Back to Journals » Journal of Inflammation Research » Volume 16
An Unusual Case of Systemic Lymphadenopathy - Kimura’s Disease
Authors Liu Y , Liu S , Xu J, Xu X, Wang M
Received 23 November 2022
Accepted for publication 25 January 2023
Published 18 February 2023 Volume 2023:16 Pages 701—705
DOI https://doi.org/10.2147/JIR.S397470
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Yongchang Liu,1 Shichang Liu,2 Jia Xu,3 Xiaocheng Xu,3 Meiyun Wang3
1Department of Vascular Surgery, Affiliated Hangzhou First People’s Hospital, ZheJiang University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China; 2Department of Pharmacy, Weifang Mental Health Center, Weifang, Shandong Province, People’s Republic of China; 3Department of Oncology, The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, Zhejiang Province, People’s Republic of China
Correspondence: Meiyun Wang, Department of Oncology, The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, Zhejiang Province, People’s Republic of China, Email [email protected]
Background: Kimura’s disease (KD) is a rare, chronic inflammatory disease. Clinically, subcutaneous nodules of the head and neck are typical manifestations, often accompanied by local lymphadenopathy or salivary gland enlargement, but there is also systemic damage, such as kidney involvement. Due to the lack of specific markers and imaging examination is not specific, it is difficult to clinically diagnose accurately and can be easy to misdiagnose. The treatment of KD is still not standardized and overtreatment can affect the quality of life.
Case Presentation: The case of a 26-year-old man complaining of chest pain with self-conscious progressive lymphadenopathy after receiving Pfizer BioNTech COVID-19 vaccine for more than 1 month is presented. Eosinophil levels were normal and IgE elevated and the final diagnosis of KD was eventually confirmed by lymph node biopsy, which revealed lymphadenopathy with extensive eosinophilic infiltration in the right neck. Treatment was prednisone combined with methotrexate, resulting in satisfactory control.
Conclusion: This case demonstrates that that Kimura disease can involve systemic lymphadenopathy, not only in the head and face or regional lymphadenopathy, suggested that KD should be excluded in patients with systemic lymphadenopathy. The current patient’s response to treatment suggested that corticosteroid combined with disease-modifying antirheumatic drugs (DENARDs) was a promising treatment for KD patients with systemic damage. It is worth noting that the mechanism of immunity in the pathogenesis of KD still needs to be further studied.
Keywords: Kimura’s disease, lymphadenopathy, COVID-19 vaccine, granulomatous disease, lymphadenopathy
Introduction
The condition of KD is a rare chronic inflammatory disorder, whose main pathological feature is subcutaneous angioblastic lymphoid hyperplasia with peripheral eosinophilia. In China, it was first described as eosinophilic hyperplastic lymphogranuloma by Kim and Szeto in 1937. Kimura et al in 1948 described the details of the pathological features of the disease and named it KD in Japan, finding that it tended to occur in Asian men and the overall male to female ratio was 4:1.1
Typical clinical manifestations included painless subcutaneous swelling of the head and neck, with or without local lymphadenopathy, involvement of salivary glands and kidneys and the incidence of head and neck KD was as high as 73.9%.2,3 Laboratory tests showed eosinophilia and immunoglobulin E (IgE) increase in peripheral blood.4 Histopathology was the gold standard for diagnosis, including marked lymphoid follicular hyperplasia with enlargement of germinating centers, eosinophilic infiltration, accumulation of eosinophilic microabscesses, proliferation of lymphocytes and mast cells around capillaries and venules, with surrounding deposition of circular collagen fibers and varying degrees of fibrosis.5
The cause of Kimura disease is still unknown, but inflammation, fungal and viral infections, allergic reactions, autoimmune and endocrine diseases have been considered as potential causes and helper T cell proliferation and cytokine overproduction play important roles in disease progression.6,7 Due to the rarity of the disease and its high recurrence rate, this benign inflammatory disease is difficult to identify and diagnose and is easily misdiagnosed as parotid gland tumors, lymphoma and other diseases and can result in inappropriate, excessive or delayed treatment.
This study presented an unusual case of a patient with KD characterized by generalized lymphadenopathy as the main clinical manifestation. The basic characteristics, laboratory examination, imaging data and pathological features of the patient were described in detail, to improve the understanding of KD, broaden the differential diagnosis of multiple lymphadenopathy and provide evidence for its treatment.
Case Report
A 26-year-old Chinese male was admitted to hospital due to recurrent chest pain with by self-conscious lymphadenopathy for over 1 month and the aggravation lasted for 3 weeks. The patient was diagnosed with Hodgkin’s lymphoma by lymph node puncture pathological examination in the UK and was admitted after returning to China for further diagnosis and treatment. He was otherwise healthy, with no other significant relevant medical, personal history or family history. There was a history of receiving a third dose of the Pfizer BioNTech COVID-19 vaccine in the UK on January 21, 2022 and had a long history of staying up late and falling asleep past 3am every night for one month before and after vaccination.
After admission, examination of the right neck detected four swollen lymph nodes, with more of similar size in the left neck, arm pits and groin. The patient had poor mobility, no sternal tenderness, and normal respiratory sounds. No positive findings were found in any other physical examination.
Laboratory analysis showed normal levels of liver and kidney function, serum lactate dehydrogenase, coagulation factors, antinuclear antibodies, antiglomerular basement membrane antibody, antibody against cyclocitrullin, immunoglobulin G subtype 4, antineutrophil cytoplasmic antibody, immunoglobulin, complement, Coomb’s test, rheumatoid factor, antistreptococcus hemolysin O, male tumor markers, viral hepatitis test and thyroid function. Elevated factors included thyroglobulin antibody TG-I (12.07 IU/ML) and thyroid TPO antibody I (197.43 IU/ML). The complete blood count showed elevated white blood cell (WBC) levels of 10.63×109, neutrophil count of 7.53×109, elevated levels of C-reactive protein (CRP) of 36.1 mg/L, erythrocyte sedimentation rate (ESR) of 58 mm/h and IgE of 119KU/L. The procalcitonin (PCT), T lymphocyte and NK cell levels were normal, while the number of B lymphocytes at 66M/L was low (90 to 323M/L). Virology tests indicated increased levels of cytomegalovirus IgG (18.68U/mL) and herpes simplex virus type I IgG (573.48AU/mL), but Epstein-Barr and rubella virus were normal. Urinalysis was normal without proteinuria.
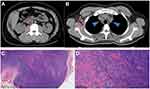
Imaging diagnosis using normal B ultrasound of liver, bile, pancreas, spleen and kidneys was normal. Chest and abdominal computed tomography (CT) showed multiple enlarged lymph nodes in the retroperitoneum (Figure 1A), right axilla and mediastinum (Figure 1B), the largest of which was 2.0 by 1.8 cm.
Histopathological examination of lymph node biopsy tissue from the right neck revealed the lymph node structure is preserved and the subcortical area is dominated by Lymphoid follicular hyperplasia (Figure 1C) with background eosinophil deposition. The paracortical area between the germinal center and the follicles has marked high endothelial venules with angiogenesis, infiltration of plasma cells and eosinophils, and localized fibrosis (Figure 1D). Immunohistochemistry and molecular detection: a small amount of CD20+, CD21(+), PAX-5(part +), CD7(part +), MUM-1(part +), CD3(+), CD30(-), and Ki-67 (40%). In situ hybridization showed EBER(-).
Gene scanning showed that lymphoma gene rearrangement correlation tests detected the presence of IGH A, IGH B, IGH C, IGH D, IGH E, IGK A, IGK B, IGL, TCRB TubeA, TCRB TubeB, TCRB TubeC, TCRG TubeA and TCRG TubeB, while TCRD was polyclonal.
Based on the above patient’s history and according to morphology, immunohistochemistry and molecular detection, KD was diagnosed.
The patient was treated with Prednisone at a starting dose of 0.5mg/kg/day combined with methotrexate 10 mg/day for 1 month, supplemented with calcium and folic acid.
Following that treatment, his laboratory analysis showed that ESR, CRP and IgE were reduced to normal and the neck, axillary, and inguinal lymph nodes were undetectable on physical examination. Color ultrasound of neck, axilla and groin showed no abnormality and no lymph node enlargement was detected.
Discussion
Kimura’s disease, also known as eosinophilic lymphogranuloma, is a rare granulomatous disease with unknown etiology and chronic progression. Most of the reported lesions are asymmetrically distributed in the head and neck and 30 to 40% of the cases also have regional lymphadenopathy.6,8
This study reported a case of Kimura’s disease, characterized by systemic lymphadenopathy without facial and salivary gland involvement, recent COVID-19 vaccination and extremely mild clinical presentation, and it was diagnosed as Hodgkin’s lymphoma in the UK, which was easily misdiagnosed. After extensive literature review, this phenotype has not been reported in the past.
The pathogenesis of KD remains a mystery, but known allergic reactions, candida infections, insect bites, eosinophil and immunoglobulin E (IgE) disorders and alterations in systemic immune regulation are all considered potential causes of KD. In recent years, increasing attention has been paid to the relationship between immune factors and KD, with a previous study showing that the levels of IgE, type 2 helper T (Th2) cytokines such as interleukin-4, IL-5, IL-13 and thymic stromal lymphopoietin (TSLP) are elevated in the peripheral blood or affected tissues of KD patients.9 Some cases of rare immune thrombocytopenic purpura (ITP) have been related to the deterioration of Kimura’s disease10 and some scholars proposed that Kimura’s disease was related to adolescent temporal arteritis (JTA) and reviewed the relevant literature. Forty-eight JTA cases were analyzed and six cases were found to have Kimura’s disease. Histopathological examination showed marked eosinophilic infiltration with marked intimal hyperplasia and vascular lumen narrowing.11 Watanabe believed that juvenile temporal arteritis was a manifestation of Kimura disease and provided detailed pathological evidence to further demonstrate and clarify his views.12
This patient had a clear history of Pfizer BioNTech COVID-19 vaccine prior to onset and had a long history of staying up late at this stage. It is well known that vaccination and staying up late can affect the immune system in the body, for example, T cells are induced to multiple viral proteins following infection and that T cells possessing anti- viral signatures associated with safety and protection and can achieved by vaccination (13), which further confirmed that immune factors may be involved in the pathogenesis of KD. But, on the other hand, the association of COVID-19 immunization with this disease is unclear because most people worldwide were vaccinated against COVID-19 (more than 60%). Therefore, the specific mechanism remains to be further studied.
The characteristics of Kimura disease are often confusing and overlap with other solid diseases, the most common of which is angiolymphoid hyperplasia with eosinophilia (ALHE). Both diseases tend to affect the head and neck, presenting clinically as subcutaneous masses with lymphoid infiltration, eosinophils and angioplasia, but ALHE occurs in middle-aged women, presenting as multiple small papules or erythema nodules with pruritus. Kimura disease has renal involvement in 10 to 60% of patients with nephrotic syndrome,13 whereas ALHE rarely presents systemically.14,15 It was confirmed that KD and IgG4RD have overlapping clinical, pathological and immunohistological features and their correlation with clinicopathological images are necessary for differential diagnosis. Overlapping features also suggest a close relationship between KD and IgG4RD, which may represent various aspects of the fibroinflammatory disease spectrum.16 Other studies used single-cell RNA sequencing, in situ sequencing and immunofluorescence polychromatic analysis to detect B cells, T follicular (Tfh) cells and infiltrating type 2 cells in the pathological tissues of patients with KD or IGG4-RD and found that many LAG3+ Tfh cells expressing il-10 were found in IGG4-RD patients, but Tfh cells of IL-13 were located in the affected tissues of KD patients, showing that il-10 +LAG3+ Tfh cells were distributed in the affected organs of IGG4-RD patients. The KD patient tissues were also infiltrated by IL-13+ Tfh cells and type 2 immune cells.17
These studies can help understand the pathogenesis of IGG4-RD and KD and at the same time improve clinical differential diagnosis and diagnostic ability. Other differential diagnoses include reactive lymphadenopathy, Kikuchi disease, hemangioma, lymphangioma, hemangioma, Mikulicz’s disease, lymphoma, and salivary gland tumors and histopathology was required to differentiate the diagnosis.
Currently, KD has no standard diagnosis and treatment and various treatment methods have been reported, including surgery, which is only suitable for localized lesions but the reporting rate of recurrence is still about 60%.18 Chemotherapy, corticosteroids, cytotoxic drugs and monoclonal antibodies were the main medical treatment methods. However, due to the disease, many patients were treated in different departments, so the drugs used (methotrexate, leflunomide, azathioprine, cyclosporine, etc.) and dose were different.19–23 It is believed that with the understanding of Kimura disease, biological agents will also be possible to become the main means to control the disease.24 This patient had systemic lymphadenopathy and was treated with prednisone combined with methotrexate to achieve satisfactory results. This appears to be a promising treatment for KD patients with systemic damage, with tolerable adverse reactions, low toxicity and no effect on quality of life.
This case had systemic lymphadenopathy. There was a clear history of Pfizer BioNTech COVID-19 and staying up late for a long time, so the effects of immune system should be considered as a trigger for the development of KD. This case demonstrates that that Kimura disease can involve multiple lymph node enlargement, not only in the head and face or regional lymphadenopathy. Laboratory examination of eosinophils can be normal and biopsy of the lesion site is the ultimate method to determine the nature of the lesion.
Consent Statement
The patient provided informed consent to publish their case details and any accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kakehi E, Kotani K, Otsuka Y, et al. Kimura’s disease: effects of age on clinical presentation. QJM. 2020;113(5):336–345. doi:10.1093/qjmed/hcz312
2. Wang X, Ma Y, Wang Z. Kimura’s Disease. J Craniofac Surg. 2019;30(5):e415–e418. doi:10.1097/SCS.0000000000005430
3. Kung IT, Gibson JB, Bannatyne PM. Kimura’s disease: a clinico-pathological study of 21 cases and its distinction from angiolymphoid hyperplasia with eosinophilia. Pathology. 1984;16(1):39–44. doi:10.3109/00313028409067909
4. Kok KYY, Lim ECC. Kimura’s disease: a rare cause of chronic neck lymphadenopathy. J Surg Case Rep. 2021;2021(7):rjab318. doi:10.1093/jscr/rjab318
5. Zhang G, Li X, Sun G, Cao Y, Gao N, Qi W. Clinical analysis of Kimura’s disease in 24 cases from China. BMC Surg. 2020;20(1):1. doi:10.1186/s12893-019-0673-7
6. Muniraju M, Dechamma S. Kimura’s disease: a rare cause of parotid swelling. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 1):589–593. doi:10.1007/s12070-018-1421-5
7. Armstrong WB, Kim JK, Pena F, Kim JK, Kim JKV. Kimura’s disease: two case reports and a literature review. Ann Otol Rhinol Laryngol. 1998;107(12):1066–1071. doi:10.1177/000348949810701212
8. Sato R, Bandoh N, Goto T, et al. Kimura disease presenting with buccal mass: a case report and literature review. Head Neck Pathol. 2021;15(2):657–662. doi:10.1007/s12105-020-01206-3
9. Sakitani E, Nonaka M, Shibata N, Furukawa T, Yoshihara T. Increased expression of thymic stromal lymphopoietin and its receptor in Kimura’s disease. ORL J Otorhinolaryngol Relat Spec. 2015;77(1):44–54. doi:10.1159/000371424
10. Oga S, Katayama O, Ogata Y, Nakazono Y. 木村病に合併した免疫性血小板減少性紫斑病 [Immune thrombocytopenia associated with Kimura’s disease]. Rinsho Ketsueki. 2021;62(12):1666–1671. Japanese. doi:10.11406/rinketsu.62.1666
11. Tomizuka T, Kikuchi H, Asako K, et al. Is Kimura’s disease associated with juvenile temporal arteritis? A case report and literature review of all juvenile temporal arteritis cases. Mod Rheumatol Case Rep. 2021;5(1):123–129. doi:10.1080/24725625.2020.1818366
12. Watanabe C, Koga M, Honda Y, Oh IT. Juvenile temporal arteritis is a manifestation of Kimura disease. Am J Dermatopathol. 2002;24(1):43–49. doi:10.1097/00000372-200202000-00009
13. Fouda MA, Gheith O, Refaie A, et al. Kimura disease: a case report and review of the literature with a new management protocol. Int J Nephrol. 2011;2010:673908. doi:10.4061/2010/673908
14. Buder K, Ruppert S, Trautmann A, Brocker EB, Goebeler M, Kerstan A. Angiolymphoid hyperplasia with eosinophilia and Kimura’s disease - a clinical and histopathological comparison. J Dtsch Dermatol Ges. 2014;12(3):224–228. doi:10.1111/ddg.12257_suppl
15. Zou A, Hu M, Niu B. Comparison between Kimura’s disease and angiolymphoid hyperplasia with eosinophilia: case reports and literature review. J Int Med Res. 2021;49(9):3000605211040976. doi:10.1177/03000605211040976
16. Wang X, Ng CS, Yin W. A comparative study of Kimura’s disease and IgG4-related disease: similarities, differences and overlapping features. Histopathology. 2021;79(5):801–809. doi:10.1111/his.14428
17. Munemura R, Maehara T, Murakami Y, et al. Distinct disease-specific Tfh cell populations in 2 different fibrotic diseases: igG4-related disease and Kimura disease. J Allergy Clin Immunol. 2022;150:440–455. doi:10.1016/j.jaci.2022.03.034
18. Hosaka N, Minato T, Yoshida S, et al. Kimura’s disease with unusual eosinophilic epithelioid granulomatous reaction: a finding possibly related to eosinophil apoptosis. Hum Pathol. 2002;33(5):561–564. doi:10.1053/hupa.2002.124037
19. Williams G, Neblett C, Arscott J, McLean S, Warren S, Blake G. Kimura disease: a rare cause of a recurrent cheek mass in a Jamaican man. J Surg Case Rep. 2021;2021(4):rjab100. doi:10.1093/jscr/rjab100
20. Ma H. Treatment of Kimura’s disease with oral corticosteroid and methotrexate. An Bras Dermatol. 2020;95(1):115–117. doi:10.1016/j.abd.2019.03.006
21. Vissing-Uhre R, Hansen A, Frevert S, Hansen D. Rituximab treatment in a patient with Kimura disease and membranous nephropathy: case report. Case Rep Nephrol Dial. 2021;11(2):116–123. doi:10.1159/000515644
22. Kinoshita M, Ogawa Y, Onaka M, Shimada S, Kawamura T. Mepolizumab-responsive Kimura disease. J Allergy Clin Immunol Pract. 2021;9(7):2928–2930. doi:10.1016/j.jaip.2021.02.049
23. Persechino S, Bartolazzi A, Persechino F, et al. Remission of Kimura disease with carotid hypervascularization after cyclosporine treatment. Dermatol Pract Concept. 2020;10(2):e2020030. doi:10.5826/dpc.1002a30
24. Teraki Y, Terao A. Treatment of Kimura disease with dupilumab. JAMA Dermatol. 2022;158(3):329–330. doi:10.1001/jamadermatol.2021.5885
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.