensive Care Unit (ICU),12–15 and the ultimate in-hospital mortality rate for such patients is 12–24%,16–18 with 35% of surviving patients expected to be readmitted to the hospital 3 months after discharge.19,20 Owing to various factors, such as the large population of people with COPD in China and their widespread distribution, limited awareness of the disease among most patients, and unstandardized diagnosis and treatment of COPD primary medical care, most patients are unable to recognize AE at an early stage and cannot receive timely and effective standardized treatment and management. To this end, China has gradually implemented a hierarchical system for COPD diagnosis and treatment by formulating relevant work plans and promoting health programs to improve the early diagnosis and management of COPD at the primary level.
The 2023 edition of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) for the treatment of COPD states that the goal of AE treatment is to minimize its negative effects and prevent its recurrence. Early prevention, timely detection, and aggressive interventional treatment can significantly reduce the readmission and death disability rates of patients with COPD, significantly shorten their lost life years, and substantially increase their quality of life after hospital discharge. Therefore, understanding the risk factors that may cause AE and strengthening the prevention of AE can improve the outcomes of COPD treatment and reduce the occurrence of adverse events during the course of the patients’ illnesses.
Risk Factors
Infection
Bacterial infection
Bacterial infections are generally considered to be an important factor in the exacerbation of COPD, and bacteria can be isolated from sputum specimens of 40−60% of patients with AECOPD.21,22 The occurrence of AE involves a variety of bacterial pathogens, with common bacterial pathogens including Haemophilus influenza, Moraxella catarrhalis, streptococcus pneumonia, pseudomonas aeruginosa, etc.21 However, there are differences in the prevalence of bacteria among different countries, with Pseudomonas aeruginosa, Klebsiella pneumoniae, and Haemophilus influenzae being more common in China,23 and Haemophilus influenza, Pseudomonas aeruginosa, and moraxella catarrhalis being more common in Australia and other countries.24 The bacterial infection leads to neutrophil and interleukin-8 (IL-8) production and increased production of matrix metalloproteinases (MMPs) by neutrophils and macrophages, which promote disease progression by participating in the pulmonary inflammatory response, airway remodeling, and development of emphysema.25–28
The strains colonizing the airways in the stable phase of COPD highly overlap with those isolated in the acute phase. However, strain population changes in the acute phase are more strongly associated with the degree of respiratory infection and systemic inflammatory response than those in the stable phase.29 Although bacterial infections are associated with poor clinical outcomes in cases of COPD, there is a quantitative relationship between levels of airway inflammatory markers.30 A microbiota homeostasis generated by the balance of bacterial migration and elimination already exists in the lungs; however, during AE, there is ciliary dysfunction in the lungs, increased mucus secretion, and enhanced bacterial migration, and the original airway flora balance is disrupted, leading to bacterial proliferation beyond the clearance capacity of the respiratory tract.31 Based on this, it may be hypothesized that AE triggers a homeostatic imbalance in which the microflora attacks the host’s respiratory and systemic immune systems. This therefore suggests that bacterial infections are unidirectionally associated with AE. However, regardless of the direction of the association, most clinical studies still find traces of bacterial infection in samples from respiratory secretions or tissues of patients in acute exacerbation.24,32,33
Viral Infection
Advances in detection technology have shown that viral infections are more closely associated with AE than bacterial infections.34–37 Additionally, 40−80% of AE cases with high-frequency hospitalization are attributed to viral infections.38 Common viruses isolated from the secretion specimens of patients with AE include rhinovirus, coronavirus, influenza virus, parainfluenza virus, adenovirus, and respiratory syncytial virus,21 with rhinovirus, influenza virus, and respiratory syncytial virus being the most common.39 In addition, there are geographical differences in the prevalence of respiratory viruses; for example, small RNA viruses are most common in the West, while the influenza viruses are most common in the East (Asia).40
Among these respiratory viruses, rhinoviruses are thought to play an important role in the occurrence of AE.41,42 When the body is infected with rhinovirus, the production of interferons and neutrophils is reduced, leading to the occurrence of viral AE, which eventually aggravates the physical changes and symptoms in the patient’s body.43 In addition, the impact of the 2019 novel coronavirus (2019-nCoV) pandemic on patients with COPD cannot be ignored. The 2019-nCoV promotes viral binding to the angiotensin-converting enzyme 2 (ACE-2) receptors and cell entry by carrying envelope burst proteins. As mRNA expression of ACE-2 receptors is increased in patients with COPD, the 2019-nCoV and other coronaviruses have a deleterious effect on respiratory health and pose a significant threat to patients with COPD.44
In addition to common respiratory viral infections, human immunodeficiency virus (HIV) infections increase the risk of AE by accelerating lung aging and promoting chronic lung inflammation, oxidative stress, immune activation, and alteration of lung microbiota.45,46 HIV attacks the human immune system by targeting CD4+T lymphocytes, resulting in decreased immune function and a significant increase in the risk of respiratory tract infections. One study found that HIV carriers and patients with COPD and low CD4+ T lymphocyte counts had >4-fold higher risk of AE compared to patients not infected with HIV.47
Respiratory symptoms, length of hospital stay, and systemic symptoms were also significantly worse in patients with AE and viral infections, compared to those without viral infection.36,48 Some viruses are still detectable in patients with stable COPD, owing to the presence of lung microbiota. However, the detection rates of viruses and bacteria in patients with AECOPD was significantly higher than those in the stable phase.49 This finding demonstrates the important role of viral infections in the development of AE. Acute viral infections increase airway reactivity and airway epithelial damage, causing the development of airway inflammatory edema, thus worsening airflow obstruction, which, in turn, leads to systemic inflammation.50–52
In most cases of AECOPD, mixed viral and bacterial infections occur more frequently than single viral or bacterial infections. Approximately 73% of patients with viral infections develop secondary bacterial infections within 14 days of the onset of worsening symptoms, suggesting that bacterial infections may be more important in the later stages of viral-induced deterioration.53 The percentage of bacterial and viral detection was positively correlated with the levels of hypersensitive C-reactive proteins (CRP), leukocytes, and other biological markers.35,54 Therefore, clinical practitioners’ can determine the AE status of patients with COPD by testing the levels of the corresponding biomarkers.
Tobacco Smoke Inhalation
Tobacco smoke inhalation is widely recognized as an essential factor in the development and progression of AECOPD. GOLD 2023 shows that smoking is a major risk factor for COPD, accounting for more than 70% of the total factors in high-income countries, whereas in lower-middle-income countries, smoking accounts for 30−40% of the total factors. A CPH Study states that the incidence of COPD is 13.7% in smokers, 10.9% in former smokers, and 6.2% in never-smokers,7 and significantly higher in smokers compared to non-smokers. Tobacco smoke is the main risk factor for frequent exacerbations in patients with COPD and can enhance the risk of hospital visits or readmissions due to AE.55,56 Research at the Nantong University demonstrated that active smokers with AE had more severe clinical symptoms, impaired lung function, and worse follow-up treatment outcomes, compared to non-smokers.57 Most AE patients with a smoking history of >30 pack-years use non-invasive ventilation (NIV), which increased during hospitalization.58 Notably, smoking cessation significantly reduced COPD readmission rates.59
Tobacco smoke causes immune deficiency, impairs immune homeostasis and defense mechanisms, and has a reverse effect on the antimicrobial signaling cascade, which influences inflammatory response to microorganisms.60 Another study showed that tobacco smoke affects intrapulmonary mechanical barriers (such as intraepithelial cells and cilia) by decreasing the frequency of ciliary oscillations and inducing squamous metaplasia, leading to a decrease in the number of ciliated cells and an increase in the number of cup cells and submucosal glands, causing excessive mucus production and favoring the growth of microbial pathogens,61,62 which indirectly proves the correlation between tobacco smoke inhalation and AE.
Air Pollution
Outdoor Air Pollution
Air pollution is also a crucial risk factor in the course of COPD.7,63–65 The prevalence of COPD in an PM2.5 exposed group was significantly increased by two-fold compared to that in the general and never-smoking populations.7 Additionally, there was a positive correlation between airborne fine particulate matter concentrations and COPD incidence, hospitalizations, and mortality.66–68 A study that selected air samples from seven randomized areas in the Guangdong Province of China found that particulate matter concentrations were negatively associated with some lung function indicators (FEV1, FVC, and FEV1/FVC).69 In addition, increased PM2.5 exposure was associated with increased COPD hospitalizations and mortality;67 for every 10 µg/m3 increase in PM2.5, patients with COPD had a 2.5% increased risk of emergency department visits and hospitalizations for AE.66 Several regional studies in China reported that airborne pollutants may increase the occurrence of AE.70–74 Part of this theory suggests that air pollutants can trigger AE by damaging the airway epithelium and macrophages, thereby affecting ciliary clearance, or by inducing alveolar inflammation and disturbing respiratory homeostasis.71,74,75 It is evident that the influence of outdoor air pollution in the development and deterioration of COPD should not be underestimated.67,76–78
Indoor Air Pollution
Household biomass exposure was correlated with an increased risk of COPD, with an additive effect and no significant sex differences.79 In recent years, the prevalence of COPD in females has gradually become equal to that of males, with 80% of non-smoking COPD patients being female.80,81 Exposure to household biomass in real life is common in the female population. Particulate matter, CO, SO2, NO2, and other components of biomass fumes have irritating effects on the respiratory tract and are strongly oxidizing. This can cause destructive changes in the airway and alveolar structure, inducing cupped cell hyperplasia of the airway mucosa and excessive secretion of airway mucus, resulting in persistent airway hyperreactivity and respiratory inflammation.82,83 The clinical signs of AE from biomass smoke were slightly different from those of tobacco smoke. Patients with COPD under former exposure have a less emphysematous phenotype and more often present with a small airway disease phenotype and more clinical symptoms such as cough, sputum, and shortness of breath, often with comorbidities such as bronchial asthma or allergic rhinitis, which aggravates clinical symptoms in patients with AE.83–85
Comorbidities
More than 80% of patients are estimated to have at least one chronic comorbidity, which can affect patient quality of life and disease prognosis, increase the risk of AE, and reduce patient survival.86–89 The Charlson Comorbidity Index (CCI) was first proposed in 1987, and current research suggests that CCI can be an independent risk factor for AE or death.90 Patients with COPD had lower survival rates and higher CCI scores, and patients with CCI scores ≥5 (patients with ≥4 comorbidities) had five or more times the mortality rate than did patients without comorbidities.91 A randomized controlled trial of elderly patients with COPD after hospital discharge also found that the risk of death, length of stay, and readmission increased in COPD populations with a higher CCI.92 In addition, the number of comorbidities was significantly associated with the risk of readmission in patients with COPD; that is, each additional comorbidity was associated with a 47% higher risk of readmission.93
COPD comorbidities mainly involve the cardiovascular system, respiratory system, mental status, or endocrine metabolism, and include ischemic heart disease, coronary artery disease, heart failure, pulmonary hypertension, dyslipidemia, lung cancer, pulmonary fibrosis, anxiety and depression disorders, osteoporosis, malnutrition, and diabetes.89,94 Among them, cardiovascular disease is more common and at a higher risk than other systemic diseases.87
Severe pulmonary hypertension is a complication of advanced COPD. Long-term pulmonary hypertension can stimulate proliferation of the vascular smooth muscle layer, leading to thickening of the pulmonary artery, which is currently being evaluated as a marker of pulmonary vascular disease.95 The mechanism of AE caused by pulmonary artery thickening may be related to cardiac systolic and diastolic dysfunction, a pulmonary parenchymal disease with the absence of a capillary bed, etc.96 The ratio of pulmonary artery diameter to aortic diameter (PA: A) correlates with right ventricular hypertrophy, right ventricular enlargement, and reduced right ventricular function; thus, cardiac-pulmonary interactions are critical during acute exacerbations.97 Most studies have shown a significant increase in AE risk when the PA: A ratio exceeds a threshold of 1.98 Pulmonary artery thickening (PA: A>1) is closely associated with AE and predicts the development of AECOPD.96,97
Chronic Bronchitis (CB) is also a more common comorbidity. A previous study showed a significant increase in annual AE rates and hospital admissions in COPD patients with combined CB and a higher probability of combined CB in patients with GOLD grade 2–4.99 CB is strongly associated with annual AE rates, adverse events, and accelerated disease progression in patients with COPD.
In addition, patients with chronic diseases are prone to electrolyte disorders, and hyponatremia is a common electrolyte disorder in patients with AECOPD.100,101 Hyponatremia causes a decrease in the body’s serum osmolality, which leads to the development of pulmonary edema and pleural effusion by modulation of the transient receptor potential ion channel 4, further exacerbating COPD.102,103 Most studies have concluded that the severity of hyponatremia severity is associated with longer hospital stays, higher mortality rates, higher readmission rates, and increased need for post-discharge care in patients with AECOPD, and can be used as a predictor of the occurrence of an adverse clinical outcome in AECOPD.104–106
Airflow Limitation
The primary pathophysiological alteration observed in individuals with COPD is the restriction of airflow. This is primarily caused by inflammation in the small airways, the accumulation of mucus, and the development of fibrous tissue, all of which contribute to an increase in resistance within the small airways. In cases of emphysema, the normal ability of alveoli to exert a pulling force on the small airways is diminished, along with a significant reduction in the elastic retraction force of the alveoli. Consequently, this leads to obstructive ventilation dysfunction, resulting in persistent limitations in expiratory flow, the retention of carbon dioxide within the lungs, and an adverse impact on the body’s metabolic processes and gas exchange function, particularly during AE.107 The FEV1%Pred, which serves as a lung function indicator, is frequently employed to assess the extent of airflow limitation in individuals diagnosed with COPD. Based on the decline in FEV1%Pred, the GOLD Grades categorize patients into four distinct grades. Research conducted in Japan revealed that COPD patients experiencing AE exhibited more severe FEV1 impairment, higher GOLD Grades, and elevated modified Medical Research Council (mMRC) scores compared to those without AE.108 The presence of high airflow limitation and low FEV1 has been found to potentially elevate the likelihood of recurrent AE. FEV1 can serve as an independent prognostic factor for AE, as well as being associated with predicting adverse clinical outcomes such as mortality and the need for mechanical ventilation in individuals with COPD. Furthermore, additional research has indicated a positive correlation between the frequency of AE and the decline in FEV1, suggesting a mutually causal relationship between airflow limitation and AE.109,110 Hence, in the diagnosis and management of COPD, consistent utilization of bronchodilators and regular monitoring of pulmonary function indicators assume paramount significance.
Biomarkers
Abnormal Levels of Eosinophils
It is well known that an important part of the inflammatory response in COPD is the activation and aggregation of neutrophils, however, in recent years several studies have shown that eosinophilic inflammation also plays an essential role. A total of 10%-40% of COPD patients with have eosinophilic airflow limitation.111 Since induced sputum is highly correlated with the proportion of eosinophils in the peripheral blood, increased blood eosinophils may be somewhat predictive of sputum eosinophils.111,112 Studies have shown that lung function is worsens and the risk of AEs is increased when sputum and peripheral blood eosinophils levels are elevated.112–114 The risk of AE is increased 1.85-fold when the percentage of peripheral blood eosinophils is more than 2%, the risk of AE is higher in patients with moderate to severe COPD with blood eosinophil counts ≥150 cells/μL, and the risk of AE is increased 3.21-fold with blood eosinophil counts ≥340 cells/μL.111,113,115,116 There is a degree of eosinophilic airflow limitation in any period of COPD.117,118 High level of eosinophils in the stable phase may predict an increased risk of progression,113 however, the proportion of eosinophils in AE is more than 30 times higher than that in the stable phase.119 Eosinophils regulate type 2 immune response, and when eosinophils are recruited, they can mediate a stronger inflammatory response, activating the secretion of various cytokines and cytotoxic granules that can cause airway damage.120 Eosinophilis has also been associated with a variety of disorders such as metaplastic, infectious, and rheumatologic disorders, which may also contribute to the development of AEs and increased readmission rate.
Moreover, a low eosinophil status predicts a decline in lung function and an increased risk of mortality due to critical illness. It was positively correlated with the length of hospitalization and mortality within 12 months in patients with AECOPD.121 In contrast to eosinophilia, low levels of eosinophilia indicate that the body is susceptible to infection and should be treated with antibiotics to prevent infection.122
Vitamin D (VitD) Deficiency
Several studies have confirmed the prevalence of low serum VitD levels in patients; that is, the higher the severity of COPD, the lower the serum VitD levels.123,124 The risk of disease deterioration is three times higher in COPD patients with VitD deficiency than in those without.123 Although Vit D supplementation does not increase the risk of upper respiratory tract infections, it has a protective effect against severe exacerbations in patients.125 Low vitamin D levels increase the risk of frequent respiratory infections, and reduced levels of VitD, an immunomodulatory effector in the body, can affect the body’s immune capacity, increase airway smooth muscle hyperplasia, and aggravate airflow limitation.123,126,127
High Fibrinogen Levels
Fibrinogen (FIB) is the primary plasma protein produced by hepatocytes and serves as an acute-phase reactant in the human body, with its expression being upregulated in response to inflammatory mediators. In patients with COPD, the occurrence of airflow limitation, thromboembolism, emphysema, and atherosclerosis are more prevalent when FIB levels exceed 4g/L.128,129 Several studies have demonstrated a negative correlation between FIB level and FEV1, as well as a positive correlation with AE rate, readmission rate, exercise tolerance, and COPD mortality. This suggests that FIB level can serve as a biomarker for predicting the failure of NIV in COPD patients.129–132 Furthermore, a meta-analysis consisting of 45 studies further supports the notion that higher FIB concentration is associated with greater severity of COPD.133 Mannino et al conducted a study on the relationship between FIB level and mortality, revealing that COPD mortality significantly increased when FIB levels reached or exceeded 4.03g/L.134 Another consistent study also indicated that elevated level of FIB (≥3.5g/L) was linked to increased rates of readmission within one year and higher mortality rates within three years.131 Currently, FIB is recognized as one of the most diverse inflammatory biomarkers in the progression of COPD.129,135–137 It possesses the potential to predict individuals at a heightened risk of COPD exacerbation and mortality, while also offering a less invasive means of measurement. Consequently, the Food and Drug Administration (FDA) has acknowledged FIB as a valuable tool in the management of COPD.137
Hyperuricemia
Uric acid (UA) is a byproduct of purine metabolism that can generate reactive oxygen free radicals in the human body. Consequently, UA serves as a potential indicator of oxidative stress.138 Hyperuricemia has been linked to systemic inflammation and cardiovascular incidents. Hypoxia caused by exacerbation of COPD can stimulate increased UA synthesis.139 Moreover, increased blood UA level can impair renal function, disrupt the prognosis of hypercapnic AECOPD patients, and increase the risk of cardiovascular events, and exacerbate the burden of comorbidities in AECOPD patients.140 According to the study’s findings, individuals with stage GOLD 3–4 and hypoxemia had greater levels of serum UA than patients with stage GOLD 1–2 and non-hypoxemia.141,142 Furthermore, it was observed that there existed a positive correlation between the elevation of serum UA level and the deterioration of lung function, an increased susceptibility to adverse clinical events (AE), and the serum UA level upon admission could be utilized as a standalone prognostic indicator for mortality within a 30-day timeframe.143,144 Consequently, given its accessibility and affordability, serum UA can be regarded as a valuable biomarker for assessing the severity of COPD in the clinic.
Previous Deterioration History
A history of disease progression is one of the most influential risk factors for future exacerbation, readmission, and death.145 The higher the risk of AE, the more frequent the deterioration and increased mortality of patients. Therefore, a history of previous deterioration can be an important independent predictor of frequent COPD deterioration.146–150 In a follow-up trial study of 73,106 patients selected for the first hospitalization for COPD, the risk of re-AE was found to increase approximately three times after experiencing a second exacerbation compared to the first exacerbation population; when patients experienced a tenth severe exacerbation, the risk of re-AE increased to 24 times. Mortality after the second exacerbation was 1.9 times higher compared to after the first exacerbation, and up to 5 times higher after the tenth exacerbation.151 Notably, 85.5% of patients with a history of AE in the previous year will experience one AE in the following year.148 As each AE occurs, the median survival time between exacerbations decreases, and the patients’ quality of survival and health status gradually decline after each AE, with the risk of re-AE peaking three months after discharge.151
Natural Killer (NK) Cell Abnormalities
As an essential component of the body’s innate immune system, NK cells are involved in the mechanisms of airway remodeling and emphysema, regulating antiviral, antitumor, and immune processes in the body. Therefore, abnormal NK cell status in patients with COPD substantially increases the risk of AE. Abnormal NK cell status can lead to an inflammatory response and tissue damage in the body, affecting a patient’s stability and susceptibility status.152,153 A majority view is that patients have insufficient expression of inhibitory CD94 receptors on the surface of NK cells, which are the main sources of pro-inflammatory cytokines and granzymes. Notably, the expression and release of granzyme B and perforin are increased in patients with COPD, with a consequent increase in NK cell-killing activity.154 A study has found that the NK cell-killing activity was significantly enhanced in alveolar lavage fluid specimens from patients with COPD,155 which supports the above view. Additionally, other study has reported that the proportion of NK cells is higher in patients with severe airway obstruction, frequent AE episodes, and poor nutritional status.156
Smoking, the number of AE episodes, and certain viral infections affect NK cell stability,152,156,157 and FPR3 expression levels in NKT cells are low in former smokers. Insufficient FPR3 expression is directly related to severe airflow limitation in COPD.158 Therefore, abnormalities in the NK cell status not only directly affect the stability of the respiratory system itself but also indirectly affect the course of COPD by altering other risk factors. Recent studies have indicated that NK cells may serve as specific biomarkers of AECOPD.155–157 As NK cell research continues to advance, it is expected that NK cells or NKT-like cells will be used as therapeutic entry targets to interfere with cytotoxicity and related inflammatory mediator production.
Immunoglobulin G (IgG) Deficiency
The relationship between nonspecific and acquired immunities and the onset and progression of chronic obstructive pulmonary disease (COPD) is evident.159 B-lymphocytes play a crucial role in the mediation of humoral immunity, which serves as a prominent immune defense mechanism against infections. Immunoglobulins, as integral constituents of the humoral immune response, hold significant importance.160 Immunoglobulins, as integral constituents of the humoral immune response, hold significant importance. IgG is the predominant serum immunoglobulin that actively engages in the process of phagocytosis, thereby facilitating the eradication of a diverse array of viruses, bacteria, and toxins within the human body.161 The most common cause of AE is infection. The compromised immune system of the patients heightens the susceptibility to infection, consequently leading to detrimental consequences on the patients’ development and prognosis through recurrent infections.162 Moreover, 25% of patients with COPD have humoral immunodeficiency and reduced IgG levels and are prone to hypogammaglobulinemia (<7.0 g/L).159,161 This presentation increases the risk of COPD hospitalization by 30%, the risk of future hospitalization by 60%, and the risk of AE by 50%-100%.159,161,163 Additionally, it positively correlates with the risk of death in hospitalized patients with COPD.164,165 As the number of AE increased, the proportion and frequency of hypogammaglobulinemia also increased. In COPD patients are more than two AE, hypogammaglobulinemia increased to 35.9%.161,163 Furthermore, the presence of IgG deficiency is associated with an elevated susceptibility to non-respiratory ailments, including allergic and autoimmune diseases, as well as cardiovascular system disorders. This deficiency also heightens the likelihood of complications and indirectly exacerbates the risk of AE. Consequently, the implementation of immunoglobulin replacement therapy among individuals with IgG deficiency may effectively mitigate the occurrence of AECOPD within clinical settings.
Genetics
To date, over 20 genes have been identified as significantly linked to the status of COPD.166 Mannose-Binding Lectin (MBL), a protein that activates the complement system in the body to eliminate pathogens, holds a crucial position in the immune system.167 The presence of MBL2 polymorphism is associated with inadequate levels of serum MBL, and defects in the MBL gene resulting from MBL2 polymorphism impact the frequency of recurrent respiratory tract infections and the duration of hospitalization for individuals with COPD.168,169 A comprehensive study comprising a sample size of 1796 individuals was conducted to explore the association between MBL and the susceptibility to COPD exacerbations. The results indicated that individuals with COPD who possessed the MBL2 gene demonstrated a diminished likelihood of experiencing AE, a heightened variety of lung microorganisms, and decreased levels of airway obstruction.170 Furthermore, three smaller studies have demonstrated that polymorphisms in the human MBL2 gene, leading to MBL deficiency, are associated with a heightened susceptibility to AE and a less favorable prognosis for the ailment. Conversely, studies have indicated that increased level of serum MBL are associated with improved survival rates among patients with COPD and a reduced risk of exacerbations.171–173
Furthermore, several studies have demonstrated the involvement of human hedgehog interacting protein (HHIP) in the process of lung development, whereby diminished level of HHIP result in lung hypoplasia.174 Additionally, the presence of single nucleotide polymorphisms within HHIP has been linked to an increased susceptibility to COPD, airflow limitation, and alterations in lung function.175 Moreover, a prospective study conducted across 46 clinical centers in 12 countries (n=1719) observed a significant correlation between genetic variants of HHIP and the occurrence of moderate to severe AE within the past year.176 However, the current investigation into genetic factors linked to AECOPD is still relatively limited in its scope of examining the entire genome. Our comprehension of the genetic factors that contribute to AECOPD remains restricted, thus requiring further exploration of a wider array of genetic variations.
Muscle and Nutritional Status Abnormalities
The most common extrapulmonary manifestations in COPD patients are muscle disorders and wasting, which can become more severe as the patient’s condition worsens.177 Malnutrition leads to abnormalities in muscle mass and function, with a significant positive correlation between COPD progression and adverse events, which can have a significant impact on the respiratory system and the whole body.177–180 BMI is a common clinical measure of a patient’s nutritional status, and studies have shown that a BMI <20 kg/m2 can be an independent risk factor for mortality in partial respiratory disease.181 Patients with COPD who are malnourished or potentially malnourished have increased readmission rates, length of stay, other comorbidities, and four times increased risk of death after 2 years.182–185
Patients with COPD are prone to nutritional events.186 The clinical outcome of Malnutrition leads to dysfunction of the respiratory muscles and diaphragm, resulting in decreased lung function, increased dyspnea, limitation of daily activities, and muscle atrophy or weakness. Damage to the respiratory muscles and surrounding muscle groups is significantly correlated with the severity of the disease at the time and frequency of admission, and in severe cases, respiratory failure or more complex cachexia.177,179
Other Factors
Negative Mentalities
Patients with depression or anxiety are at an increased risk of COPD progression and death.187–189 In a clinical study of 376 patients with AECOPD, the prevalence of depression at admission was found to be 44.4%. Furthermore, patients with COPD and depression had lower survival rates, longer hospital stays, and a higher disease burden at one-year follow-up.190 COPD patients with high depression and anxiety (anxiety > 50, depression > 53) were at higher risk of AE.191 Depression is significantly associated with poorer social functioning and economic status, low family or marital happiness, and low educational attainment.93,190 Some studies have suggested that the pathological mechanisms of negative psychological states effects on AE, among others, may be related to systemic inflammatory responses or neuronal necrosis in the hippocampus.
Seasonal Temperature Variations
A global-scale study of COPD exacerbation and seasonal correlation indicated a nearly two-fold increase in AE risk in winter compared to summer. AE was common between December and February in 9% of patients in the northern region, compared to 5% in summer, and 12% of patients in the southern region had higher AE rates between December and February, compared to 7% in summer.192 Excluding the tropic regions, COPD has a higher AE rate in winter, and the seasonality of AE is more pronounced. Another Spanish regional study showed that COPD was particularly prevalent in winter, followed by autumn, spring, and summer; additionally, the number of hospitalizations also increased with lower temperatures, with a 4.7% increase for every 1 °C drop in temperature,193 supporting the effect of seasonal temperature changes on AE.
Conclusions
AE is the outcome of multiple risk factors, including individual and environmental factors, which act together in the body to trigger AE (Figure 1, Table 1). The majority of these AE are initiated by respiratory pathogens, including viruses and bacteria. Currently, global research predominantly concentrates on the microscopic level, delving extensively into the mechanisms underlying AECOPD. The objective is to identify novel regulatory pathways and pharmaceutical treatments utilizing genomic and biomarker technologies.
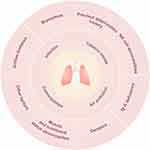 |
Figure 1 Diagram of risk factors associated with chronic obstructive pulmonary disease. |
 |
Table 1 Risk Factors Associated with Acute Exacerbation of COPD |
Due to the large size of the COPD population, and with the world population gradually aging and multiple risk factors continuing to attack, the mortality and disability rates of COPD patients will continue to increase in the future. For these reasons, preventive diagnosis and treatment of COPD, early identification, timely diagnosis and treatment, and whole process intervention are particularly important. Long-term standardized management and intervention in COPD patients will likely improve the negative impact of AE in the course of COPD to a greater extent, and improve patients’ disease prognosis and quality of life. Currently, there remains a dearth of research literature pertaining to the long-term standardized management intervention for patients with COPD in the context of investigating risk factors and predicting AE. Furthermore, completed studies on standardized management factors may exhibit limitations, including inadequate sample sizes, incomplete incorporation and analysis of indicators, and suboptimal patient adherence.
With the increasing emphasis on chronic diseases in the international health community, most countries are gradually implementing standardized training for respiratory physicians, public health education, early screening of high-risk groups for COPD, and actively build standardized supervision and management system for primary care. These efforts aim to investigate novel medical intervention strategies that can effectively mitigate the occurrence of AE. With the popularization and improvement of network information, the combination of the Internet and medical treatment will become the mainstream trend for precise treatment, full management, and prevention of AE for patients with COPD in both urban and rural communities in the future.
Abbreviations
AECOPD, Acute exacerbations of chronic obstructive pulmonary disease; VitD, Vitamin D; CB, Chronic Bronchitis; CCI, Charlson Comorbidity Index; NIV, non-invasive ventilation; CRP, C-reactive proteins; ACE-2, angiotensin-converting enzyme 2; MMPs, matrix metalloproteinases; NK, Natural killer; HIV, human immunodeficiency virus; mMRC, modified Medical Research Council; FIB, fibrinogen; UA, uric acid; IgG, Immunoglobulin G; MBL, Mannose-Binding Lectin; HHIP, human hedgehog interacting protein.
Acknowledgments
This work was supported by Ningbo Science And Technology Bureau (Project Number: 202003N4023).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Venkatesan P. GOLD COPD report: 2023 update. Lancet Respir Med. 2023;11(1):18. doi:10.1016/S2213-2600(22)00494-5
2. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. doi:10.7189/jogh.05.020415
3. Vos T, Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
4. Heron M. Deaths: leading Causes for 2017. Natl Vital Stat Rep. 2019;68(6):1–77.
5. Global. regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi:10.1016/S0140-6736(18)32279-7
6. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/S0140-6736(22)00470-6
7. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
8. Ko FW, Chan KP, Hui DS, et al. Acute exacerbation of COPD. Respirology. 2016;21(7):1152–1165. doi:10.1111/resp.12780
9. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi:10.1016/S2213-2600(17)30207-2
10. Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11(1):181. doi:10.1186/1741-7015-11-181
11. Celli BR, Fabbri LM, Aaron SD, et al. Differential Diagnosis of Suspected Chronic Obstructive Pulmonary Disease Exacerbations in the Acute Care Setting: best Practice. Am J Respir Crit Care Med. 2023;207(9):1134–1144. doi:10.1164/rccm.202209-1795CI
12. Marcos PJ, Sanjuán P, Huerta A, et al. Relationship Between Severity Classification of Acute Exacerbation of Chronic Obstructive Pulmonary Disease and Clinical Outcomes in Hospitalized Patients. Cureus. 2017;9(1):e988. doi:10.7759/cureus.988
13. Pozo-Rodríguez F, López-Campos JL, Alvarez-Martínez CJ, et al. Clinical audit of COPD patients requiring hospital admissions in Spain: AUDIPOC study. PLoS One. 2012;7(7):e42156. doi:10.1371/journal.pone.0042156
14. Escarrabill J, Torrente E, Esquinas C, et al. Clinical audit of patients hospitalized due to COPD exacerbation. MAG-1 Study. Arch Bronconeumol. 2015;51(10):483–489. doi:10.1016/j.arbres.2014.06.023
15. Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): a Systematic Literature Review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–460. doi:10.2147/COPD.S234942
16. Afessa B, Morales IJ, Scanlon PD, Peters SG. Prognostic factors, clinical course, and hospital outcome of patients with chronic obstructive pulmonary disease admitted to an intensive care unit for acute respiratory failure. Crit Care Med. 2002;30(7):1610–1615. doi:10.1097/00003246-200207000-00035
17. Berenyi F, Steinfort DP, Abdelhamid YA, et al. Characteristics and Outcomes of Critically Ill Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Australia and New Zealand. Ann Am Thorac Soc. 2020;17(6):736–745. doi:10.1513/AnnalsATS.201911-821OC
18. Prediletto I, Giancotti G, Nava S. COPD Exacerbation: why It Is Important to Avoid ICU Admission. J Clin Med. 2023;12(10):3369. doi:10.3390/jcm12103369
19. Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: european COPD Audit. Eur Respir J. 2016;47(1):113–121. doi:10.1183/13993003.01391-2014
20. Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. 2011;37(3):508–515. doi:10.1183/09031936.00043710
21. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi:10.1016/S0140-6736(07)61382-8
22. Ritchie AI, Wedzicha JA. Definition, Causes, Pathogenesis, and Consequences of Chronic Obstructive Pulmonary Disease Exacerbations. Clin Chest Med. 2020;41(3):421–438. doi:10.1016/j.ccm.2020.06.007
23. Ye F, He LX, Cai BQ, et al. Spectrum and antimicrobial resistance of common pathogenic bacteria isolated from patients with acute exacerbation of chronic obstructive pulmonary disease in mainland of China. Chin Med J. 2013;126(12):2207–2214.
24. Wark PA, Tooze M, Powell H, Parsons K. Viral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmission. Respirology. 2013;18(6):996–1002. doi:10.1111/resp.12099
25. Hou HH, Wang HC, Cheng SL, Chen YF, Lu KZ, Yu CJ. MMP-12 activates protease-activated receptor-1, upregulates placenta growth factor, and leads to pulmonary emphysema. Am J Physiol Lung Cell Mol Physiol. 2018;315(3):L432–L442. doi:10.1152/ajplung.00216.2017
26. Churg A, Zhou S, Wright JL. Series “matrix metalloproteinases in lung health and disease”: matrix metalloproteinases in COPD. Eur Respir J. 2012;39(1):197–209. doi:10.1183/09031936.00121611
27. Liu J, Ran Z, Wang F, Xin C, Xiong B, Song Z. Role of pulmonary microorganisms in the development of chronic obstructive pulmonary disease. Crit Rev Microbiol. 2021;47(1):1–12. doi:10.1080/1040841X.2020.1830748
28. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi:10.1056/NEJMra0800353
29. Hurst JR, Perera WR, Wilkinson TMA, Donaldson GC, Wedzicha JA. Systemic and Upper and Lower Airway Inflammation at Exacerbation of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2006;173(1):71–78. doi:10.1164/rccm.200505-704OC
30. Sapey E. COPD exacerbations.2: aetiology. Thorax. 2006;61(3):250–258. doi:10.1136/thx.2005.041822
31. Wypych TP, Wickramasinghe LC, Marsland BJ. The influence of the microbiome on respiratory health. Nat Immunol. 2019;20(10):1279–1290. doi:10.1038/s41590-019-0451-9
32. Rohde G. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi:10.1136/thorax.58.1.37
33. Choi KJ, Cha SI, Shin KM, et al. Prevalence and predictors of pulmonary embolism in Korean patients with exacerbation of chronic obstructive pulmonary disease. Respiration. 2013;85(3):203–209. doi:10.1159/000335904
34. Aaron SD, Angel JB, Lunau M, et al. Granulocyte Inflammatory Markers and Airway Infection during Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2001;163(2):349–355. doi:10.1164/ajrccm.163.2.2003122
35. Clark TW, Medina MJ, Batham S, Curran MD, Parmar S, Nicholson KG. C-reactive protein level and microbial aetiology in patients hospitalised with acute exacerbation of COPD. Eur Respir J. 2015;45(1):76–86. doi:10.1183/09031936.00092214
36. Hosseini SS, Ghasemian E, Jamaati H, Tabaraie B, Amini Z, Cox K. Association between respiratory viruses and exacerbation of COPD: a case-control study. Infect Dis. 2015;47(8):523–529. doi:10.3109/23744235.2015.1022873
37. Hewitt R, Farne H, Ritchie A, Luke E, Johnston SL, Mallia P. The role of viral infections in exacerbations of chronic obstructive pulmonary disease and asthma. Ther Adv Respir Dis. 2016;10(2):158–174. doi:10.1177/1753465815618113
38. Stolz D, Papakonstantinou E, Grize L, et al. Time-course of upper respiratory tract viral infection and COPD exacerbation. Eur Respir J. 2019;54(4):1900407. doi:10.1183/13993003.00407-2019
39. Zwaans WA, Mallia P, van Winden ME, Rohde GG. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease-a systematic review. J Clin Virol. 2014;61(2):181–188. doi:10.1016/j.jcv.2014.06.025
40. Mohan A, Chandra S, Agarwal D, et al. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology. 2010;15(3):536–542. doi:10.1111/j.1440-1843.2010.01722.x
41. Biancardi E, Fennell M, Rawlinson W, Thomas PS. Viruses are frequently present as the infecting agent in acute exacerbations of chronic obstructive pulmonary disease in patients presenting to hospital. Intern Med J. 2016;46(10):1160–1165. doi:10.1111/imj.13213
42. Kwak HJ, Park DW, Kim JE, et al. Prevalence and Risk Factors of Respiratory Viral Infections in Exacerbations of Chronic Obstructive Pulmonary Disease. Tohoku J Exp Med. 2016;240(2):131–139. doi:10.1620/tjem.240.131
43. Mallia P, Message SD, Kebadze T, Parker HL, Kon OM, Johnston SL. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res. 2006;7(1). doi:10.1186/1465-9921-7-116
44. Leung JM, Niikura M, Yang CWT, Sin DD. COVID-19 and COPD. Eur Respir J. 2020;56(2):2002108. doi:10.1183/13993003.02108-2020
45. Cribbs SK, Crothers K, Morris A. Pathogenesis of HIV-Related Lung Disease: immunity, Infection, and Inflammation. Physiol Rev. 2020;100(2):603–632. doi:10.1152/physrev.00039.2018
46. Liu JC, Leung JM, Ngan DA, et al. Absolute leukocyte telomere length in HIV-infected and uninfected individuals: evidence of accelerated cell senescence in HIV-associated chronic obstructive pulmonary disease. PLoS One. 2015;10(4):e0124426. doi:10.1371/journal.pone.0124426
47. Lambert AA, Kirk GD, Astemborski J, Mehta SH, Wise RA, Drummond MB. HIV Infection Is Associated With Increased Risk for Acute Exacerbation of COPD. J Acquir Immune Defic Syndr. 2015;69(1):68–74. doi:10.1097/QAI.0000000000000552
48. Dimopoulos G, Lerikou M, Tsiodras S, et al. Viral epidemiology of acute exacerbations of chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2012;25(1):12–18. doi:10.1016/j.pupt.2011.08.004
49. Choi J, Oh JY, Lee YS, et al. Bacterial and Viral Identification Rate in Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Korea. Yonsei Med J. 2019;60(2):216–222. doi:10.3349/ymj.2019.60.2.216
50. Almansa R, Socias L, Andaluz-Ojeda D, et al. Viral infection is associated with an increased proinflammatory response in chronic obstructive pulmonary disease. Viral Immunol. 2012;25(4):249–253. doi:10.1089/vim.2011.0095
51. Perera WR, Hurst JR, Wilkinson TM, et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;29(3):527–534. doi:10.1183/09031936.00092506
52. Hegele RG, Hayashi S, Hogg JC, Paré PD. Mechanisms of airway narrowing and hyperresponsiveness in viral respiratory tract infections. Am J Respir Crit Care Med. 1995;151(5):1659–1664. doi:10.1164/ajrccm/151.5_Pt_1.1659
53. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87–96. doi:10.1183/09031936.00223113
54. Mackay AJ, Kostikas K, Murray L, et al. Patient-reported Outcomes for the Detection, Quantification, and Evaluation of Chronic Obstructive Pulmonary Disease Exacerbations. Am J Respir Crit Care Med. 2018;198(6):730–738. doi:10.1164/rccm.201712-2482CI
55. Montserrat-Capdevila J, Godoy P, Marsal JR, Barbe F, Galvan L. Risk factors for exacerbation in chronic obstructive pulmonary disease: a prospective study. Int J Tuberc Lung Dis. 2016;20(3):389–395. doi:10.5588/ijtld.15.0441
56. Eisner M. The impact of SHS exposure on health status and exacerbations among patients with COPD. Int J Chron Obstruct Pulmon Dis. 2009;169. doi:10.2147/COPD.S4681
57. Li X, Wu Z, Xue M, Du W. Smoking status affects clinical characteristics and disease course of acute exacerbation of chronic obstructive pulmonary disease: a prospectively observational study. Chron Respir Dis. 2020;17:1479973120916184. doi:10.1177/1479973120916184
58. Saad AB, Adhieb A, Migaou A, et al. Effect of intensity of smoking intoxication on severity parameters of acute exacerbations of chronic obstructive pulmonary disease treated in a hospital milieu. Pan Afr Med J. 2021;38:91. doi:10.11604/pamj.2021.38.91.21512
59. Godtfredsen NS. Risk of hospital admission for COPD following smoking cessation and reduction: a Danish population study. Thorax. 2002;57(11):967–972. doi:10.1136/thorax.57.11.967
60. Bauer CMT, Morissette MC, Stampfli MR. The influence of cigarette smoking on viral infections: translating bench science to impact COPD pathogenesis and acute exacerbations of COPD clinically. Chest. 2013;143(1):196–206. doi:10.1378/chest.12-0930
61. Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflammation Res. 2008;57(11):497–503. doi:10.1007/s00011-008-8078-6
62. Johnston S, Molyneaux P, Singanayagam A, Joshi B, Mallia P. Lung microbiology and exacerbations in COPD. Int J Chron Obstruct Pulmon Dis. 2012;555. doi:10.2147/COPD.S28286
63. Jin Y, Cheng Y, Wang H, Zhao C. Effects of air pollution from burning coal on respiratory diseases in adults. Wei Sheng Yan Jiu. 2001;30(4):241–243, 246.
64. Schikowski T, Mills IC, Anderson HR, et al. Ambient air pollution: a cause of COPD? Eur Respir J. 2014;43(1):250–263. doi:10.1183/09031936.00100112
65. Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378(9795):991–996. doi:10.1016/S0140-6736(11)60990-2
66. DeVries R, Kriebel D, Sama S. Outdoor Air Pollution and COPD-Related Emergency Department Visits, Hospital Admissions, and Mortality: a Meta-Analysis. COPD. 2017;14(1):113–121. doi:10.1080/15412555.2016.1216956
67. Li MH, Fan LC, Mao B, et al. Short-term Exposure to Ambient Fine Particulate Matter Increases Hospitalizations and Mortality in COPD: a Systematic Review and Meta-analysis. Chest. 2016;149(2):447–458. doi:10.1378/chest.15-0513
68. Wordley J, Walters S, Ayres JG. Short term variations in hospital admissions and mortality and particulate air pollution. Occup Environ Med. 1997;54(2):108–116. doi:10.1136/oem.54.2.108
69. Liu S, Zhou Y, Liu S, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax. 2017;72(9):788–795. doi:10.1136/thoraxjnl-2016-208910
70. Sun XW, Chen PL, Ren L, et al. The cumulative effect of air pollutants on the acute exacerbation of COPD in Shanghai, China. Sci Total Environ. 2018;622-623:875–881. doi:10.1016/j.scitotenv.2017.12.042
71. Xie J, Teng J, Fan Y, Xie R, Shen A. The short-term effects of air pollutants on hospitalizations for respiratory disease in Hefei, China. Int J Biometeorol. 2019;63(3):315–326. doi:10.1007/s00484-018-01665-y
72. Hwang SL, Lin YC, Guo SE, Chou CT, Lin CM, Chi MC. Fine particulate matter on hospital admissions for acute exacerbation of chronic obstructive pulmonary disease in southwestern Taiwan during 2006-2012. Int J Environ Health Res. 2017;27(2):95–105. doi:10.1080/09603123.2017.1278748
73. Wang W, Ying Y, Wu Q, Zhang H, Ma D, Xiao W. A GIS-based spatial correlation analysis for ambient air pollution and AECOPD hospitalizations in Jinan, China. Respir Med. 2015;109(3):372–378. doi:10.1016/j.rmed.2015.01.006
74. Liang L, Cai Y, Barratt B, et al. Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in Beijing, 2013–17: an ecological analysis. Lancet Planetary Health. 2019;3(6):e270–e279. doi:10.1016/S2542-5196(19)30085-3
75. Kelly FJ, Fussell JC. Air pollution and airway disease. Clin Exp Allergy. 2011;41(8):1059–1071. doi:10.1111/j.1365-2222.2011.03776.x
76. Morantes-Caballero JA, Fajardo Rodriguez HA. Effects of air pollution on acute exacerbation of chronic obstructive pulmonary disease: a descriptive retrospective study (pol-AECOPD). Int J Chron Obstruct Pulmon Dis. 2019;14:1549–1557.
77. Peacock JL, Anderson HR, Bremner SA, et al. Outdoor air pollution and respiratory health in patients with COPD. Thorax. 2011;66(7):591–596. doi:10.1136/thx.2010.155358
78. Lin MT, Kor CT, Chang CC, et al. Association of meteorological factors and air NO2 and O3 concentrations with acute exacerbation of elderly chronic obstructive pulmonary disease. Sci Rep. 2018;8(1):10192. doi:10.1038/s41598-018-28532-5
79. Hu G, Zhou Y, Tian J, et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138(1):20–31. doi:10.1378/chest.08-2114
80. Gut-Gobert C, Cavailles A, Dixmier A, et al. Women and COPD: do we need more evidence? Eur Respir Rev. 2019;28(151):180055. doi:10.1183/16000617.0055-2018
81. Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers: results from the Third National Health and Nutrition Examination Survey. Am J Med. 2005;118(12):1364–1372. doi:10.1016/j.amjmed.2005.06.041
82. Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138(1):3–6. doi:10.1378/chest.10-0645
83. Cheng LL, Liu YY, Su ZQ, Liu J, Chen RC, Ran PX. Clinical characteristics of tobacco smoke-induced versus biomass fuel-induced chronic obstructive pulmonary disease. J Transl Int Med. 2015;3(3):126–129. doi:10.1515/jtim-2015-0012
84. Golpe R, Mengual-Macenlle N, Sanjuán-López P, Cano-Jiménez E, Castro-Añón O, Pérez-de-Llano LA. Prognostic Indices and Mortality Prediction in COPD Caused by Biomass Smoke Exposure. Lung. 2015;193(4):497–503.
85. Cho J, Lee C-H, Hwang SS, et al. Risk of acute exacerbations in chronic obstructive pulmonary disease associated with biomass smoke compared with tobacco smoke. BMC Pulm Med. 2019;19(1). doi:10.1186/s12890-019-0833-7
86. Putcha N, Drummond MB, Wise RA, Hansel NN. Comorbidities and Chronic Obstructive Pulmonary Disease: prevalence, Influence on Outcomes, and Management. Semin Respir Crit Care Med. 2015;36(4):575–591. doi:10.1055/s-0035-1556063
87. Sievi NA, Senn O, Brack T, et al. Impact of comorbidities on physical activity in COPD. Respirology. 2015;20(3):413–418. doi:10.1111/resp.12456
88. McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62(5):411–415. doi:10.1136/thx.2006.072348
89. Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454–475. doi:10.1183/09059180.00008612
90. Lin Z, Chunmei P, Xiuhong N. Predictive Value of Charlson Comorbidity Index in Prognosis of Aged Chronic Obstructive Pulmonary Disease Patients. Chine J Respir Critical Care Med. 2016;15(4):333–336.
91. Almagro P, Calbo E, Ochoa de Echagüen A, et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448. doi:10.1378/chest.121.5.1441
92. Almagro P, Cabrera FJ, Diez J, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: the EPOC en Servicios de medicina interna (ESMI) study. Chest. 2012;142(5):1126–1133. doi:10.1378/chest.11-2413
93. Wong AW, Gan WQ, Burns J, Sin DD, van Eeden SF. Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of hospital stay and readmission rates. Can Respir J. 2008;15(7):361–364. doi:10.1155/2008/569496
94. Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: impact, measurement and mechanisms. Respirology. 2015;20(8):1160–1171. doi:10.1111/resp.12642
95. Wells JM, Dransfield MT. Pathophysiology and clinical implications of pulmonary arterial enlargement in COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:509–521. doi:10.2147/COPD.S52204
96. Cuttica MJ, Bhatt SP, Rosenberg SR, et al. Pulmonary artery to aorta ratio is associated with cardiac structure and functional changes in mild-to-moderate COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1439–1446. doi:10.2147/COPD.S131413
97. Wells JM, Morrison JB, Bhatt SP, Nath H, Dransfield MT. Pulmonary Artery Enlargement Is Associated With Cardiac Injury During Severe Exacerbations of COPD. Chest. 2016;149(5):1197–1204. doi:10.1378/chest.15-1504
98. Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi:10.1056/NEJMoa1203830
99. Corhay JL, Vincken W, Schlesser M, Bossuyt P, Imschoot J. Chronic bronchitis in COPD patients is associated with increased risk of exacerbations: a cross-sectional multicentre study. Int J Clin Pract. 2013;67(12):1294–1301. doi:10.1111/ijcp.12248
100. Ramírez E, Rodríguez A, Queiruga J, et al. Severe Hyponatremia Is Often Drug Induced: 10-Year Results of a Prospective Pharmacovigilance Program. Clin Pharmacol Ther. 2019;106(6):1362–1379. doi:10.1002/cpt.1562
101. Cuesta M, Slattery D, Goulden EL, et al. Hyponatraemia in patients with community-acquired pneumonia; prevalence and aetiology, and natural history of SIAD. Clin Endocrinol (Oxf). 2019;90(5):744–752. doi:10.1111/cen.13937
102. García-Sanz MT, Martínez-Gestoso S, Calvo-álvarez U, et al. Impact of Hyponatremia on COPD Exacerbation Prognosis. J Clin Med. 2020;9(2):503. doi:10.3390/jcm9020503
103. Urso C, Brucculeri S, Caimi G. Physiopathological, Epidemiological, Clinical and Therapeutic Aspects of Exercise-Associated Hyponatremia. J Clin Med. 2014;3(4):1258–1275. doi:10.3390/jcm3041258
104. Chalela R, González-García JG, Chillarón JJ, et al. Impact of hyponatremia on mortality and morbidity in patients with COPD exacerbations. Respir Med. 2016;117:237–242. doi:10.1016/j.rmed.2016.05.003
105. Al Mawed S, Pankratz VS, Chong K, Sandoval M, Roumelioti ME, Unruh M. Low serum sodium levels at hospital admission: outcomes among 2.3 million hospitalized patients. PLoS One. 2018;13(3):e0194379. doi:10.1371/journal.pone.0194379
106. De Vecchis R, Di Maio M, Di Biase G, Ariano C. Effects of Hyponatremia Normalization on the Short-Term Mortality and Rehospitalizations in Patients with Recent Acute Decompensated Heart Failure: a Retrospective Study. J Clin Med. 2016;5(10):92. doi:10.3390/jcm5100092
107. Anzueto A, Miravitlles M. Pathophysiology of dyspnea in COPD. Postgrad Med. 2017;129(3):366–374. doi:10.1080/00325481.2017.1301190
108. Yamaya M, Usami O, Nakayama S, et al. Malnutrition, Airflow Limitation and Severe Emphysema are Risks for Exacerbation of Chronic Obstructive Pulmonary Disease in Japanese Subjects: a Retrospective Single-Center Study. Int J Chron Obstruct Pulmon Dis. 2020;15:857–868. doi:10.2147/COPD.S238457
109. Gulati S, Wells JM. Bringing Stability to the Chronic Obstructive Pulmonary Disease Patient: clinical and Pharmacological Considerations for Frequent Exacerbators. Drugs. 2017;77(6):651–670. doi:10.1007/s40265-017-0713-5
110. Miravitlles M, Guerrero T, Mayordomo C, Sánchez-Agudo L, Nicolau F, Segú JL. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000;67(5):495–501. doi:10.1159/000067462
111. Gao J, Chen B, Wu S, Wu F. Blood cell for the differentiation of airway inflammatory phenotypes in COPD exacerbations. BMC Pulm Med. 2020;20(1):50. doi:10.1186/s12890-020-1086-1
112. Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi:10.1016/S2213-2600(17)30432-0
113. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193(9):965–974. doi:10.1164/rccm.201509-1869OC
114. Brusselle G, Pavord ID, Landis S, et al. Blood eosinophil levels as a biomarker in COPD. Respir Med. 2018;138:21–31. doi:10.1016/j.rmed.2018.03.016
115. Bafadhel M, Pavord ID, Russell REK. Eosinophils in COPD: just another biomarker? Lancet Respir Med. 2017;5(9):747–759. doi:10.1016/S2213-2600(17)30217-5
116. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi:10.1016/S2213-2600(15)00106-X
117. Papi A, Romagnoli M, Baraldo S, et al. Partial reversibility of airflow limitation and increased exhaled NO and sputum eosinophilia in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(5):1773–1777. doi:10.1164/ajrccm.162.5.9910112
118. Gao P, Zhang J, He X, Hao Y, Wang K, Gibson PG. Sputum inflammatory cell-based classification of patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One. 2013;8(5):e57678. doi:10.1371/journal.pone.0057678
119. Saetta M, Di Stefano A, Maestrelli P, et al. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1646–1652. doi:10.1164/ajrccm.150.6.7952628
120. Eltboli O, Bafadhel M, Hollins F, et al. COPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophils. BMC Pulm Med. 2014;14:112. doi:10.1186/1471-2466-14-112
121. Abidi K, Khoudri I, Belayachi J, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care. 2008;12(2):R59. doi:10.1186/cc6883
122. Pavord ID, Lettis S, Anzueto A, Barnes N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir Med. 2016;4(9):731–741. doi:10.1016/S2213-2600(16)30148-5
123. Lokesh KS, Chaya SK, Jayaraj BS, et al. Vitamin D deficiency is associated with chronic obstructive pulmonary disease and exacerbation of COPD. Clin Respir J. 2021;15(4):389–399. doi:10.1111/crj.13310
124. Burkes RM, Ceppe AS, Doerschuk CM, et al. Associations Among 25-Hydroxyvitamin D Levels, Lung Function, and Exacerbation Outcomes in COPD: an Analysis of the SPIROMICS Cohort. Chest. 2020;157(4):856–865. doi:10.1016/j.chest.2019.11.047
125. Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3(2):120–130. doi:10.1016/S2213-2600(14)70255-3
126. Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5(7):2502–2521. doi:10.3390/nu5072502
127. Jung JY, Kim YS, Kim SK, et al. Relationship of vitamin D status with lung function and exercise capacity in COPD. Respirology. 2015;20(5):782–789. doi:10.1111/resp.12538
128. Sun W, Cao Z, Ma Y, Wang J, Zhang L, Luo Z. Fibrinogen, a Promising Marker to Evaluate Severity and Prognosis of Acute Exacerbation of Chronic Obstructive Pulmonary Disease: a Retrospective Observational Study. Int J Chron Obstruct Pulmon Dis. 2022;17:1299–1310. doi:10.2147/COPD.S361929
129. Miller BE, Tal-Singer R, Rennard SI, et al. Plasma Fibrinogen Qualification as a Drug Development Tool in Chronic Obstructive Pulmonary Disease. Perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Am J Respir Crit Care Med. 2016;193(6):607–613. doi:10.1164/rccm.201509-1722PP
130. Valvi D, Mannino DM, Müllerova H, Tal-Singer R. Fibrinogen, chronic obstructive pulmonary disease (COPD) and outcomes in two United States cohorts. Int J Chron Obstruct Pulmon Dis. 2012;7:173–182. doi:10.2147/COPD.S29892
131. Mannino DM, Tal-Singer R, Lomas DA, et al. Plasma Fibrinogen as a Biomarker for Mortality and Hospitalized Exacerbations in People with COPD. Chronic Obstr Pulm Dis. 2015;2(1):23–34. doi:10.15326/jcopdf.2.1.2014.0138
132. Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1065–1072. doi:10.1164/rccm.201110-1792OC
133. Zhou B, Liu S, He D, et al. Fibrinogen is a promising biomarker for chronic obstructive pulmonary disease: evidence from a meta-analysis. Biosci Rep. 2020;40(7). doi:10.1042/BSR20193542
134. Mannino DM, Valvi D, Mullerova H, Tal-Singer R. Fibrinogen, COPD and mortality in a nationally representative U.S. cohort. Copd. 2012;9(4):359–366. doi:10.3109/15412555.2012.668249
135. Wang J, Pathak R, Garg S, Hauer-Jensen M. Fibrinogen deficiency suppresses the development of early and delayed radiation enteropathy. World J Gastroenterol. 2017;23(26):4701–4711. doi:10.3748/wjg.v23.i26.4701
136. Bellou V, Belbasis L, Konstantinidis AK, Evangelou E. Elucidating the risk factors for chronic obstructive pulmonary disease: an umbrella review of meta-analyses. Int J Tuberc Lung Dis. 2019;23(1):58–66. doi:10.5588/ijtld.18.0228
137. Duvoix A, Dickens J, Haq I, et al. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax. 2013;68(7):670–676. doi:10.1136/thoraxjnl-2012-201871
138. Zhang Y, Yamamoto T, Hisatome I, et al. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells. Mol Cell Endocrinol. 2013;375(1–2):89–96. doi:10.1016/j.mce.2013.04.027
139. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi:10.7326/0003-4819-155-3-201108020-00008
140. Kahnert K, Alter P, Welte T, et al. Uric acid, lung function, physical capacity and exacerbation frequency in patients with COPD: a multi-dimensional approach. Respir Res. 2018;19(1):110. doi:10.1186/s12931-018-0815-y
141. Li H, Chen Y. Serum uric acid level as a biomarker for chronic obstructive pulmonary disease: a meta-analysis. J Int Med Res. 2021;49(1):300060520983705. doi:10.1177/0300060520983705
142. Kir E, Güven Atici A, Güllü YT, Köksal N, Tunçez H. The relationship between serum uric acid level and uric acid/creatinine ratio with chronic obstructive pulmonary disease severity (stable or acute exacerbation) and the development of cor pulmonale. Int J Clin Pract. 2021;75(8):e14303. doi:10.1111/ijcp.14303
143. Bartziokas K, Papaioannou AI, Loukides S, et al. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur Respir J. 2014;43(1):43–53. doi:10.1183/09031936.00209212
144. Horsfall LJ, Nazareth I, Petersen I. Serum uric acid and the risk of respiratory disease: a population-based cohort study. Thorax. 2014;69(11):1021–1026. doi:10.1136/thoraxjnl-2014-205271
145. Hogea SP, Tudorache E, Fildan AP, Fira-Mladinescu O, Marc M, Oancea C. Risk factors of chronic obstructive pulmonary disease exacerbations. Clin Respir J. 2020;14(3):183–197. doi:10.1111/crj.13129
146. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
147. Cardoso J, Coelho R, Rocha C, Coelho C, Semedo L, Bugalho Almeida A. Prediction of severe exacerbations and mortality in COPD: the role of exacerbation history and inspiratory capacity/total lung capacity ratio. Int J Chron Obstruct Pulmon Dis. 2018;13:1105–1113. doi:10.2147/COPD.S155848
148. Margüello MS, Garrastazu R, Ruiz-Nuñez M, et al. Independent effect of prior exacerbation frequency and disease severity on the risk of future exacerbations of COPD: a retrospective cohort study. NPJ Prim Care Respir Med. 2016;26:16046. doi:10.1038/npjpcrm.2016.46
149. Dixit D, Bridgeman MB, Andrews LB, et al. Acute exacerbations of chronic obstructive pulmonary disease: diagnosis, management, and prevention in critically ill patients. Pharmacotherapy. 2015;35(6):631–648. doi:10.1002/phar.1599
150. Montserrat-Capdevila J, Godoy P, Marsal JR, Barbé F, Galván L. Risk of exacerbation in chronic obstructive pulmonary disease: a primary care retrospective cohort study. BMC Fam Pract. 2015;16(1):173. doi:10.1186/s12875-015-0387-6
151. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi:10.1136/thoraxjnl-2011-201518
152. Osterburg AR, Lach L, Panos RJ, Borchers MT. Unique natural killer cell subpopulations are associated with exacerbation risk in chronic obstructive pulmonary disease. Sci Rep. 2020;10(1):1238. doi:10.1038/s41598-020-58326-7
153. Rao Y, Le Y, Xiong J, Pei Y, Sun Y. NK Cells in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Front Immunol. 2021;12:666045. doi:10.3389/fimmu.2021.666045
154. Suzuki M, Sze MA, Campbell JD, et al. The cellular and molecular determinants of emphysematous destruction in COPD. Sci Rep. 2017;7(1):9562. doi:10.1038/s41598-017-10126-2
155. Hodge G, Mukaro V, Holmes M, Reynolds PN, Hodge S. Enhanced cytotoxic function of natural killer and natural killer T-like cells associated with decreased CD94 (Kp43) in the chronic obstructive pulmonary disease airway. Respirology. 2013;18(2):369–376. doi:10.1111/j.1440-1843.2012.02287.x
156. Pascual-Guardia S, Ataya M, Ramírez-Martínez I, et al. Adaptive NKG2C+ natural killer cells are related to exacerbations and nutritional abnormalities in COPD patients. Respir Res. 2020;21(1):63. doi:10.1186/s12931-020-1323-4
157. Cong J, Wei H. Natural Killer Cells in the Lungs. Front Immunol. 2019;10:1416. doi:10.3389/fimmu.2019.01416
158. Chen YC, Lin MC, Lee CH, et al. Defective formyl peptide receptor 2/3 and annexin A1 expressions associated with M2a polarization of blood immune cells in patients with chronic obstructive pulmonary disease. J Transl Med. 2018;16(1):69. doi:10.1186/s12967-018-1435-5
159. Leitao Filho FS, Won Ra S, Mattman A, et al. Serum IgG and risk of exacerbations and hospitalizations in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2017;140(4):1164–1167.e1166. doi:10.1016/j.jaci.2017.01.046
160. Palikhe NS, Niven M, Fuhr D, et al. Low immunoglobulin levels affect the course of COPD in hospitalized patients. Allergy Asthma Clin Immunol. 2023;19(1):10. doi:10.1186/s13223-023-00762-x
161. Leitao Filho FS, Mattman A, Schellenberg R, et al. Serum IgG Levels and Risk of COPD Hospitalization: a Pooled Meta-analysis. Chest. 2020;158(4):1420–1430. doi:10.1016/j.chest.2020.04.058
162. Jang JH, Kim JH, Park HS. Current Issues in the Management of IgG Subclass Deficiencies in Adults With Chronic Respiratory Diseases. Allergy Asthma Immunol Res. 2023;15(5):562–579. doi:10.4168/aair.2023.15.5.562
163. Leitao Filho FS, Ra SW, Mattman A, et al. Serum IgG subclass levels and risk of exacerbations and hospitalizations in patients with COPD. Respir Res. 2018;19(1):30. doi:10.1186/s12931-018-0733-z
164. Alotaibi NM, Filho FSL, Mattman A, et al. IgG Levels and Mortality in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2021;204(3):362–365. doi:10.1164/rccm.202102-0382LE
165. Lee H, Kovacs C, Mattman A, et al. The impact of IgG subclass deficiency on the risk of mortality in hospitalized patients with COPD. Respir Res. 2022;23(1):141. doi:10.1186/s12931-022-02052-3
166. Ragland MF, Benway CJ, Lutz SM, et al. Genetic Advances in Chronic Obstructive Pulmonary Disease. Insights from COPDGene. Am J Respir Crit Care Med. 2019;200(6):677–690. doi:10.1164/rccm.201808-1455SO
167. Ingebrigtsen TS, Marott JL, Vestbo J, et al. Characteristics of undertreatment in COPD in the general population. Chest. 2013;144(6):1811–1818. doi:10.1378/chest.13-0453
168. Ingebrigtsen TS, Marott JL, Nordestgaard BG, Lange P, Hallas J, Vestbo J. Statin use and exacerbations in individuals with chronic obstructive pulmonary disease. Thorax. 2015;70(1):33–40. doi:10.1136/thoraxjnl-2014-205795
169. Lipson DA, Crim C, Criner GJ, et al. Reduction in All-Cause Mortality with Fluticasone Furoate/Umeclidinium/Vilanterol in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2020;201(12):1508–1516. doi:10.1164/rccm.201911-2207OC
170. Dicker AJ, Crichton ML, Cassidy AJ, et al. Genetic mannose binding lectin deficiency is associated with airway microbiota diversity and reduced exacerbation frequency in COPD. Thorax. 2018;73(6):510–518. doi:10.1136/thoraxjnl-2016-209931
171. Lin CL, Siu LK, Lin JC, et al. Mannose-binding lectin gene polymorphism contributes to recurrence of infective exacerbation in patients with COPD. Chest. 2011;139(1):43–51. doi:10.1378/chest.10-0375
172. Mandal J, Malla B, Steffensen R, et al. Mannose-binding lectin protein and its association to clinical outcomes in COPD: a longitudinal study. Respir Res. 2015;16(1):150. doi:10.1186/s12931-015-0306-3
173. Vogt S, Leuppi JD, Schuetz P, et al. Association of mannose-binding lectin, ficolin-2 and immunoglobulin concentrations with future exacerbations in patients with chronic obstructive pulmonary disease: secondary analysis of the randomized controlled REDUCE trial. Respir Res. 2021;22(1):227. doi:10.1186/s12931-021-01822-9
174. Shi W, Chen F, Cardoso WV. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6(7):558–563. doi:10.1513/pats.200905-031RM
175. Van Durme YM, Eijgelsheim M, Joos GF, et al. Hedgehog-interacting protein is a COPD susceptibility gene: the Rotterdam Study. Eur Respir J. 2010;36(1):89–95. doi:10.1183/09031936.00129509
176. Pillai SG, Kong X, Edwards LD, et al. Loci identified by genome-wide association studies influence different disease-related phenotypes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(12):1498–1505. doi:10.1164/rccm.201002-0151OC
177. Vilaró J, Ramirez-Sarmiento A, Martínez-Llorens JM, et al. Global muscle dysfunction as a risk factor of readmission to hospital due to COPD exacerbations. Respir Med. 2010;104(12):1896–1902. doi:10.1016/j.rmed.2010.05.001
178. Gea J, Agusti A, Roca J. Pathophysiology of muscle dysfunction in COPD. J Appl Physiol. 2013;114(9):1222–1234. doi:10.1152/japplphysiol.00981.2012
179. Keogh E, Mark Williams E. Managing malnutrition in COPD: a review. Respir Med. 2021;176:106248. doi:10.1016/j.rmed.2020.106248
180. Martinez CH, Diaz AA, Meldrum CA, et al. Handgrip Strength in Chronic Obstructive Pulmonary Disease. Associations with Acute Exacerbations and Body Composition. Ann Am Thorac Soc. 2017;14(11):1638–1645. doi:10.1513/AnnalsATS.201610-821OC
181. Liang Y, Sun YC. Nutrition management of patients with acute exacerbation of chronic obstructive pulmonary disease hospitalized in Respiratory Intensive Care Unit. Zhonghua Jie He He Hu Xi Za Zhi. 2022;45(9):920–925. doi:10.3760/cma.j.cn112147-20220422-00342
182. Marco E, Sánchez-Rodríguez D, Dávalos-Yerovi VN, et al. Malnutrition according to ESPEN consensus predicts hospitalizations and long-term mortality in rehabilitation patients with stable chronic obstructive pulmonary disease. Clin Nutr. 2019;38(5):2180–2186. doi:10.1016/j.clnu.2018.09.014
183. Limpawattana P, Putraveephong S, Inthasuwan P, Boonsawat W, Theerakulpisut D, Chindaprasirt J. Frailty syndrome in ambulatory patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1193–1198. doi:10.2147/COPD.S134233
184. Ter Beek L, van der Vaart H, Wempe JB, et al. Coexistence of malnutrition, frailty, physical frailty and disability in patients with COPD starting a pulmonary rehabilitation program. Clin Nutr. 2020;39(8):2557–2563. doi:10.1016/j.clnu.2019.11.016
185. Jingrong LI. Analysis of the prevalence and influencing factors of chronic obstructive pulmonary disease in elderly hospitalized patients: a study based on a Comprehensive Geriatric Assessment System in Yunnan Province. Chinese General Practice. 2022;25(11):1320–1326.
186. Tramontano A, Palange P. Nutritional State and COPD: effects on Dyspnoea and Exercise Tolerance. Nutrients. 2023;15(7):1786. doi:10.3390/nu15071786
187. Yohannes AM, Mülerová H, Lavoie K, et al. The Association of Depressive Symptoms With Rates of Acute Exacerbations in Patients With COPD: results From a 3-year Longitudinal Follow-up of the ECLIPSE Cohort. J Am Med Dir Assoc. 2017;18(11):955–959.e956. doi:10.1016/j.jamda.2017.05.024
188. Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766–777. doi:10.1378/chest.12-1911
189. Holas P, Michalowski J, Gaweda L, Domagala-Kulawik J. Agoraphobic avoidance predicts emotional distress and increased physical concerns in chronic obstructive pulmonary disease. Respir Med. 2017;128:7–12. doi:10.1016/j.rmed.2017.04.011
190. T-P N. Depressive Symptoms and Chronic Obstructive Pulmonary Disease. Arch Intern Med. 2007;167(1):60. doi:10.1001/archinte.167.1.60
191. Huang J, Bian Y, Zhao Y, Jin Z, Liu L, Li G. The Impact of Depression and Anxiety on Chronic Obstructive Pulmonary Disease Acute Exacerbations: a prospective cohort study. J Affect Disord. 2021;281:147–152. doi:10.1016/j.jad.2020.12.030
192. Jenkins CR, Celli B, Anderson JA, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39(1):38–45. doi:10.1183/09031936.00194610
193. Almagro P, Hernandez C, Martinez-Cambor P, Tresserras R, Escarrabill J. Seasonality, ambient temperatures and hospitalizations for acute exacerbation of COPD: a population-based study in a metropolitan area. Int J Chron Obstruct Pulmon Dis. 2015;10:899–908. doi:10.2147/COPD.S75710
194. Li X, Wu Z, Xue M, Du W. An observational study of the effects of smoking cessation earlier on the clinical characteristics and course of acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med. 2022;22(1):390. doi:10.1186/s12890-022-02187-5
195. Hosking L, Yeo A, Hoffman J, et al. Genetics plays a limited role in predicting chronic obstructive pulmonary disease treatment response and exacerbation. Respir Med. 2021;187:106573. doi:10.1016/j.rmed.2021.106573
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
