Back to Journals » Drug Design, Development and Therapy » Volume 17
Analgesic Efficacy of Nalbuphine as an Adjuvant to Ropivacaine in Erector Spinae Plane Block for Percutaneous Nephrolithotomy: A Randomized, Double-Blinded, Clinical Trial
Authors Sun M , Wu Z, Wang R , Xia R, Sun Y, Esmaeili E, Xia Z, Wu Z , Wang T
Received 27 July 2023
Accepted for publication 3 November 2023
Published 14 November 2023 Volume 2023:17 Pages 3385—3395
DOI https://doi.org/10.2147/DDDT.S432600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianbo Sun
Meng Sun,1– 3,* Zhouyang Wu,1– 3,* Rong Wang,1– 3 Rui Xia,1– 3 Yi Sun,1– 3 Elham Esmaeili,1– 3 Zhengyuan Xia,4,5 Zhilin Wu,1– 3 Tingting Wang1– 3
1Department of Anesthesiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 2Institute of Anesthesia and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 3Key Laboratory of Anesthesiology and Resuscitation (Huazhong University of Science and Technology), Ministry of Education, Wuhan, People’s Republic of China; 4State Key Laboratory of Pharmaceutical Biotechnology, The University of Hong Kong, Hong Kong, People’s Republic of China; 5Department of Anesthesiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tingting Wang; Zhilin Wu, Department of Anesthesiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China, Tel +862785351643, Email [email protected]; [email protected]
Background: Erector spinae plane block (ESPB) is an easy and safe method for postoperative analgesia. However its effect lasts only for several hours. This trial was to investigate the effectiveness of different doses of nalbuphine as an adjuvant to ropivacaine in ESPB for patients undergoing percutaneous nephrolithotomy (PCNL).
Methods: Patients scheduled for PCNL were randomized into three groups and received ultrasound-guided ESPB at T10 level for postoperative analgesia. Each subject received 28 mL of 100 mg ropivacaine solution mixed with 2 mL of normal saline (Group R), 2 mL of 10 mg nalbuphine (Group RNL), or 2 mL of 20 mg nalbuphine (Group RNH). Primary outcome was the time to first opioid demand. Secondary outcomes were morphine consumption, VAS scores within 24 h postoperatively, rescue analgesic requirements, and length of hospital stay.
Results: The median [interquartile range, IQR] time to first opioid demand was significantly longer in group RNH (8.70 [6.90,14.85] h) than that of group R and group RNL (2.90 [2.00,6.30] h and 5.80 [2.95,7.00] h, respectively). VAS scores (either resting or active) within 24 h postoperatively were comparable between the three groups, with the most significant differences especially at 4, 6, 8 h. Morphine consumption at 24 h postoperatively was significant for R group vs RNH group (median difference, 9; 95% confidence interval [CI], 1.57 to 16.43; p = 0.02).
Conclusions: Adding 20mg nalbuphine to ropivacaine in ESPB could significantly improve the effect of analgesia and prolong the duration of nerve blocks for PCNL.
Keywords: adjuvant, erector spinae plane block, nalbuphine, percutaneous nephrolithotomy, postoperative pain
Introduction
Percutaneous nephrolithotomy (PCNL) is the most common surgical procedure for addressing kidney stones. Though PCNL is mini-invasive, patients still complain moderate to severe pain after the operation.1 As a crucial component of multimodal analgesia scheme for pain control after PCNL, peripheral nerve blocks become increasingly popular. It was reported that nerve blocks including epidural block,2 paravertebral nerve block,3 transmuscular quadratus lumborum block4 etc. could significantly reduce the consumption of opioids and provide effective analgesia for PCNL patients. However, these operations can be challenging to perform and pose an increased risk of complications, such as pneumothorax and spinal cord injury.5,6
Erector spinae plane block (ESPB) is a commonly used method to provide analgesia for different sites of surgery depending on the spinal levels chosen. It is based on the principle of blocking the ventral and dorsal rami by applying an ultrasound-guided local anesthetic injection between the erector spinae muscle and the transverse process. Compared to the other nerve blocks, the maneuver of advancing the needle tip toward the transverse process under the guidance of an ultrasound in ESPB is easier to perform and brings less complications such as pneumothorax. However, it has been reported that ESPB was effective only in a very short postoperative period,7 which limited its use for analgesia after PCNL.
Nalbuphine, a semi-synthetic opioid with mixed properties of κ receptor agonist and µ receptor antagonist, provides comparable analgesic efficacy to morphine but with fewer opioid-induced adverse effects.8,9 Studies have shown that nalbuphine could be safely used as an adjuvant to prolong the duration of analgesia for subarachnoid blocks,10 epidural blocks11 and peripheral nerve blocks.12,13
Till now, there is few data on nalbuphine for prolonging the effect of ESPB. This was previously explored by Rao et al that 20 mg of nalbuphine as an adjuvant for ESPB can control the onset of acute pain and prolong the duration of sensory blockade after video-assisted thoracoscopic lobectomy surgeries.13 However, due to the scarcity of available studies, it is not known whether the efficacy of nalbuphine as an adjuvant for nerve block is stable. Therefore, this study was designed to investigate the effect of different doses of nalbuphine as an adjuvant to ESPB for patients undergoing PCNL.
Methods
Study Design and Randomization
This prospective, double-blinded, randomized, controlled study was performed after receiving approval from the Ethics Committee of Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology (Approval NO. 20211038-01). The study was registered in the Chinese Clinical Trials Registry on March 14, 2022 (ChiCTR2200057493, https://www.chictr.org.cn/edit.aspx?pid=143147&htm=4) and used a CONSORT flow diagram for the enrollment and allocation of patients (Figure 1). After written informed consent was obtained, the study began on April 1, 2022.
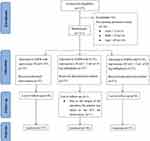 |
Figure 1 Consort flow diagram of patients. |
Patients aged 18–65 years with ASA physical status I–II and scheduled for elective unilateral PCNL in our hospital were recruited. Exclusion criteria were failure to cooperate, allergy to local anesthetics, history of chronic analgesia, BMI>35, and skin infection at the injection site.
Patients were randomly assigned into three different groups according to a computer-generated randomization list, which was concealed in sealed opaque envelopes. Based on the randomization results, patients with ultrasound-guided ESPB at T10 level received 28 mL of 100 mg ropivacaine solution mixed with 2 mL of normal saline (Group R), 2 mL of 10 mg nalbuphine (Group RNL), or 2 mL of 20 mg nalbuphine (Group RNH). The doses of ropivacaine and nalbuphine were chosen in accordance with the published literatures.12,14 All subjects and an investigator who was in charge of aftercare during the first 24 h after surgery were blinded to group allocation.
General Anesthesia and Surgical Technique
On admission, all patients underwent routine monitoring with electrocardiography, pulse oximetry, non-invasive blood pressure, and bispectral index (BIS). Penehyclidine hydrochloride 0.3 mg and tropisetron 5 mg were administered intravenously for secretion reduction, and antiemetic prophylaxis. Each patient was given the same anesthesia induction with a medication scheme of sufentanil 0.5 μg kg−1, propofol 1–2 mg kg−1, and rocuronium 0.6 mg kg−1. After endotracheal intubation, the general anesthesia was maintained with a continuous infusion of propofol 0.5–0.75 mg kg−1 h−1 and remifentanil 30–45 mg kg−1.-1 After general anesthesia, the patient is placed in the lateral position, the skin is disinfected, and an experienced anesthesiologist performs all blocks. Intraoperatively, the remifentanil dosage was adjusted according to the hemodynamic changes of the patient’s heart rate (HR) or mean blood pressure that were not higher than 20% of the baseline value before the start of the procedure. Patients were placed in the prone position and received PCNL surgeries. After the operation, a nephrostomy tube was inserted.
Ultrasound-Guided Erector Spinae Plane Block
Subjects were posed in the lateral decubitus position with a single injection of ESPB at the T10 level of the spine. A linear probe (Wisonic Navis, Shenzhen, China) was placed approximately 3 cm lateral to the midline with a sagittal scan to identify the ultrasound landmarks, which consisted of the T10 transverse process and the erector spinae muscle. Then the ultrasound apparatus’ midline button was turned on to ensure the T10 transverse process is centered in the ultrasound image (Figure 2). Under aseptic conditions, a 22-gauge, 10-cm block needle was inserted at the midpoint of the probe in a cranial-to-caudal direction until the contact of the T10 transverse process. After the position of the needle tip was confirmed with 1–2 mL saline injection, 30 mL of prepared solution was injected. A successful ESPB was considered when local anesthetics diffused in a linear pattern subsurface to the erector spinae muscle (Figure 2).
 |
Figure 2 Technique of ESPB. (A) Anatomical basis of the erector spinae plane block. (B) Ultrasound image taken after the erector spinae plane block (ESPB). |
Postoperative Analgesia Protocol and Rescue Analgesic
After the procedure, subjects were extubated in Post Anesthesia Care Unit (PACU). The time of extubation was defined as 0 h. Postoperative pain management protocol was the same for the three groups. Then a patient-controlled analgesia (PCA) device (BCDB-200; BCM, Shanghai, China) was connected to the venous cannula, and patients were instructed to initiate PCA when the visual analog scale (VAS) score was greater than 3. The PCA device was programmed to administer a standard dose of morphine 5 mg only as needed by the patient.4 It was available with a 20 min lockout time and up to 8 boluses per 4 h.4 A specialized person within the research team was responsible for the preparation of the PCA solution. 50 mg of diclofenac sodium suppositories were prescribed as rescue analgesia when the VAS rest score > 3 despite PCA demand.15
Outcome Measurements
The primary outcome was the time to first PCA opioid bolus during the first 24 h postoperatively. The secondary outcomes included VAS scores at 0, 1, 2, 4, 6, 8, 10, 12, 16 and 24 h postoperatively, cumulative consumption of propofol and remifentanil during surgery, time from arrival in the PACU until first ambulation, rescue analgesic requirement and morphine consumption in the first 24 h postoperatively, time of extubation, time of PACU stay, length of hospital stay, and postoperative adverse effects such as nausea, vomiting and bloating.
Sample Size
The sample size calculation was based on a pilot study that showed the time to first PCA opioid bolus was 4.9 (2.8) h in the Group R, 6.4 (2.1) h in the Group RNH, 5 (2.3) h in the Group RNL. With a difference of 2.1, a power of 80% and a significance level of 0.05, a suitable sample size of 32 patients per group was calculated by PASS 15.0. Considering the anticipated dropouts, we planned to recruit 37 patients in each group.
Statistical Analysis
All analyses were performed with SPSS 22.0 (IBM Corporation, USA). Continuous variables with normal distribution were presented as mean (SD) and analyzed using χ²-tests or analysis of variance (ANOVA). Data following non-normal distribution were described as median (interquartile range) and analyzed using Kruskal–Wallis test. Categorical variables are expressed as number (percentage) and compared using Fisher’s exact test or χ²-test. In addition, pairwise comparisons were evaluated with the Mann–Whitney U-test. Bonferroni correction was used to accommodate multiple comparisons. P < 0.05 was considered statistically significant.
Results
One hundred and eleven subjects were assessed for eligibility. Only one patient was sent to the ICU for observation due to the length of the procedure resulting in a loss to follow-up. Therefore, 110 patients were eventually included in the analysis (Figure 1). The patients’ baseline characteristics and perioperative parameters were similar in the three groups. No significant difference was found between intraoperative propofol and remifentanil consumption (Table 1).
 |
Table 1 Baseline Characteristics of the Patients and Secondary Outcomes, Data Presented as Mean (SD), Number of Patients or Median (IQR) |
The mean (SD) morphine consumption at 24 h postoperatively was significantly different among the three groups (R, 17 [8.19] mg; RNL, 12.22 [5.65] mg; RNH, 8 [4.47] mg; p = 0.045). For the R group vs the RNH group, this difference was statistically significant (median difference, 9; 95% confidence interval [CI], 1.57 to 16.43; p = 0.020), but not for R vs RNL (median difference, 4.8; 95% [CI], −1.29 to 10.85; p = 0.118) and RNL group vs RNH group (median difference, 4.2; 95% [CI], −3.81 to 12.25; p = 0.290). Moreover, fifteen, nine, and five patients in group R, group RNL, group RNH respectively used morphine during 24 postoperative hours (40.5% vs 25% vs 13.5%, p = 0.030). These results indicate that ropivacaine compounded with 20 mg nalbuphine significantly reduces postoperative opioid consumption (Table 2).
 |
Table 2 Postoperative Analgesic Requirements, Data Presented as Mean (SD), Number of Patients or Median (IQR) |
The time to first opioid demand in these three groups was shown in Table 2. This indicator varies across groups (R vs RNL vs RNH; 2.90 [2.00, 6.30] vs 5.80 [2.95, 7.00] vs 8.70 [6.90, 14.85] h, p = 0.023). Time to first opioid demand was longer in RNH group compared to R group and RHL group (R vs RNH, p = 0.006; RNH vs RHL, p = 0.061; R vs RNL, p = 0.375) (Figure 3).
 |
Figure 3 Kaplan-Meier survival plot. Time to first PCA from tracheal tube removal (T0). Kaplan-Meier survival plot of time to first PCA (min), defined as time from T0 until first request for opioid. |
As expected, we found substantial differences in VAS scores between the three groups both at rest and during activity (Figure 4). VAS scores at rest were significantly lower for group RNH compared to group RNL and group R at 4, 6, 8, 10 h postoperatively. However, there was no statistical difference between the group R and group RNL (Table 3). VAS scores during activity showed a great advantage for the RNH group, with the RNH group scoring lower than the R group for the first ten hours postoperatively, and lower than the RNL group at 4, 6, 8 h postoperatively. Besides, VAS scores at 0 and 4 h were also significantly different between the group R and group RNL (p = 0.017, p = 0.021) (Table 3).
 |
Table 3 Pairwise Comparisons of VAS, Values are Median (IQR) and Median Differences with 95% CI |
As indicated in Table 2, the frequency of rescue analgesia during the first 24 hours postoperatively was similar in all groups (p = 0.821). No significant differences were found between the three groups regarding the time of PACU stay, the time to first ambulation, length of hospital stay, time of extubation, and postoperative adverse effects (Table 1).
Discussion
The main finding of this study was that the addition of nalbuphine to 0.33% ropivacaine for ESPB in PCNL patients significantly extended the duration of postoperative analgesia, reduced the consumption of morphine and prolonged the time to first opioid requirement. In addition, it also showed that 20 mg of nalbuphine exhibited a more noticeable effect compared to 10 mg of nalbuphine.
It was reported that EPSB could provide better short-term (1–2 h) and long-term (24 h) pain control in PCNL patients.16 In our study, the median time to first press of PCA was 2.9 h for ESPB with ropivacaine, which was in line with previous researches.16 While the time to first press of PCA was prolonged in the other two groups in which different doses of nalbuphine were used. There was no significant difference in long-term (24 h) VAS scores between the three groups. Previous study conducted by Rao et al substantiated the benefits of nalbuphine as an adjuvant to ropivacaine in ESPB for video-assisted thoracoscopic lobectomy surgery.13 The average duration of sensory blockade in patients who received nalbuphine during video-assisted thoracoscopic lobectomy surgery was found to be 14.1 hours, which exhibited a significant increase of 5.2 hours compared to the ropivacaine group. Interestingly, the results of this experiment showed that resting VAS scores were significantly lower in the nalbuphine group at 24 h after surgery. This disparity in outcomes may be attributed to variations in the concentration of ropivacaine employed, as Rao et al opted for a concentration of 0.5%. In addition, the type of surgery may also have an influence on pain scores, with video-assisted thoracoscopic lobectomy surgery causing a worse pain experience for patients compared to PCNL. Based on the aforementioned experiments, it can be inferred that the utilization of ESPB with nalbuphine as an adjunctive medication has the potential to provide extended pain relief for patients undergoing PCNL.
The safety and efficacy of nalbuphine as an adjuvant to peripheral nerve blocks has been well established.17,18 Clinically, the commonly used doses of nalbuphine are 10 mg and 20 mg,18,19 and no study has yet explored the appropriate dosage of nalbuphine as an adjuvant in ESPB. Kalika et al found that nalbuphine, as an adjunct to ropivacaine in brachial plexus blocks, provided durable analgesia and reliable sensory blockade.19 They found that 10 mg nalbuphine was a better dosing agent compared to 20 mg nalbuphine, providing similar analgesia with fewer adverse effects such as nausea and vomiting.19 However, a 20 mg dose of nalbuphine seems to be more supportive in our study. VAS scores at rest were significantly lower in the 20 mg nalbuphine group than in the 10 mg group (4, 6, 8, 10 h postoperatively). The time to first PCA press was also significantly longer in the 20 mg nalbuphine group. The different effect in our results may be ascribed to the diverse mechanisms of the nerve blocks. As Kalika et al described, nalbuphine was applied precisely to brachial plexus, thus the drug worked directly to the nerve roots. While ESPB is a fascial plane block that relies on the penetration of local anesthetic in the muscle to block the nerve traveling within it. It is reasonable that a large dose of nalbuphine may be more beneficial for postoperative analgesia than a small dose of nalbuphine. An obvious side effect of nalbuphine is nausea and vomiting. However, with prophylactic antiemetic treatment, there were no differences between these groups. The time to ambulation and length of hospital stay were also similar. This might be explained by the fact that patients in our hospital were asked to stay in bed for at least two days after PCNL to decrease the risk of bleeding and ensure a safe discharge from the hospital.
The minimally invasive channels established during PCNL are usually in the 10 th and 11 th intercostal space or subcostal region, and their nociceptive innervations are derived from T10-12 spinal nerves. The visceral sensory nerves that transmit renal and urethral pain are T10-L1 and T10-L2, respectively.20 Previous literature has shown that local anesthetics in ESPB can spread 3 to 4 paravertebral spaces cephalad and caudal to block the transduction of visceral and somatic pain.21 Therefore, T10 transverse process was selected as the target of ESPB in this study. Theoretically, ESPB performed in the T10 plane can provide adequate analgesia for PCNL. However, the effect of ESPB lasts for only several hours, which has greatly limited its wide application. The acting mechanism of ESPB is controversial. Schwartzmann et al supported the view that local anesthetics injected in ESPB could leak into the paravertebral space and act on the ventral and dorsal branches of the spinal nerve roots.22,23 However, an opposite perspective was held by Ivanusic et al whose cadaveric studies showed that local anesthetics in ESPB failed to penetrate into the paravertebral space and the anterior branches of the intercostal nerves could not be blocked.24 Local anesthetic diffusing into the paravertebral space is also limited, which contributes to the fact that the duration of ESPB is inferior to that of paravertebral block (PVB).15,25 However, ESPB is a relatively easy approach with fewer complications than PVB, which requires a more skilled and experienced anesthesiologist to perform.
Continuous ESPB is a useful technique to prolong the effect of analgesia. Finneran et al carried out continuous ESPB in patients undergoing PCNL postoperatively.26 All five patients who received continuous ESPB demonstrated excellent analgesia with minimal or no supplementation of oral opioid analgesics. However, this approach needs an extra time to be completed. In addition, the risk of infection, catheter knitting, kinking or even being broken should not be ignored. In this study, single ESPB with a mixture of nalbuphine and ropivacaine achieved a significantly prolonged duration of analgesia but also effectively avoided the risks of continuous ESPB.
There are several mechanisms for the enhanced effect of ESPB with nalbuphine. First, nalbuphine is an opioid with a mixed effect of complete κ-receptor agonist and partial μ-receptor antagonist.27 The activation of κ receptor produces significant analgesic efficacy for visceral pain, which is the chief derivative of post-PCNL pain. Secondly, opioid receptors are present in peripheral neurons. Surgical and inflammatory stimulation can induce the upregulation of opioid receptors at peripheral nerve terminals. And nalbuphine binds to opioid receptors in peripheral nerves that allow for increasing the synthesis of opioid receptors in the dorsal root ganglion neurons and enhancing the analgesic effect of ESPB.28–30 Another potential mechanism might be explained by the direct action of nalbuphine on spinal opioid receptors through penetration into the paravertebral space. In our study, by adding nalbuphine to ropivacaine, the duration of ESPB was significantly prolonged, which remained to be effective at 12 h postoperatively. With its simplicity and safety of performance, ESPB with adjuvant of nalbuphine showed great promises for postoperative analgesia in PCNL patients.
Some limitations in this study should be taken into account. First, we chose to perform ESPB on patients after general anesthesia in order to alleviate their discomfort and panic about the block, so we were unable to assess the extent of dermatomal sensory block to confirm successful ESPB. Second, the optimal concentration and volume of ropivacaine for unilateral ESPB are still controversial. It was also suggested by Tsui et al that 0.5% ropivacaine with a volume of 10–30 mL was a good choice of local anesthetic concentration for unilateral ESPB.14 Whether a higher concentration of ropivacaine with nalbuphine could further extend the effect of analgesia needs to be explored. Third, the blood concentration of nalbuphine was not determined. The possibility that systemically absorbed nalbuphine prolongs the duration of analgesia and improves the analgesic effect should be considered. The exact pathway through which nalbuphine acts may need to be clarified with an additional group of intravenous nalbuphine injections, and this is an area where future experiments could be further refined.
Conclusion
Nalbuphine as an adjuvant to ropivacaine in ESPB was effective for pain relief in patients undergoing PCNL. A dose of 20 mg nalbuphine for ESPB could significantly extend the duration of analgesia, and contribute to the reduction of postoperative opioid consumption. Our results showed that the single injection of a mixture of nalbuphine and local anesthetic for ESPB could be a simple method for postoperative analgesia in PCNL patients.
Code Availability
The sample size was calculated with PASS 15.0, and the statistical analyses were performed using SPSS 22.0 software.
Data Sharing Statement
The data set used during the current study is available from the corresponding authors upon reasonable request.
Ethics Approval
This trial was performed after receiving approval from the Ethics Committee of Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology (Approval NO. 20211038-01). This study was conducted in accordance with the declaration of Helsinki.
Consent to Participate
Written informed consents were obtained from all patients.
Consent for Publication
All authors read and approved the final manuscript and were in agreement with the content of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (NO. 81770824), Bethune Charitable Foundation (NO. bnmr-2022-009), and Natural Science Foundation of Hubei Province (NO. 2020CFB797).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Wang J, Zhang C, Tan D, et al. The effect of local anesthetic infiltration around nephrostomy tract on postoperative pain control after percutaneous nephrolithotomy: a systematic review and meta-analysis. Urol Int. 2016;97(2):125–133. doi:10.1159/000447306
2. Kamal M, Sharma P, Singariya G, Jain R. Feasibility and complications of spinal anaesthesia in percutaneous nephrolithotomy: our experience. J Clin Diagn Res. 2017;11(6):Uc08–uc11. doi:10.7860/JCDR/2017/26425.10111
3. Baldea KG, Patel PM, Delos Santos G, et al. Paravertebral block for percutaneous nephrolithotomy: a prospective, randomized, double-blind placebo-controlled study. World J Urol. 2020;38(11):2963–2969. doi:10.1007/s00345-020-03093-3
4. Dam M, Hansen CK, Poulsen TD, et al. Transmuscular quadratus lumborum block for percutaneous nephrolithotomy reduces opioid consumption and speeds ambulation and discharge from hospital: a single centre randomised controlled trial. Br J Anaesth. 2019;123(2):e350–e358. doi:10.1016/j.bja.2019.04.054
5. Cook TM, Counsell D, Wildsmith JA. Major complications of central neuraxial block: report on the third national audit project of the royal college of anaesthetists. Br J Anaesth. 2009;102(2):179–190. doi:10.1093/bja/aen360
6. Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade. Incidence of failed block and complications. Anaesthesia. 2001;56(12):1184–1188. doi:10.1046/j.1365-2044.2001.02084-2.x
7. Bryniarski P, Bialka S, Kepinski M, Szelka-Urbanczyk A, Paradysz A, Misiolek H. Erector spinae plane block for perioperative analgesia after percutaneous nephrolithotomy. Int J Environ Res Public Health. 2021;18(7):3625. doi:10.3390/ijerph18073625
8. Zeng Z, Lu J, Shu C, et al. A comparision of nalbuphine with morphine for analgesic effects and safety: meta-analysis of randomized controlled trials. Sci Rep. 2015;5(1):10927. doi:10.1038/srep10927
9. Mao Y, Cao Y, Mei B, et al. Efficacy of nalbuphine with flurbiprofen on multimodal analgesia with transverse abdominis plane block in elderly patients undergoing open gastrointestinal surgery: a randomized, controlled, double-blinded trial. Pain Res Manag. 2018;2018:3637013. doi:10.1155/2018/3637013
10. Chetty DK, Ahmed F, Chatterjee R, Rathore M. Comparison of intrathecal nalbuphine hydrochloride and clonidine hydrochloride as an adjuvant to hyperbaric bupivacaine in abdominal hysterectomy. Anesth Essays Res. 2018;12(2):402–406. doi:10.4103/aer.AER_5_18
11. Babu S, Gupta BK, Gautam GK. A comparative study for post operative analgesia in the emergency laparotomies: thoracic epidural ropivacaine with nalbuphine and ropivacaine with Butorphanol. Anesth Essays Res. 2017;11(1):155–159. doi:10.4103/0259-1162.186593
12. Ren Y, Liu H, Wang Y, Fu X, You F. Efficacy of nalbuphine as an adjuvant to ropivacaine in ultrasound-guided supraclavicular brachial block: a prospective randomized controlled study. Clin J Pain. 2021;37(2):158–159. doi:10.1097/AJP.0000000000000899
13. Rao J, Gao Z, Qiu G, et al. Nalbuphine and dexmedetomidine as adjuvants to ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Medicine. 2021;100(32):e26962. doi:10.1097/MD.0000000000026962
14. Tsui BCH, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae plane (ESP) block: a pooled review of 242 cases. J Clin Anesth. 2019;53:29–34. doi:10.1016/j.jclinane.2018.09.036
15. Chen N, Qiao Q, Chen R, Xu Q, Zhang Y, Tian Y. The effect of ultrasound-guided intercostal nerve block, single-injection erector spinae plane block and multiple-injection paravertebral block on postoperative analgesia in thoracoscopic surgery: a randomized, double-blinded, clinical trial. J Clin Anesth. 2020;59:106–111. doi:10.1016/j.jclinane.2019.07.002
16. Ma Y, Lin L, Xiao K, Luo Z, Jin T. Efficiency and safety of erector spinae plane block in percutaneous nephrolithotomy: a meta-analysis based on randomized controlled trials. Urology. 2022;168:64–71. doi:10.1016/j.urology.2022.07.017
17. Borah TJ, Dey S, Yunus M, Dev P, Karim HMR, Bhattacharyya P. Effect of different doses of intrathecal nalbuphine as adjuvant to ropivacaine in elective lower limb surgeries: a dose finding study. Indian J Anaesth. 2018;62(11):865–870. doi:10.4103/ija.IJA_278_18
18. Jiang C, Xie W, Xie H, Xie W, Kang Z, Liu N. Nalbuphine exhibited a better adjuvant than dexmedetomidine in supraclavicular brachial plexus block in youths. Clin Neuropharmacol. 2020;43(5):134–138. doi:10.1097/WNF.0000000000000410
19. Kalika P, Xue R, Zheng J, Xiao Y, Zhen M, Ran R. Efficacy of nalbuphine as an adjuvant to ropivacaine in ultrasound-guided supraclavicular Brachial block: a prospective randomized controlled study. Clin J Pain. 2020;36(4):267–272. doi:10.1097/AJP.0000000000000803
20. Liu Y, Yu X, Sun X, et al. Paravertebral block for surgical anesthesia of percutaneous nephrolithotomy: care-compliant 3 case reports. Medicine. 2016;95(28);e4156. doi:10.1097/MD.0000000000004156
21. Chin KJ, Adhikary S, Sarwani N, Forero M. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia. 2017;72(4):452–460. doi:10.1111/anae.13814
22. Schwartzmann A, Peng P, Maciel MA, Forero M. Mechanism of the erector spinae plane block: insights from a magnetic resonance imaging study. Can J Anaesth. 2018;65(10):1165–1166. doi:10.1007/s12630-018-1187-y
23. Bonvicini D, Boscolo-Berto R, De Cassai A, et al. Anatomical basis of erector spinae plane block: a dissection and histotopographic pilot study. J Anesth. 2021;35(1):102–111. doi:10.1007/s00540-020-02881-w
24. Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. 2018;43(6):567–571. doi:10.1097/AAP.0000000000000789
25. Vogt A, Stieger DS, Theurillat C, Curatolo M. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesth. 2005;95(6):816–821. doi:10.1093/bja/aei250
26. Finneran JJ, Alexander B, Bechis SK, Sur RL, Ilfeld BM. Continuous erector spinae plane blocks with automated boluses for analgesia following percutaneous nephrolithotomy. Korean J Anesthesiol. 2021;74(2):178–180. doi:10.4097/kja.20398
27. Larson PC, Miller’s anesthesia. Anesth Analg. 2010;1110:263–265. doi:10.1213/ane.0b013e3181b7c799
28. Stein C, Yassouridis A. Peripheral morphine analgesia. Pain. 1997;71(2):119–121. doi:10.1097/00006396-199706000-00001
29. Likar R, Koppert W, Blatnig H, et al. Efficacy of peripheral morphine analgesia in inflamed, non-inflamed and perineural tissue of dental surgery patients. J Pain Symptom Manage. 2001;21(4):330–337. doi:10.1016/S0885-3924(01)00251-2
30. Sehgal N. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011;14(3):249–258. doi:10.36076/ppj.2011/14/249
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

