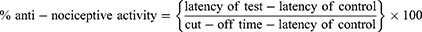Back to Journals » Drug Design, Development and Therapy » Volume 16
Analagesic and Anti-Inflammatory Potentials of a Less Ulcerogenic Thiadiazinethione Derivative in Animal Models: Biochemical and Histochemical Correlates
Authors Rahman K, Ali G , Khan R, Khan I, Ali I, Mosa OF, Ahmed A , Ayaz M , Nawaz A, Murthy HCA
Received 18 December 2021
Accepted for publication 31 March 2022
Published 21 April 2022 Volume 2022:16 Pages 1143—1157
DOI https://doi.org/10.2147/DDDT.S354779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Khista Rahman,1 Gowhar Ali,1,2 Rasool Khan,3 Imad Khan,3 Izaz Ali,1 Osama F Mosa,4,5 Alshebli Ahmed,4,6 Muhammad Ayaz,7 Asif Nawaz,7 HC Ananda Murthy8
1Department of Pharmacy, University of Peshawar, Peshawar, Pakistan; 2The Ken and Ruth Davee Department of Neurology, Department of Neurology and Clinical Neurosciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA; 3Institute of Chemistry Sciences, University of Peshawar, Peshawar, Pakistan; 4Public Health Department,Health Sciences College at Lieth, Umm Al Qura University, Makkah, Kingdom of Saudi Arabia; 5Biochemistry Department Bukhara State Medical Institute named after Abu Ali ibn Sino, Bukhara, Uzbekistan; 6University of Khartoum, Faculty of Public and environmental Health, Khartoum, Sudan; 7Department of Pharmacy, Faculty of Biological Sciences, University of Malakand, Chakdara, 18000, Dir (L), KP, Pakistan; 8Department of Applied Chemistry, School of Applied Natural Science, Adama Science and Technology University, Adama, Ethiopia
Correspondence: Gowhar Ali; HC Ananda Murthy, Email [email protected]; [email protected]
Purpose: Gastric ulcer induced by NSAIDs is the major medical concern and researchers are utilizing several approaches to combat this medical issue. In the current study, we investigated the efficacy of thiadiazinethione derivative (2,2’(2-thioxo-1,3,5-thiadiazinane-3,5-diyl) diacetic acid, as new less ulcerogenic compound.
Methods: 2,2’(2-thioxo-1,3,5-thiadiazinane-3,5-diyl) diacetic acid was evaluated using standard animal models including hot plate, writhing test and formalin induced nociceptive models. Anti-inflammatory activity was assessed via carrageenan-induced paw oedema model. Involvement of opioidergic nociceptive mechanism was confirmed via naloxone administration in hot plat assay. The gastro-ulcerogenic potential of test and standard compounds were evaluated via NSAID-induced pyloric ligation model followed by standard histopathological and biochemical analysis.
Results: In acetic acid-induced writhing test, our compound significantly reduced abdominal constrictions at the tested doses of 15 (p < 0.05), 30 (p < 0.01) and 45 mg kg− 1 (p < 0.001) as compared to control (p < 0.001). In hot plate test, after 30 min of administration, our test compound showed significant anti-nociceptive potential (p < 0.05 at 15 and 30 mg kg− 1 and p < 0.01 at 45 mg kg− 1) and tramadol (p ˂ 0.001) at 30 mg kg− 1 dose. After 60 min tramadol (30 kg− 1) and test sample (30, 45 mg kg− 1) exhibited significant anti-nociceptive activity p < 0.001. In Formalin-induced nociceptive response, a significant decline (p ˂ 0.001) was observed for aspirin and test compound during acute and chronic phases. Decline in the anti-nociceptive potential of tramadol and test sample via administration of naloxone indicate the involvement of opioidergic mechanism. Our compound exhibited significant anti-inflammatory activity in second phase of carrageenan induced paw oedema model. Histological and biochemical parameters exhibited less ulcerogenic potential as compared to aspirin.
Conclusion: Our findings suggests that our test compound has desirable anti-nociceptive and anti-inflammatory potentials with less propensity to cause gastric ulcer.
Keywords: NSAIDs, nociception, inflammation, gastric ulcer, hot plate
Introduction
Inflammation is the protective response of the vascularised tissues to a harmful stimuli which can be triggered by a number of noxious stimuli, including injury and infection, affecting normal function of tissues.1,2 Inflammation is among the major causes for the development of different diseases including cardiovascular diseases, cancer, asthma, diabetes, obesity, rheumatoid arthritis, osteoporosis, inflammatory bowel disease, and CNS related diseases including depression and parkinson’s disease.3 Anti-inflammatory drugs include nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, Disease-modifying antirheumatic drugs (DMARDs) and other nutraceuticals.4 Non-steroidal anti-inflammatory drugs (NSAIDs) are the most commonly used drugs throughout the world for the management of pain and inflammation.4,5 Unfortunately, prolong use of NSAIDs is associated with undesirable effects upon gastric mucosa including gastric mucosal injury.6 The gastric ulcerogenic side effects of NSAIDs if not treated appropriately may cause gastric ulcer leading to severe problems like perforation and bleeding.7 Various clinical practice guidelines have suggested strategies for the management of NSAID-induced gastric injury. American College of Gastroenterology recommended a number of strategies to reduce NSAID-induced gastric injury including the use of COX-2 selective inhibitors, co-administration of gastro protective medicines like PPIs, misoprostol and histamine-2 receptor blockers.8 Yet, these alternatives have limited efficacy and are associated with side effects.7
There is no complete cure till now to address effectively NSAID-induced gastric injury and discovery of more effective drugs is serious challenge.9,10 Consequently, development of a new therapeutic agent that have anti-inflammatory and anti-nociceptive activities as well as no or less gastric ulcerogenic effects is an overwhelming need of the present age. Therefore, a search for finding new compounds which are free from gastric ulcerogenic effects of NSAIDs is in progress.
The thiadiazine thione nucleus and its derivatives have get special focus because of its wide spectrum of biological activities most importantly anti-bacterial, anti-fungal, anti-tubercular, anthelmintic, leishmanicidal, anti-mycobacterial and anti-viral.11 It is also been used for the treatment of atherosclerosis. Recently, its antiepileptic pro-drug applications has also been reported. This diverse heterocycle has found many applications in the design of drug delivery systems in the research area as a bio-labile pro-drug because of its lipid solubility and high enzymatic hydrolysis. Another important beneficial effect of this class is their stability in gastric fluid, which enhance their absorption in a less ionized form. The excellent physic-chemical properties of this class delivered its use in many essential projects for new bioactive drug development.12 In the past few decades there are remarkable advances occur in the synthesis and investigation of the pharmaceutical and biomedical characteristics of the thiadiazine thione moiety, therefore this research study is designed to investigate anti-nociceptive, anti-inflammatory and ulcerogenic potential of our selected compound.
Methodology
Apparatus and Chemicals
Hot plate analgesiometer (Harvard apparatus, USA), digital plethysmometer (Model LE 7500 Plane lab S.L), microtome (SLEE Mainz Cut 5062, Germany) and compound microscope (Labomed L×400 USA) linked to a digital camera (Labomedivu 3100), were used in our study. Acetyl salicylic acid (Sigma, USA), bovine serum albumin (Sigma, USA), lambda carrageenan (sigma, USA), glacial acetic acid (Panreac, Spain), formaldehyde (Merck, Germany) and ketamine hydrochloride from (global pharmaceuticals) were obtained. All solvents and chemicals used were of analytical standard.
Experimental Animals
Sprague Dawley rats (150–200 gm each) and albino mice (18–30 gm) were utilized in our experiments. Six animals were included in each group. These experimental rats and mice were nurtured in the animal house and bioassay laboratory of pharmacy department, university of Peshawar. Standardized laboratory foods and water ad libitum were provided to animals. Temperature was maintained at 22°C in the room with 12 by 12 hours light and dark cycle. All experimental procedures were carried out according to the animals scientific procedure Act 1986 UK.13,14
Ethical Approval
The research work was approved by the ethical committee of faculty of life and environmental sciences university of Peshawar under a project entitled “Investigation of gastroulcerogenic potential of 2,2’(2-thioxo-1,3,5-thiadiazinane-3,5-diyl) diacetic acid: in vivo and histopathological approach in form number 415/EC/F.LIFE/UOP-2021.
Acute Toxicity Test
The acute toxicity test was conducted to evaluate the safe dose range of selected synthetic compound. Mice either male or female were arranged in six groups and the test compound were injected intraperitoneally in the dose ranges from 25 to 1000 mg kg−1 body weight (six animals were used for each dose). Animals were observed for 2 hours initially and then up to 24 hours for any abnormal behaviour like ataxia, aggressiveness, writhing, cyanosis, tail punch response, righting reflex, catalepsy, convulsions and bizarre behaviour.15,16
Doses Selection: (15, 30 and 45 mg kg−1 doses justification)
After acute toxicity test, the therapeutic doses (15, 30 and 45 mg kg−1) of the test compound were selected according to the previously reported method.17 The therapeutic effects was shown at 15 mg kg−1 dose, therefore we selected 15 mg kg−1 as a lowest dose, then the dose was doubled and tripled to further study the compound effects.
In-Vivo Anti-Nociceptive Studies
Writhing Test Induced by Acetic Acid
Albino mice either male or female having weight in the range of 18–22 g were utilized for this activity. Food was removed from animals two hours before to the beginning of experiment. Acetic acid (1%) at dose of 10 mL kg−1 was injected intraperitoneally to induce writhing behaviour. Randomly, the mice were arranged into five groups and six mice were included in every group.18
Group-I: Saline-treated group
Group-II: Aspirin (standard) treated group (45 mg kg−1)
Group-III: Selected compound (test) treated group (15 mg kg−1)
Group-IV: Selected compound (test) treated group (30 mg kg−1)
Group-V: Selected compound (test) treated group (45 mg kg−1)
Test compound, aspirin standard drug and normal saline were administered via intraperitoneal route 30 min before 1% acetic acid injection. Post 5 min of acetic acid injection, number of writhing were counted for twenty min.19,20
Hot Plate Test
Albino mice were used in the weight range of 18–22 g. Hot plate analgesiometer was set at temperature of 54±0.1°C. Food was withdrawn two hours before to make the start of each experiment. Mice were tested before drug administration on a hot plate for their response latencies. The response latencies were indicated by hind limb licking, flicking or jumping from cylinder. When the response shows the animals were removed instantly from the surface of hot plate for the purpose to prevent tissue damage. A cut-off time of 30 seconds was enforced, the mice were removed instantly from the hot plate stimulus if they did not show response within 30 seconds. Animals were randomly selected and at least 6 animals were included in every group.21
Group-I: Normal saline treated group
Group-II: Tramadol treated (standard) group (30 mg kg−1)
Group-III: Selected compound (test) treated group (15 mg kg−1)
Group-IV: Selected compound (test) treated group (30 mg kg−1)
Group V: Selected compound (test) treated group (45 mg kg−1)
Group VI: Naloxone treated group (1mg kg−1)
Post thirty min of pre-testing, the mice were administered with the test compound and tramadol through intraperitoneal route and hot plate response time was observed after 30 min, 60 min and 90 min with the help of hot plate analgesiometer. To study antagonism, naloxone (1 mg kg−1) was administered via subcutaneous route 10 min before administration of test compound and tramadol.22 Finally, anti-nociceptive activity (%) was determined via formula;
Formalin Induced Nociceptive Model
Albino mice either male or female having weight in the range of 25–30 g were utilized for this study. Randomly, the mice were arranged into five groups and six mice were included in every group as per following details.23
Group-I: Saline-treated group
Group-II: Aspirin treated (standard) group (45 mg kg−1)
Group-III: Selected compound (test) treated group (15 mg kg−1)
Group-IV: Selected compound (test) treated group (30 mg kg−1)
Group-V: Selected compound (test) treated group (45 mg kg−1)
Test compound, standard drug aspirin and normal saline were given via oral route. After 60 min of standard drug, normal saline and test compound administration, 20 μL of 2.5% formalin solution freshly prepared was injected by sub planter route to induce pain. The animals were observed in 2 phases for nociceptive action like licking, biting and lifting of paw. For first five min in acute phase and for 15–30 min in chronic phase.
Carrageenan-Induced Paw Oedema Model
Albino mice either male or female having weight in the range of 25–30 g were used for this model. The mice were fasted during night and were provided with water. Mice were arranged into five groups randomly and six mice were included in each group.24
Group-I: Saline-treated group
Group-II: Aspirin treated (standard) group (50 mg kg−1)
Group-III: Selected compound (test) treated group (15 mg kg−1)
Group-IV: Selected compound (test) treated group (30 mg kg−1)
Group-V: Selected compound (test) treated group (45 mg kg−1)
Test compound, standard drug and normal saline were injected intraperitoneally. After thirty min of drugs administration, 1% carrageenan (0.05 mL) was intravenously administered into the hind paw of right side via sub planter route. Digital plethysmometer was used for the measurement of paw volume before carrageenan administration and after carrageenan administration at 1, 2, 3, 4 and 5 hours’ intervals respectively.25 Extent of paw oedema was measured via increase in paw volume (V0 – Vt) where V0 is the volume of paw prior to carrageenan injection at zero hour and Vt is the volume of paw after carrageenan injection at 1, 2, 3, 4 and 5 hours.26
Comparative Gastric Ulcerogenecity Study on Aspirin and Test Compound
In this study Sprague Dawley rats were utilized in the weight range of 150–200 g. Randomly, the rats were arranged into five groups and six rats were included in every group. Treatment details including administration route and doses are encapsulated in Table 1.
 |
Table 1 Treatment Details of Animals Groups |
Administration of Drugs and Surgical Procedures
Test compound, standard drug (aspirin) and normal saline were administered orally through feeding tube once daily for six continues days. The last doses were given to thirty-six hour fasted animals before 60 min to a pyloric ligation procedure under 50 or 100 mg kg−1 ketamine anaesthesia. In this procedure animals were anaesthetized via ketamine injection (50 or 100 mg kg-1). Then cautiously the stomach was exposed and with the help of surgical silk, pyloric sphincter was ligated instantly preventing damage to blood vessels and then with the help of sutures the abdominal wall was reclosed before recovery. After recovery water and food were provided for four hours to accumulate gastric juice. The animals were then killed through cervical dislocation and their stomachs were exposed again. At the oesophageal end a knot was applied to prevent discharge of gastric juice. Gastric juice were collected for biochemical studies and stomach samples were taken for histological and macroscopic examination.27,28
Macroscopic Examination of Gastric Mucosa
The stomach tissues were exposed along the greater curvature to examine score of ulceration with the help of 10X magnifying glass. Score of ulcer was determined by the method reported previously.27 The mean score of ulcer was calculated for all animals as given below in Table 2.
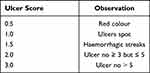 |
Table 2 Interpretation Criteria of Gastric Ulceration Score |
Gastric Juice Biochemical Analysis
Gastric juice was obtained from each stomach and the volume were measured through graduated cylinder. The gastric juice was then centrifuged for ten min at a speed of 1000 rpm at room temperature. After centrifugation, the supernatant was isolated for the determination of total acidity, free acidity, pH and pepsin activity. The pH of gastric juice was measured through digital PH meter. Free acidity was measured by adding 2–3 drops of Topfer’s reagent to 1 mL of supernatant and mix well. For the titration of mixture 0.01N NaOH solution was utilized. When the light red color of the mixture changed to canary yellow it shows the end point. The amount of alkali volume consumed in changing color of the mixture indicated free acidity and calculated with the help of the formula given below.27
Free acidity = NaOH volume × N × 100 mEq/0.1
N denotes NaOH normality. Total acidity of the gastric juice was determined by mixing 2 drops phenolphthalein indicator to the above mixture and then the mixture was titrated against 0.1N sodium hydroxide until the color changes to red color. The alkali volume utilized substituted in the above equation indicate total acidity.27
Gastric juice pepsin concentration was determined by mixing gastric juice (0.1 mL) with bovine serum albumin solution (1 mL), blended in 0.01 N HCL and subjected to incubation for 20 min at 37°C. After incubation, 10% trichloroacetic acids was incorporated to the tubes and heated for 5 min in the water bath for the purpose to precipitate the undigested proteins. The tubes were then allowed to cool and subsequently the tubes were centrifuged for 20 min at 4500 rpm. Then isolated the supernatant from all the tubes. Folin’s reagent (0.1 mL) and sodium hydroxide (0.4 mL) was added to 0.1 mL of supernatant. Final volume was made 10 mL by the addition of distilled water. Then the absorbance of pepsin was examined by UV spectrophotometer at 700 nm with reference to mg/dl BSA or tyrosine/mL.27
Comparative Histological Assessment of Ulcerogenecity
For Histological assessment, the stomach tissues were rinsed with normal saline to remove the debris. Then the gastric tissues were fixed in 10% solution of formalin (neutrally buffered) for forty eight hours. The gastric tissues in ethanol graded solutions (50, 70, 80, 90, 2 changes each of 100%), in automatic tissue processor were dehydrated. Then the gastric tissues were cleared in 2 changes one by one of 100% xylene. The tissues were then infiltrated and embedded in paraffin wax. After this the gastric tissues were sectioned at 4µm through rotary microtome and then were adhered to the slides. The tissues were then stained with harris hematoxyllin and eosin for observation via microscope. The changes observed in gastric histology were scored as (-) for no changes, (+) for mild changes, (++) for moderate changes and (+++) for severe changes.29
Statistical Analysis
In order to determine the statistical significance, the data was analyzed by graph pad version 5 by suitable post hoc test after applying one-way ANOVA analysis to all sets of data. The p-value less than 0.05 was deemed as statistically significant. Data was expressed as mean ± SEM (n=six).
Results
Acute Toxicity
In acute toxicity test the compound (Figure 1) was evaluated in 6 groups of mice at various increasing doses of 25, 50, 100, 200, 500 and 1000 mg kg−1. The test compounds up to 500 mg kg−1 dose did not show any mortality or abnormal behavioral changes while abnormal behavioral changes and mortality was observed at higher dose (1000 mg kg−1). Hence it was concluded that the test compound was safe up to 500 mg kg−1 dose and was toxic at a dose of 1000 mg kg−1.
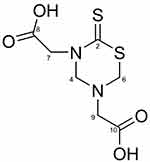 |
Figure 1 Chemical structure of 2,2’(2-thioxo-1,3,5-thiadiazinane-3,5-diyl) diacetic acid. |
Comparative Anti-Nociceptive Outcomes in Acetic Acid-Induced Writhing Paradigm
Acetic acid (1%) induced abdominal constrictions via intraperitoneal injection were significantly decreased with the administration of our sample at the tested doses of 15, 30 and 45 mg kg−1. Standard drug aspirin at 45 kg−1 significantly decreased number of writhing (Figure 2).
Ani-Nociceptive Potentials Using Hot Plate Paradigm
In hot plate test, after thirty min of test compound and tramadol administration, tramadol showed significant anti-nociceptive potential (p ˂ 0.001) at 30 mg kg−1 dose and test compound also demonstrated significant anti-nociceptive potential with p-value less than 0.05 at 15 and 30 mg kg−1 dose and with p-value less than 0.01 at 45 mg kg−1 dose. Post sixty min tramadol showed significant anti-nociceptive activity with p-value less than 0.001 at 30 kg−1dose and test compound at the dose of 30 and 45 mg kg−1 with p-value less than 0.001 also showed significant anti-nociceptive potential while test compound at 15 mg kg−1 dose the anti-nociceptive effect was not shown significantly. After 90 minutes tramadol (30 mg kg−1 dose) and test compound (45 mg kg−1 dose) showed significant anti-nociceptive effect with p-value less than 0.001 and 0.01 respectively, whereas test compound at 15 and 30 mg kg−1 doses were not significantly shown anti-nociceptive effect (Figure 3).
Anti-Nociceptive Potential of Test Compound and Aspirin in Formalin-Induced Nociception Model
Formalin (2.5%) induced nociceptive response via sub planter route was significantly decreased by aspirin (45 mg kg−1 dose) and selected test compounds (15, 30 and 45 mg kg−1 doses) in both acute and chronic phase and shows significant anti-nociceptive activity (p ˂ 0.001) (Figure 4).
Antagonism Result for the Determination of Opioidergic Mechanism Using Naloxone
For the determination of proposed mechanism of action, the test compound was evaluated by using naloxone as antagonist to determine the possible opioidergic receptors involvement. Thermally induced nociceptive model (Hot plate analgesiometer) was used for this study. Naloxone reduced the anti-nociceptive potential of tramadol (30 mg kg−1) significantly with p-value less than 0.001. Similarly, naloxone also reduced the anti-nociceptive potential of our sample at the doses of 30 and 45 mg kg−1 with p-value less than 0.05. The decrease in nociceptive response of test compounds (30 and 45 mg kg−1 doses) gives some idea about the test compound opioidergic mechanism (Figure 5).
Anti-Inflammatory Study Using Carrageenan-Induced Paw Edema Model
During 1st and 2nd hour of the aspirin (standard drug) at 50 mg kg−1 dose and selected test compound at 15, 30 and 45 mg kg−1 administration, no significant decline in the paw volume of mice was observed. During 3rd, 4th and 5th hour of the study, aspirin (standard drug) at 50 mg kg-1 dose and selected test compound at various doses (15, 30, 45 mg kg−1) reduced the paw volume significantly (p ˂0.001). Hence it suggests that during 1st and 2nd hour of the study both aspirin and test compound did not show anti-inflammatory activity while after 3, 4 and 5 hour of the study both aspirin and test compound at all selected doses reveals significant anti-inflammatory effects (Figure 6).
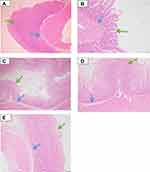
Effect of Test Sample on Gross Appearance of Gastric Mucosa
In aspirin treated group, significant changes were observed like ulcer spots, red coloration, and hemorrahagic streaks while these changes were not observed in saline group and test compound (15, 30 mg kg−1) treated groups. However, hemorrhagic streaks were observed in test compound 45 mg kg−1 treated group (Figure 7).
Effect of Test Compound and Aspirin on Biochemical Parameters
The effect of Aspirin, test compound and normal saline on biochemical parameters of gastric juice such as total acidity, free acidity, pH, volume, and pepsin concentration were observed among all groups. Aspirin (150 mg kg−1), test compound (15, 30 and 45 mg kg−1) and normal saline were given orally (OD) for six consecutive days. Final dose was administered to thirty six hour fasted animals in all groups prior to one hour of pyloric ligation procedure. After 4 hours of the surgery, all the animals were killed and the gastric juice were collected and then checked for volume (Figure 8A), pH (Figure 8B), free acidity (Figure 8C), total acidity (Figure 8D) and pepsin concentration (Figure 8E).
Effect of Aspirin and Test Compound on Histology of Stomach
Histological examination shows normal mucosal epithelial lining in the saline treated as well as sample treated groups. However, disruption and erosion of mucosal epithelial lining along with inflammatory cells was observed with the aspirin treated group. The slides were prepared and examined through microscope and histopathological changes in gastric tissues were noted as negative (-) for no changes, (+) for mild changes, (++) for moderate changes and (+++) for severe changes. The results were shown below in the Table 3. There were no changes observed in normal saline control group. Significant histopathological changes were observed in aspirin treated group. In test compound treated groups there were no major changes observed in gastric tissue. The photomicrographs of the glandular portion of the gastric tissues 4X after saline, aspirin and test compound treatment is shown below in (Figure 9A–E).
 |
Table 3 Effect of Treatment Groups on Histopathological Changes |
Discussion
NSAIDs are among the most extensively used anti-inflammatory, antipyretic and analgesic medicines. However, it has been reported that 25% of patients who takes NSAIDs experience long term upper GIT adverse effects.30 NSAID-induced gastrointestinal toxicity particularly gastric ulceration is still an important medical and socioeconomic issue.31 It has been accepted broadly that the main mechanism of NSAIDs is cyclooxygenase enzyme inhibition required for prostaglandin synthesis and also responsible for the major undesirable effect of gastric mucosal injury. COX-1 and COX-2 being the two isoforms of cyclooxigenases are implicated in the production of gastric-friendly prostaglandins and pain-pyrexia respectively. COX-1 isoform has involved in the production of gastric mucus and has mainly cytoprotective effects while COX-2 is expressed during inflammatory response.32 Most of the NSAIDs inhibit COX-1 and COX-2 enzymes non-selectively which results in a useful effect on pain and inflammation but exhibit potential harmful effect on GIT33.
In order to overcome the NSAID-induced gastric problems there is dire need for the discovery of novel analgesic and anti-inflammatory drugs having gastroprotective profiles. Keeping in mind the importance of thiadiazinethione nucleus in biomedical research, this study was designed to investigate the 2,2’-(2-thioxo-1,3,5-thiadiazinane-3,5-diyl)diacetic acid for its anti-inflammatory and anti-nociceptive potentials and to uncover its additional benefits of being less gastric ulcerogenic at our selected doses. During initial acute toxicity phase, our selected test compound was found safe and was subsequently subjected to anti-inflammatory and anti-nociceptive studies. The compound was evaluated at the dose range of 25–1000 mg kg−1 to assess the safety range of the selected test compound. The acute toxicity test result shows that the selected test compound up to 500 mg kg−1 dose was safe and toxic at the dose of 1000 mg kg−1. Finally, the compound was investigated for any potential gastric ulcerogenecity linked with our selected anti-inflammatory and anti-nociceptive doses.
For the investigation of anti-nociceptive activity of test compound, we selected three standard models of pain. The acetic acid induced writhing test is a sensitive method utilized for the assessment of peripherally acting anti-nociceptive compounds or drugs such as NSAIDs. Intraperitoneally injection of acetic acid causes an abdominal constriction response because of the chemoreceptors sensitization by prostaglandins. This test has been related with enhanced prostaglandins level in peritoneal fluids specifically PGE2 and PGEα and also lipoxygenase products. This increases inflammatory pain by enhancing capillary permeability.34 Acetic acid induced writhing test result shows that our selected test compound was effective at all selected doses and aspirin at 45 mg kg−1 dose decreased the number of writhing significantly. Thermally induced pain by hot plate is specific for centrally acting pain. Thereby using this model, the prolonging of reaction latency of mice to thermally induced pain implies centrally mediated anti-nociceptive activity.34 Compound that enhance the mice latency on the hot plate suggests that it possess centrally acting activity that is comparable to that of opioids.35 Our findings of the hot plate test revealed that tramadol at 30 mg kg−1 dose shows significant protection and our selected test compound at all doses shows a considerable protection against hot plate induced nociception. The formalin (2.5%) nociceptive test is used frequently to confirm the involvement of central and peripheral analgesic activities of a new investigational compound. The mice after an intra plantar injection of 2.5% formalin immediately produce abnormal repetitive behaviour such as biting or liking of injected paw. The nociceptive response can be observed in 2 phases. The acute phase for first 5 min (neurogenic mechanism) due to activation of nociceptors directly by formalin and second phase (inflammatory) occurs after formalin injection at 15 to 30 min due to the release of prostaglandins, bradykinin and histamine. Centrally acting drugs for example opioids can inhibit pain in first phase while both peripherally and centrally acting drugs can suppress pain in second phase.35 Results of formalin induced nociception test shows that our selected test compound at all doses and aspirin at 45 mg kg−1 dose decreased the effects of formalin (2.5%) injection in both phases of the study proposing that the compound can act on both inflammatory and non-inflammatory pathways. Cumulatively all the three models of nociception result show that the compound has both centrally and peripherally acting anti-nociceptive activity and also attenuates both inflammatory and non-inflammatory pain.
In order to find out the opioidergic mechanism of selected test compound, naloxone was administered to mice before tramadol and selected test compound administration. Naloxone can reverse the anti-nociceptive effect of opioids such as tramadol. Naloxone antagonism result shows that naloxone reversed the anti-nociceptive effect of tramadol significantly and notably decreased the anti-nociceptive effect of our selected test compound at 30 and 45 mg kg−1 doses which indicates that opioidergic mechanism might be involved.
Inflammation is a mammalian living tissues local response to injury. It is a defence reaction of our body in order to limit or eliminate the spread of injurious agent. Several components can contribute to the associated tissue injury and symptoms in an inflammatory reaction. These components are granuloma formation, leukocyte infiltration and oedema. Even though inflammation is a defence mechanism, the complex mediators and events involved in the inflammatory response can aggravate or induce many reactions.36 In order to investigate anti-inflammatory activity of selected test compound we selected standard model of inflammation namely carrageenan induced paw oedema model. Carrageenan induced paw oedema model is usually utilized for assessing the anti-inflammatory activities of new drugs or compounds. Inflammation induced by carrageenan is biphasic, in first phase the release of leukotrienes, serotonin, histamine and kinins occurs in the first hour of the carrageenan administration and the late phase has been associated to production of prostaglandins, bradykinin and neutrophil infiltration.37 In the current study, results of the carrageenan induced paw oedema test shows that our selected test compound at all doses and aspirin at 50 mg kg−1 dose significantly decreased paw oedema volume after 3, 4 and 5 hours. Our findings suggest that the anti-inflammatory potential of selected test compound may be due to the cyclooxygenase synthesis inhibition and this effect is same to the effect that produced by NSAIDs such as aspirin.
Gastric ulcer is caused due to the imbalance between mucosal disruption factors including long term use of NSAIDs and mucosal protective factors such as prostaglandins level, anti-oxidant enzymes activity. This imbalance results in the disruption of the defensive barrier of gastric mucosa and leads to gastric ulcer.38 For the evaluation of gastric ulcerogenecity, the test compound was evaluated in NSAID-induced pyloric ligation model. The ulcerative effect of the test compound was evaluated in comparison with aspirin. The ulcer score result shows that there were no major changes observed in the gross morphology of stomach such as ulcer spots, red coloration and haemorrhagic streaks in the normal saline control group and test compound at the dose of 15 and 30 mg kg−1 groups. Whereas, hemorrhagic streaks were observed in test compound (45 mg kg−1) treated group. However, visible ulcer spots were seen in the stomach sample of aspirin treated group. The result of gastric juice volume shows significant increase in the gastric juice volume in aspirin (150 mg kg−1) and test compound (45 mg kg−1) treated groups while no significant increase in the gastric juice volume occur in test compound (15 and 30 mg kg−1) treated groups. The result of gastric juice pH shows significant increase in gastric juice pH in aspirin 150 mg kg−1 and test compound 45 mg kg−1 treated groups while no notable increase in gastric juice pH occur in test compound 15 mg kg−1 and 30 mg kg−1 groups. The result of gastric juice free acidity shows notable increase in gastric juice free acidity in aspirin 150 mg kg−1 and test compound 45 mg kg−1 treated groups while no notable increase in gastric juice free acidity occur in test compound 15 mg kg−1 and 30 mg kg−1 groups. The result of gastric juice total acidity shows considerable increase in gastric juice total acidity in aspirin 150 mg kg−1 and test compound 30 and 45 mg kg−1 treated groups while no considerable increase in gastric juice total acidity occur in test compound 15 mg kg−1 treated group. The result of pepsin concentration shows that the concentration of pepsin is not effected in any of the treated group. The result of histopathological changes shows that there were no significant histopathological changes observed in gastric mucosa such as mucosal disruption in normal saline control group and test compound treated group at all the three doses. However, aspirin treated group shows disruption of gastric mucosa. Cumulatively ulcerogenic study result shows that the test compound at our selected doses has low tendency to cause gastric ulcer as compared to aspirin.
Conclusion
The 2.2-(2-thioxo-1,3,5-thiadiazinane-3,5-diyl)diacetic acid was selected for the purpose of investigating its anti-inflammatory and anti-nociceptive potentials and its gastroulcerogenic tendency. Our findings suggest that our selected compound has considerable anti-nociceptive potentials possibly mediated through the involvement of opioidergic mechanism. The test compound depicted desirable profile in selected standard animal models of pain, inflammation and less tendency to cause gastric ulcer when compared with aspirin.
Funding
There is no funding to report.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Okin D, Medzhitov R. Evolution of inflammatory diseases. Curr Biol. 2012;22(17):R733–R740. doi:10.1016/j.cub.2012.07.029
2. Akbar S, Subhan F, Shahid M, et al. 6-Methoxyflavanone abates cisplatin-induced neuropathic pain apropos anti-inflammatory mechanisms: a behavioral and molecular simulation study. Eur J Pharmacol. 2020;872:172972. doi:10.1016/j.ejphar.2020.172972
3. Laveti D, Kumar M, Hemalatha R, et al. Anti-inflammatory treatments for chronic diseases: a review. Inflamm Allergy Drug Targets. 2013;12:349–361.
4. Vane J. The mode of action of aspirin and similar compounds. J Allergy Clin Immunol. 1976;58(6):691–712. doi:10.1016/0091-6749(76)90181-0
5. Jan MS, Ahmad S, Hussain F, et al. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2, 5-dione derivatives as multitarget anti-inflammatory agents. Eur J Med Chem. 2020;186:111863. doi:10.1016/j.ejmech.2019.111863
6. Taha AS, Hudson N, Hawkey CJ, et al. Famotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal antiinflammatory drugs. N Engl J Med. 1996;334(22):1435–1439. doi:10.1056/NEJM199605303342204
7. Tamaddonfard E, Erfanparast A, Farshid AA, et al. Safranal, a constituent of saffron, exerts gastro-protective effects against indomethacin-induced gastric ulcer. Life Sci. 2019;224:88–94. doi:10.1016/j.lfs.2019.03.054
8. Lanza FL, Chan FK, Quigley EM, et al. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–738.
9. Jan MS, Shahid M, Ahmad S, et al. Synthesis of pyrrolidine-2, 5-dione based anti inflammatory drug: in vitro COX-2, 5-LOX inhibition and in vivo anti-inflammatory studies. Latin Am J Pharm. 2019;38:2287–2294.
10. Sinha M, Gautam L, Shukla PK, et al. Current perspectives in NSAID-induced gastropathy. Mediators Inflamm. 2013;2013:1–11. doi:10.1155/2013/258209
11. Shah MIA, Khan R, Arfan M, et al. Synthesis, in vitro urease inhibitory activity and molecular docking of 3, 5‐disubstituted thiadiazine‐2‐thiones. J Heterocycl Chem. 2019;56(11):3073–3080. doi:10.1002/jhet.3705
12. Rodríguez H, Suárez M, Albericio F. Thiadiazines, N, N-heterocycles of biological relevance. Molecules. 2012;17(7):7612–7628. doi:10.3390/molecules17077612
13. Akbar S, Subhan F, Karim N, et al. 6-Methoxyflavanone attenuates mechanical allodynia and vulvodynia in the streptozotocin-induced diabetic neuropathic pain. Biomed Pharmacother. 2016;84:962–971. doi:10.1016/j.biopha.2016.10.017
14. Tong X, Li X, Ayaz M, et al. Neuroprotective studies on Polygonum hydropiper L. essential oils using transgenic animal models. Front Pharmacol. 2020;11:2290.
15. Ahmad N, Subhan F, Islam NU, et al. Gabapentin and its salicylaldehyde derivative alleviate allodynia and hypoalgesia in a cisplatin-induced neuropathic pain model. Eur J Pharmacol. 2017;814:302–312. doi:10.1016/j.ejphar.2017.08.040
16. Saleem U, Akhtar R, Anwar F, et al. Neuroprotective potential of Malva neglecta is mediated via down-regulation of cholinesterase and modulation of oxidative stress markers. Metab Brain Dis. 2021;36(5):889–900. doi:10.1007/s11011-021-00683-x
17. Khan J, Ali G, Rashid U, et al. Mechanistic evaluation of a novel cyclohexenone derivative’s functionality against nociception and inflammation: an in-vitro, in-vivo and in-silico approach. Eur J Pharmacol. 2021;902:174091. doi:10.1016/j.ejphar.2021.174091
18. Mahmood F, Ali R, Jan MS, et al. Chemical characterization and analgesic potential of Notholirion thomsonianum extract. Lat Am J Pharm. 2019;38:807–812.
19. Abbas M, Subhan F, Mohani N, et al. The involvement of opioidergic mechanisms in the activity of Bacopa monnieriextract and its toxicological studies. Afr J Pharmacy Pharmacol. 2011;5:1120–1124.
20. Fawad K, Islam NU, Subhan F, et al. Novel hydroquinone derivatives alleviate algesia, inflammation and pyrexia in the absence of gastric ulcerogenicity. Trop J Pharm Res. 2018;17(1):53–63. doi:10.4314/tjpr.v17i1.9
21. Sadiq A, Zeb A, Ullah F, et al. Chemical characterization, analgesic, antioxidant, and anticholinesterase potentials of essential oils from Isodon rugosus Wall. ex. Benth. Front Pharmacol. 2018;9:623. doi:10.3389/fphar.2018.00623
22. Aman U, Subhan F, Shahid M, et al. Passiflora incarnata attenuation of neuropathic allodynia and vulvodynia apropos GABA-ergic and opioidergic antinociceptive and behavioural mechanisms. BMC Complement Altern Med. 2016;16(1):1–17. doi:10.1186/s12906-016-1048-6
23. Zeb A, Ahmad S, Ullah F, et al. Anti-nociceptive activity of ethnomedicinally important analgesic plant Isodon rugosus Wall. ex Benth: mechanistic study and identifications of bioactive compounds. Front Pharmacol. 2016;7:200. doi:10.3389/fphar.2016.00200
24. Ahmad S, Mahnashi MH, Alyami BA, et al. Synthesis of michael adducts as key building blocks for potential analgesic drugs: in vitro, in vivo and in silico explorations. Drug Des Devel Ther. 2021;15:1299. doi:10.2147/DDDT.S292826
25. Ali G, Subhan F, Wadood A, et al. Pharmacological evaluation, molecular docking and dynamics simulation studies of salicyl alcohol nitrogen containing derivatives. Afr J Pharmacy Pharmacol. 2013;7(11):585–596. doi:10.5897/AJPP12.893
26. Ratnayake W, Suresh T, Abeysekera A, et al. Acute anti-inflammatory and anti-nociceptive activities of crude extracts, alkaloid fraction and evolitrine from Acronychia pedunculata leaves. J Ethnopharmacol. 2019;238:111827. doi:10.1016/j.jep.2019.111827
27. Ali G, Subhan F, Islam NU, et al. Synthetically modified bioisosteres of salicyl alcohol and their gastroulcerogenic assessment versus aspirin: biochemical and histological correlates. Naunyn-Schmiedeb Arch Pharmacol. 2014;387(3):281–290. doi:10.1007/s00210-013-0941-5
28. Akhtar MF, Mehal MO, Saleem A, et al. Attenuating effect of Prosopis cineraria against paraquat-induced toxicity in prepubertal mice, Mus musculus. Environ Sci Pollut Res. 2021:1–17. doi:10.1007/s11356-020-11060-z
29. Shahid M, Subhan F, Ullah I, et al. Beneficial effects of Bacopa monnieri extract on opioid induced toxicity. Heliyon. 2016;2(2):e00068. doi:10.1016/j.heliyon.2016.e00068
30. Chan F, Graham D. Prevention of non‐steroidal anti‐inflammatory drug gastrointestinal complications–review and recommendations based on risk assessment. Aliment Pharmacol Ther. 2004;19(10):1051–1061. doi:10.1111/j.1365-2036.2004.01935.x
31. Becker JC, Domschke W, Pohle T. Current approaches to prevent NSAID‐induced gastropathy–COX selectivity and beyond. Br J Clin Pharmacol. 2004;58(6):587–600. doi:10.1111/j.1365-2125.2004.02198.x
32. Díaz‐González F, Sánchez‐Madrid F. NSAIDs: learning new tricks from old drugs. Eur J Immunol. 2015;45(3):679–686. doi:10.1002/eji.201445222
33. Traoré O, Diarra AS, Kassogué O, et al. The clinical and endoscopic aspects of peptic ulcers secondary to the use of nonsteroidal anti-inflammatory drugs of various origins. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.170.17325
34. Rege MG, Ayanwuyi LO, Zezi AU, et al. Anti-nociceptive, anti-inflammatory and possible mechanism of anti-nociceptive action of methanol leaf extract of Nymphaea lotus Linn (Nymphaeceae). J Tradit Complement Med. 2020;11(2):123–129. doi:10.1016/j.jtcme.2020.02.010
35. Ayumi RR, Mossadeq WMS, Zakaria ZA, et al. Antinociceptive activity of Asiaticoside in mouse models of induced nociception. Planta Med. 2020;86(08):548–555. doi:10.1055/a-1144-3663
36. Amdekar S, Roy P, Singh V, et al. Anti-inflammatory activity of lactobacillus on carrageenan-induced paw edema in Male Wistar Rats. Int J Inflam. 2012;2012:1–6. doi:10.1155/2012/752015
37. Gupta AK, Parasar D, Sagar A, et al. Analgesic and anti-inflammatory properties of gelsolin in acetic acid induced writhing, tail immersion and carrageenan induced paw edema in mice. PLoS One. 2015;10(8):e0135558. doi:10.1371/journal.pone.0135558
38. Zhou D, Yang Q, Tian T, et al. Gastroprotective effect of gallic acid against ethanol induced gastric ulcer in rats: involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed Pharmacother. 2020;126:110075.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.