Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
An Overview of 3D Anatomical Model Printing in Orthopedic Trauma Surgery
Authors Mendonça CJA , Guimarães RMDR, Pontim CE, Gasoto SC, Setti JAP , Soni JF, Schneider Jr B
Received 16 August 2022
Accepted for publication 9 December 2022
Published 4 April 2023 Volume 2023:16 Pages 875—887
DOI https://doi.org/10.2147/JMDH.S386406
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Celso Junio Aguiar Mendonça,1,2 Ricardo Munhoz da Rocha Guimarães,3,4 Carlos Eduardo Pontim,4 Sidney Carlos Gasoto,2 João Antonio Palma Setti,4 Jamil Faissal Soni,1,3 Bertoldo Schneider Jr2,4
1Musculoskeletal System Unit, Hospital of Federal University of Paraná, Curitiba, Paraná, Brazil; 2Postgraduate Program in Electrical Engineering and Industrial Informatics, Hospital of the Federal University of Paraná, Curitiba, Paraná, Brazil; 3Cajuru University Hospital, Pontifical Catholic University of Paraná, Curitiba, Paraná, Brazil; 4Postgraduate Program in Biomedical Engineering, Hospital of the Federal University of Paraná, Curitiba, Paraná, Brazil
Correspondence: Celso Junio Aguiar Mendonça, Postgraduate Program in Electrical Engineering and Industrial Informatics – CPGEI, Federal Technological University of Paraná – UTFPR, Av. Sete de Setembro, 3165 – Rebouças, Curitiba, Paraná, 80230-901, Brazil, Tel +55 41 999973900, Email [email protected]
Introduction: 3D object printing technology is a resource increasingly used in medicine in recent years, mainly incorporated in surgical areas like orthopedics. The models made by 3D printing technology provide surgeons with an accurate analysis of complex anatomical structures, allowing the planning, training, and surgery simulation. In orthopedic surgery, this technique is especially applied in oncological surgeries, bone, and joint reconstructions, and orthopedic trauma surgeries. In these cases, it is possible to prototype anatomical models for surgical planning, simulating, and training, besides printing of instruments and implants.
Purpose: The purpose of this paper is to describe the acquisition and processing from computed tomography images for 3D printing, to describe modeling and the 3D printing process of the biomodels in real size. This paper highlights 3D printing with the applicability of the 3D biomodels in orthopedic surgeries and shows some examples of surgical planning in orthopedic trauma surgery.
Patients and Methods: Four examples were selected to demonstrate the workflow and rationale throughout the process of planning and printing 3D models to be used in a variety of situations in orthopedic trauma surgeries. In all cases, the use of 3D modeling has impacted and improved the final treatment strategy.
Conclusion: The use of the virtual anatomical model and the 3D printed anatomical model with the additive manufacturing technology proved to be effective and useful in planning and performing the surgical treatment of complex articular fractures, allowing surgical planning both virtual and with the 3D printed anatomical model, besides being useful during the surgical time as a navigation instrument.
Keywords: 3D printing, 3D biomodel, virtual surgical planning
Graphical Abstract:
Introduction
The 3D printing technology, also known as Additive Manufacturing (AM) or Rapid Prototyping (RP), has been increasingly used in the health sciences.1 The first paper published on AM in orthopedics was in 2000 and since then the use of this technology has helped in the development of several surgical specialties, especially orthopedic surgery.2 The great impact of this technology is mainly in cases of trauma and highly complex diseases where a better understanding of the injury is needed for a more appropriate surgical treatment schedule. Surgeries in regions of complex anatomy such as the pelvis, spine, shoulder, knee, and joint regions have been the situations of greatest need for the use of this technology in orthopedic surgeries.1,3–5
With the technological advancement of radiological examinations, especially concerning the processing resources of the images of Radiography (X-ray), Computed Tomography (CT), and Magnetic Resonance (MR), the possibility of printing high-definition models in real size of organs and body parts, such as bones, is becoming more doable and more accessible.6
Due to the increasing use of 3D printing in various areas of human activity, the cost of printing is decreasing as well as access to technology is becoming easier every day.7 Both the value of the printer and the inputs are decreasing value and the possibility of printing models by the surgical team makes this technology an example of the style: “do it yourself” (DIY).8 In computing, this technology is an example of another trend: “what you see is what you get” (WYSIWYG).8,9 In addition, with the technological development in the area of 3D printing, there is a gradual rise in the accuracy and precision of biomodel and biomaterial printing. Nowadays, for better integration between biomedical engineering and orthopedics the concept of “point of care manufacturing” (POCM) emerged according to Calvo-Haro et al that means a new model production including networks hubs in hospital centers, reconciling in-house manufacturing and outsourcing.10 The workflow for 3D anatomical model printing changes by institution. Usually, a multidisciplinary team is required, for example in university hospitals the team that performs the manufacturing process of the 3D anatomical model can be composed of orthopedic surgeon, biomedical engineers and/or designers. In many cases, before printing the 3D model, all the team meeting to perform a virtual surgical planning to program the implant position and surgical strategies.12 Meanwhile, in smaller centers, the process of printing biomodels is performed only by the surgeon.
Kim et al in their study report the clinical experience with the use of 3D printing techniques in orthopedic trauma, with the following applications in 3D printing technology:11 1. A better understanding of the fracture and anatomical relationships; 2. Preoperative planning; 3. Medical Education; 4. Surgical training and simulation.
According to Bagaria et al, the advantages of the use of additive manufacturing in the surgical treatment of complex joint fractures are9 1. A better understanding of fracture morphology or anatomopathology; 2. Measurement of the real dimensions and anatomical relationships in the model; 3. Reduction of surgical time; 4. Decreased anesthesia time; 5. Decrease of intraoperative blood loss; 6. Assistance in obtaining fracture reduction; 7. Reduction of time using fluoroscopy.
The purpose of this paper is to describe and analyze the acquisition and processing of CT images for 3D printing and its applicability in orthopedic surgeries and shows some examples of surgical planning in orthopedic trauma surgery.
Materials and Methods
Clinical Cases
Four examples were selected to demonstrate the workflow and rationale throughout the process of planning and printing 3D models to be used in a variety of situations in orthopedic trauma surgeries. The cases here presented were treated in different institutions by the authors during the last two years. In all cases, the use of 3D modeling has impacted and improved the final treatment strategy. This study was carried out after approval by the Ethics Committee of the Federal Technological University of Paraná - UTFPR under CAAE: 94790518.4.0000.5547. The consent to publish term has been provided by the patient(s) to have the case details and any accompanying images published.
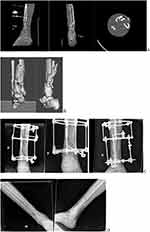
Case 1: Tibial Plafond Fracture
A 28-year-old male suffered a motorcycle accident with severe polytrauma with an open complex fracture of the right distal tibia. It was performed the damage control of the open fracture with the transarticular external fixator. The patient had extensive skin lesions in the posteromedial region that evolved with infection of soft tissues. The CT images showed high comminution of the fragments. The 3D anatomical model was printed to understanding the pattern fracture to plan the surgery. The chosen strategy to fix the fracture was to install the circular external fixator. In order to obtain the fracture healing a bone graft was necessary in the distal tibia metaphysis gap (Figure 1). The 3D anatomical model allowed the team to understand and decide on the fracture fixation method (circular external fixator), as well as predicting the use of bone graft in place of metaphyseal bone loss.
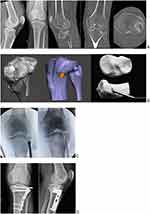
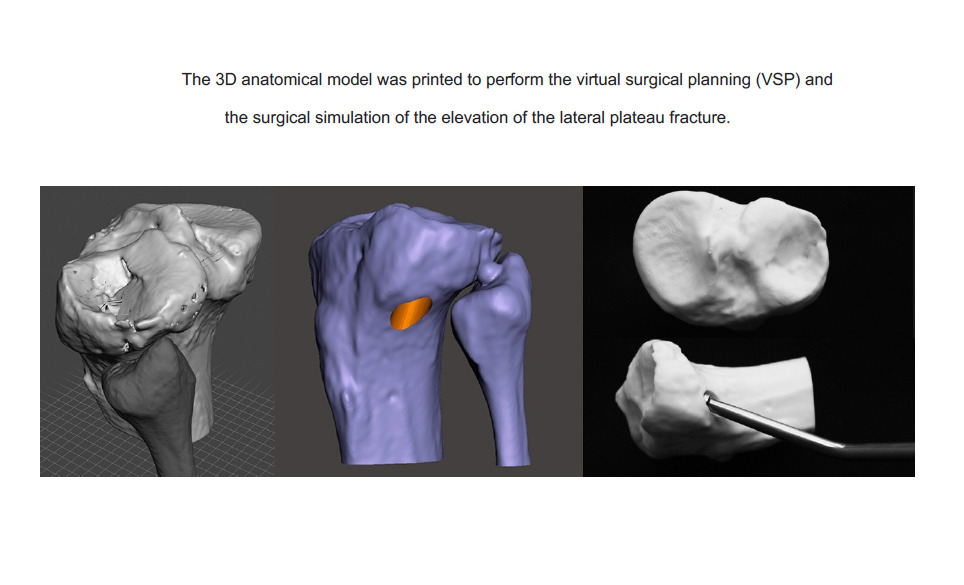
Case 2: Plateau Fracture
A 40-year-old male suffered a fall from a height with knee axial trauma. After 4 weeks after the accident, he sought medical attention due to pain and swelling in his right knee. The CT scan showed an old fracture of the knee lateral plateau with great central sinking. The virtual surgical planning (VSP) was performed, and the joint surface elevation site was defined. The 3D anatomical model was printed, and the surgical simulation of the elevation of the lateral plateau was performed. Performing the VSP, in this case, was what made it possible to determine the exact local of the hole in the lateral cortical to insert the instrument to elevate the sunken articular surface. The 3D printed model was important such as navigation during surgery to identify the exact location of the tunnel for elevation of the joint surface (Figure 2).
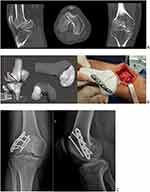
Case 3: Hoffa´s Fracture Nonunion
A 44-year-old male was involved in a motorcycle accident with severe polytrauma with multiple fractures in the left lower limb. After 6 years from the accident, the patient started to feel pain in his right knee. CT images were performed and diagnosed nonunion of the coronal fracture of the lateral femoral condyle of the right knee (Hoffa’s fracture). The CT images were used to perform the VSP and print the 3D anatomical model that was used in the surgery simulation (to choose the implants and their position on the bone). It was verified the stability of the osteosynthesis during simulation. The 3D printed model was important such as navigation during surgery to identify the nonunion line and the correct place to fit the plate (Figure 3).
Case 4: Spine Fracture
A 20 years old male involved in a car accident with overturning was admitted to our emergency department with neck pain and local tenderness. The patient was neurologically intact (ASIA E). X-rays and CT images showed a C3 right pedicle fracture with a C3-C4 subluxation and a right side locked facet. It was planned a short posterior fusion using pedicle screws in C2 and C3 (left side only) and lateral mass screws in C4. A 3D model of the cervical spine upper segment has been constructed with tunnels through the pedicle previously planned trajectories to be used as a navigation aid in the surgical field. 3D printed perforation guides for C2 and C3 pedicles have been produced as well. In this case, the biomodel was used in the surgery field to serve as a navigation tool. The pedicle screw trajectories were printed as tunnels in the model. Considering the precise dimensional properties of 3D printed models, they help in mitigating doubts and possible mistakes in trajectories and position of implants. We have also designed perforation guides that were successfully used for pedicle screw implantation, especially in C3 (Figure 4).
The source of data was CT images obtained in a high-resolution protocol by different machines and distributed by the well-known Digital Imaging and Communications in Medicine (DICOM) protocol. The images were further processed by software that was designed for image segmentation and volumetric reconstruction that produce a file that can be used by other applications in the process: InVesalius® v. 3.1.1 (CTI. Campinas, Brazil) in cases 1 to 3 and 3D Slicer® v. 4.10 and 4.11 (The Brigham and Women’s Hospital Inc. Massachusetts, USA) in case 4. After the DICOM images are segmented and the 3D object rectified, they are exported to an STL extension, which is a file format that contains data describing the layout of a 3D object. It is worth mentioning that each software used for 3D printing requires the files in a specific format (eg, with the STL extension, AMF extension, OBJ extension, 0.3MF extension, etc.).
The volumes obtained were remodeled and modified to produce very specific objects and tools using the software Meshmixer® v. 3.3 (Autodesk. San Rafael, USA) in cases 1 to 3 and Mimics® v. 22.0 (Materialise. Leuven, Belgium) in case 4. This software also allows the performing of VSP and some degree of simulation. The virtual objects to be transformed into prototypes were processed by the software Simplify3D® v. 4.1 (Cincinnati, OH, USA) in all cases to generate a code (Gcode) containing specific commands to the extruder machines.
The 3D objects in STL format were processed by the software Simplify 3D® v. 4.1 (Cincinnati, OH, USA) slicing software for 3D printers, used to prepare virtual objects according to selected parameters to generate a code format file (Gcode) containing specific commands to operate the extruder machines.
The objects created in our study were manufactured in a 3D cartesian desktop printer with Fused Deposition Modeling (FDM) technology of the model Ender 3® printer (Creality. Shenzhen, China). The thermoplastics chosen to print the biomodels were Acrylonitrile Butadiene Styrene (ABS) in cases 1 to 3 and Polylactic Acid (PLA) in case 4. The thermoplastics have a white coloration in a 1.75 mm width. The parameters chosen for printing the pieces were 0.4mm extrusion nozzle, 0.15 mm layer height, 0.4 mm layer width, 0.8 mm perimeter shells, 4 top solid layers, 4 bottom solid layers, and 50% density of filling in the recline format and 30 mm/s print speed.
Discussion
According to some authors13 currently, the 3D printing process in the medical field (used for teaching, training, and surgical planning, making guides and implants) consists of four steps (Figure 5).
 |
Figure 5 The 3D printing process in the medical area. |
The first and most important step in the printing of 3D objects is image acquisition.14 The quality of the printed models depends on the quality of the processed data (image resolution). Therefore, low-resolution images will result in inappropriate models and distortions.15,16 The width of the slices of the CT images will also influence the image quality, being one of the main limiting factors for the quality of the prototyping in the medical area.13,17 The width of the slice should be 0.5 to 2 mm depending on the anatomical region. Slices above 2mm can cause distortion when printing objects. The region of interest (ROI) must be established to reduce the work of extracting parts that will not be useful for printing.14 Bone tissues have a high contrast compared to soft tissues in CT images which makes this type of exam the most suitable for data acquisition for 3D modeling.1 The acquired data (images) in the CT scan are processed in programs observing a set of rules for treatment, storage, and transmission of information in an electronic format, structuring a DICOM protocol.6,13,14,17 It was created to standardize the formatting of diagnostic images such as X-ray, CT, MRI, Ultrasonography (US), etc. The DICOM Standard is a series of rules that allow medical images and associated information to be exchanged between imaging-generating diagnostic equipment with computers.
Segmentation is a process of separating the desired area from an unwanted area, which is determining the ROI for future image processing.5,14,17 The separation of the parts depends on the anomalous area and the chosen tissue to be studied (bone, muscle, blood vessels, etc.). Segmentation can be performed manually or through algorithms created for this purpose.18 This is the most critical and most demanding phase in the 3D printing process since the generation of low-resolution 3D images can lead to improper printing of objects.13
There are some software for this purpose, eg, OsiriX®, Horos®, 3DSlicer®, and Invesalius®.
After segmentation of the image, it is possible to perform the operative planning, using the data processing with the creation of the 3D object, performing the study of the spatial geometry of the site (anatomy), performing virtual surgery with resection of parts (eg, oncological surgery) or the possibility of simulating reduction of fractures and/or placement of implants and prostheses.18
In the first three clinical cases, the Invesalius® software was used to perform the segmentation and the 3DSlicer® software was used in case 4. In all cases, the accuracy of these programs to create the virtual 3D bone was considered very satisfactory.
After segmentation, the surface is extracted from the volumetric data by converting the voxel data into a mesh formed from a series of triangular facets.17 In other words, there is a conversion of 2D images into 3D images, for the possibility of editing the 3D object.13 At this moment, there is a 3D reconstruction of the images. In this format, the object can be manipulated and made the necessary adjustments for editing the final geometry to be printed. These data are converted into files in the STL format in all cases in this study.
The image selected in the STL file is then processed to improve the regularity of the object surface and to make the object the closest to the real situation. During this stage, it is possible to remove parts of the object that are not of interest to the study as well as perform, as in the case of fractures, the reduction of them. In addition, in this phase, with the aid of CAD software technology, it is possible to model 3D objects such as implants and prostheses for the VSP, testing their positioning and size. For this purpose, some of these software are usually used, eg, Mimics®, Meshlab®, Blender®, Rhinoceros3D®, and Meshmixer®. In cases 1 to 3 of this study, Meshmixer® was used to do the modeling. In case 4, the Mimics software was used. The following are some modeling operations performed:
Due to the complexity of geometry and CT resolution, it is necessary to form a mesh to define the places where there are gaps so that they will be corrected with image editing methods, making the surface of the object as smooth as possible.5
It is often necessary to remove artifacts from the images that make the object irregular and with deformations on the surface. Most of the time these artifacts are due to metallic materials implanted in the patient’s body or are created during the segmentation process. The spatial cleaning method, normally used, is an algorithm to reduce noise without loss of anatomical information.5
Smoothening algorithms are used to improve the definition and quality of the 3D object to be printed.5,19 Special care must be taken in this step because if the smoothing is exaggerated, the created 3D object no longer has the initial geometry.
The printing of dimensional objects that have complex shapes can be done with different characteristics of solidity and porosity during the making of the object.6 This requires choosing the type of printing technology and the type of material used.20 There are several Computer-Aided Manufacturing (CAM) technologies used in the manufacture of 3D models, among them: Stereolithography (SLA), Digital Light Processing (DLP), Selective Laser Sintering (SLS), and Fused Deposition Modeling (FDM).2,15,17,18,21
The SLA and DLP technologies use a beam of light to polymerize liquid (resin) from a vat, forming successive layers. SLS technology uses a high-powered laser to melt particles of polymers, metal, glass, or ceramics. This technology has a high resolution and high cost. FDM technology uses the deposition technique of heated polymer through an extruder nozzle, making 3D objects with a good geometric definition. This technology requires support for the printing of the structures.17 In the FDM technology, the printing speed is low and the print resolution is lower than the SLA, DLP and SLS techniques.1,21
Currently, the two most widespread techniques used in the production of biomodels for orthopedic surgeries are FDM17 and SLS. The most common technique for the manufacture of cutting and drilling guides and metal implants, mainly using titanium alloys, is SLS technology.
Some software used to perform biomodel printing are, eg, MakerBot Desktop®, Cura®, Slic3r®, and Simplify 3D®.
During the printing process, some care is needed to obtain a biomodel printed with 3D technology in real size that reproduces the original anatomical features. Establishing which printing parameters will be used is a fundamental step in this process. Our research team, after several tests, uses the parameters mentioned above, where we can reproduce 3D printed bone models with great accuracy.
Various types of materials are used for 3D printing in orthopedic surgeries such as metals, polymers (natural and synthetic), and biomaterials.17,18 The most used materials currently in the printing of biomodels in orthopedic surgery are:
Acrylonitrile Butadiene Styrene (ABS): it is a rigid and light thermoplastic, resistant and non-toxic, with a melting point of approximately 210°C to 250°C. Derived from petroleum, it is not biodegradable, and it can release unpleasant vapors during printing.22 It is also used in bone model printing for education, training, and surgical planning.5
Polylactic Acid (PLA) is a thermoplastic of vegetable origin (starch), which has a melting point of approximately 210°C to 250°C.23 PLA is easy to print, it is biocompatible and biodegradable, but its strength is degraded over time and the print has a texture with certain roughness.22 It is also used in the printing of bone models and the printing of surgical guides.15 PLA is brittle and has low mechanical resistance.
According to some authors, the biomedical use of 3D printing technology has four important areas:
- Biomodel printing (anatomical models).
- Guides and surgical instruments printing (cutting guides, drilling guides).
- Implants, prostheses printing.
- Tissue printing.
Some situations in the treatment of orthopedic problems where technology and 3D printing have been used:22
- Periarticular and articular fractures.
- Complex arthroplasties present bone defects.
- Spinal fractures and complex spine deformities.
- Congenital malformations.
- Angular deformities.
One possibility of using the anatomical model includes the orientation of patients and family members. Regarding the communication of the medical team with patients and family members, some studies show the use of anatomical models to inform about the type of surgical treatment proposed, promoting a better understanding of the patient clinical condition, surgical schedule, rehabilitation, and greater adherence to treatment, contributing to an improvement in the doctor–patient relationship.24–27
Several studies have shown the effective application of the use of 3D printing technology in education and medical training,6,14,17,21,28 mainly in surgical procedures.5,17,21,29–33 Kim et al29 in their study concluded that the 3D printing technique provided surgeons with a better understanding of the fracture pattern and anatomy and was effectively used for preoperative planning, training of interns, and performing surgical simulations to improve intraoperative technical results.
The CAD programs currently have been allowed the performing of VSP with a better understanding of spatial geometry, anatomical relationships mainly in places of complex anatomy, and the possibility of programming a less invasive surgical approach and in the case of orthopedic trauma surgeries the previous reduction of bone fragments simulating the definitive osteosynthesis.34
The printing of biomodels provides additional information to conventional images with increased knowledge of the anatomopathology of the disease to be treated.17,27,31,35 This way, 3D printing is useful in surgical simulation, surgical planning, in the preoperative choice of implants and guides to be used14,16,31,36 mainly concerning the understanding of geometry (distances, scales, shapes) and identification of complex anatomy14,17,28,30,31 and in referencing anatomical structures in the intraoperative step. Other advantages are the better choice of access to bone defects, a better understanding of the fracture pattern, and better choice in the positioning of bone implants.5,13
An accurate navigation technique is essential to transfer the preoperative 3D surgical planning to the patient during surgery. The patient-specific instruments (PSI) such as implants and guides printed in 3D are customized to fit on the patient’s bone to directly position previously planned surgical procedures.30,33,37
Xiong L et al38 and Morgan et al39 have concluded in their systematic review and meta-analysis that the use of 3D printing-assisted surgery in orthopedic trauma reduces operative time, intraoperative blood loss, and the number of time fluoroscopy used.
One important aspect of the 3D printing workflow is time. Once created the 3D printed object from medical images, the trained staff can spend many hours in the surgery planning; moreover, the printing time depends on the model size and 3D technology used.27
In our study, all the cases were performed virtual surgical planning and surgical simulation using a 3D biomodel printed in real size. As shown in the literature above, virtual surgical planning was a decisive step in the treatment of all cases, determining the surgical approach, the choice of the implants, the fracture reduction, and the position of the implants in the bone.
Some papers have demonstrated that the use of the printed 3D model can be sterilized and used such a template during the surgery. It is an example of surgical navigation, promoting important information to the surgeon. The sterilizing process depends on the kind of material used in 3D object printing.27
Conclusion
To obtain a 3D biomodel in real size that represents the original anatomy, it is necessary to follow a protocol that includes the image acquisition parameters and the printing parameters, for standardization of the 3D printing process.
The use of the virtual anatomical model and the 3D printed anatomical model with the AM technology proved to be effective and useful in planning and performing the surgical treatment of complex articular fractures, allowing surgical planning both virtual and with the 3D printed anatomical model, besides being useful during the surgical time as a navigation instrument.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wong TM, Jin J, Lau TW, et al. The use of three-dimensional printing technology in orthopaedic surgery. J Orthop Surg. 2017;25(1):2309499016684077. doi:10.1177/2309499016684077
2. Javaid M, Haleem A. Additive manufacturing applications in orthopaedics: a review. J Clin Orthop Trauma. 2018;9(3):202–206. doi:10.1016/j.jcot.2018.04.008
3. Giannetti S, Bizzotto N, Stancati A, Santucci A. Minimally invasive fixation in tibial plateau fractures using an pre-operative and intra-operative real size 3D printing. Injury. 2017;48(3):784–788. doi:10.1016/j.injury.2016.11.015
4. Yang P, Du D, Zhou Z, et al. 3D printing-assisted osteotomy treatment for the malunion of lateral tibial plateau fracture. Inj Int J Care Inj. 2016;47:2816–2821. doi:10.1016/j.injury.2016.09.025
5. Bagaria V, Chaudhary K. A paradigm shift in surgical planning and simulation using 3Dgraphy: experience of first 50 surgeries done using 3D-printed biomodels. Inj Int J Care Inj. 2017;48:2501–2508. doi:10.1016/j.injury.2017.08.058
6. Mulford JS, Babazadeh S, Mackay N. Three-dimensional printing in orthopaedic surgery: review of current and future applications. ANZ J Surg. 2016;86(9):648–653. doi:10.1111/ans.13533
7. Minto J, Zhou X, Osborn J, Zhang LG, Sarkar K, Rao RD. Three-dimensional printing: a catalyst for a changing orthopaedic landscape. JBJS Rev. 2020;8(2):1–13.
8. Chana-Rodríguez F, Mañanes RP, Rojo-Manaute J, Gil P, Martínez-Gómiz JM, Vaquero-Martín J. 3D surgical printing and pre contoured plates for acetabular fractures. Injury. 2016;47(11):2507–2511. doi:10.1016/j.injury.2016.08.027
9. Bagaria V, Deshpande S, Rasalkar DD, Kuthe A, Paunipagar BK. Use of rapid prototyping and three-dimensional reconstruction modeling in the management of complex fractures. Eur J Radiol. 2011;80(3):814–820. doi:10.1016/j.ejrad.2010.10.007
10. Calvo-Haro JA, Pascau J, Mediavilla-Santos L, et al. Conceptual evolution of 3D printing in orthopedic surgery and traumatology: from “do it yourself” to “point of care manufacturing”. BMC Musculoskelet Disord. 2021;22(360):1–10. doi:10.1186/s12891-021-04224-6
11. Kim JW, Lee Y, Seo J, et al. Clinical experience with three-dimensional printing techniques in orthopedic trauma. J Orthop Sci. 2017:1–6. doi:10.1016/j.jos.2017.12.010
12. Ijpma FFA, Meesters AML, Merema BBJ, et al. Feasibility of imaging-based 3-dimensional models to design patient-specific osteosynthesis plates and drilling guides. JAMA Netw Open. 2021;4:2. doi:10.1001/jamanetworkopen.2020.37519
13. Van EM, Van DR, Dobbe J, Streekstra G, Koivisto J, Wolff J. CT image segmentation methods for bone used in medical additive manufacturing. Med Eng Phys. 2018;51:6–16. doi:10.1016/j.medengphy.2017.10.008
14. Shui W, Zhou M, Chen S, et al. The production of digital and printed resources from multiple modalities using visualization and three-dimensional printing techniques. Int J Comput Assist Radiol Surg. 2017;12(1):13–23. doi:10.1007/s11548-016-1461-9
15. Mok SW, Nizak R, Fu SC, et al. From the printer: potential of three-dimensional printing for orthopaedic applications. J Orthop Transl. 2016;6:42–49. doi:10.1016/j.jot.2016.04.003
16. Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3-dimensional printing in surgery: a systematic review. Surgery. 2016;159(6):1485–1500. doi:10.1016/j.surg.2015.12.017
17. Marro A, Bandukwala T, Mak W. Three-dimensional printing and medical imaging: a review of the methods and applications. Curr Probl Diagn Radiol. 2016;45(1):2–9. doi:10.1067/j.cpradiol.2015.07.009
18. Chen X, Xu L, Wang W, Li X, Sun Y, Politis C. Computer-aided design and manufacturing of surgical templates and their clinical applications: a review. Expert Rev Med Devices. 2016;13(9):853–864. doi:10.1080/17434440.2016.1218758
19. Favier V, Zemiti N, Mora OC, et al. Geometric and mechanical evaluation of 3D-printing materials for skull base anatomical education and endoscopic surgery simulation – a first step to create reliable customized simulators. PLoS One. 2017;12(12):e0189486. doi:10.1371/journal.pone.0189486
20. Mishra A, Verma T, Vaish A, Vaish R, Vaishya R, Maini L. Virtual preoperative planning and 3D printing are valuable for the management of complex orthopaedic trauma. Chinese J Traumatol. 2019;22:350–355. doi:10.1016/j.cjtee.2019.07.006
21. Malik HH, Darwood ARJ, Shaunak S, et al. Three-dimensional printing in surgery: a review of current surgical applications. J Surg Res. 2015;199(2):512–522. doi:10.1016/j.jss.2015.06.051
22. Bagaria V, Bhansali R, Pawar P. 3D printing creating a blueprint for the future of orthopedics: current concept review and the road ahead! J Clin Orthop Trauma. 2018;9(3):207–212. doi:10.1016/j.jcot.2018.07.007
23. Hoang D, Perrault D, Stevanovic M, Ghiassi A. Surgical applications of three-dimensional printing: a review of the current literature & how to get started. Ann Transl Med. 2016;4(23):456. doi:10.21037/atm.2016.12.18
24. Tack P, Victor J, Gemmel P, Annemans L. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online. 2016;15(115):1–21. doi:10.1186/s12938-016-0236-4
25. Wilcox B, Mobbs RJ, Wu A-M, Phan K. Systematic review of 3D printing in spinal surgery: the current state of play. J Spine Surg. 2017;3(3):433–443. doi:10.21037/jss.2017.09.01
26. Zheng W, Su J, Cai L, et al. Application of 3D printing technology in the treatment of humeral intercondylar fractures. Orthop Traumatol Surg Res. 2018;104(1):83–88. doi:10.1016/j.otsr.2017.11.012
27. Jiang M, Chen G, Coles-Black J, Chuen J, Hardidge A. Three-dimensional printing in orthopaedic preoperative planning improves intraoperative metrics: a systematic review. ANZ J Surg. 2020;90(3):243–250. doi:10.1111/ans.15549
28. Langridge B, Momin S, Coumbe B, Woin E, Griffin M, Butler P. Systematic review of the use of 3-dimensional printing in surgical teaching and assessment. J Surg Educ. 2018;75(1):209–221. doi:10.1016/j.jsurg.2017.06.033
29. Kim JW, Lee Y, Seo J, et al. Clinical experience with three-dimensional printing techniques in orthopedic trauma. J Orthop Sci. 2018;23(2):383–388. doi:10.1016/j.jos.2017.12.010
30. Fadero PE, Shah M. Three dimensional (3D) modelling and surgical planning in trauma and orthopaedics. Surg. 2014;12:328–333. doi:10.1016/j.surge.2014.03.008
31. Zhang Y, Wen L, Zhang J, Yan G, Zhou Y, Huang B. Three-dimensional printing and computer navigation assisted hemipelvectomy for en bloc resection of osteochondroma: a case report. Medicine. 2017;96(12):1–6. doi:10.1097/MD.0000000000006414
32. Sallent A, Vicente M, Reverté MM, et al. How 3D patient-specific instruments improve accuracy of pelvic bone tumour resection in a cadaveric study. Bone Jt Res. 2017;6(10):577–583. doi:10.1302/2046-3758.610.BJR-2017-0094.R1
33. Caiti G, Dobbe JGG, Strijkers GJ, Strackee SD, Streekstra GJ. Positioning error of custom 3D-printed surgical guides for the radius: influence of fitting location and guide design. Int J CARS. 2017;1–12. doi:10.1007/s11548-017-1682-6
34. Egger J, Wallner J, Gall M, et al. Computer-aided position planning of miniplates to treat facial bone defects. PLoS One. 2017;12(8):e182839. doi:10.1371/journal.pone.0182839
35. Punyaratabandhu T, Liacouras PC, Pairojboriboon S. Using 3D models in orthopedic oncology: presenting personalized advantages in surgical planning and intraoperative outcomes. 3D Print Med. 2018;4(12):1–13. doi:10.1186/s41205-018-0035-6
36. Van den Broeck J, Vereecke E, Wirix-Speetjen R, Sloten VJ. Segmentation accuracy of long bones. Med Eng Phys. 2014;36:949–953. doi:10.1016/j.medengphy.2014.03.016
37. Woo S-H, Sung M-J, Park K-S, Yoon T-R. Three-dimensional-printing technology in hip and pelvic surgery: current landscape. Hip Pelvis. 2020;32(1):1–10. doi:10.5371/hp.2020.32.1.1
38. Xiong L, Li X, Li H, Chen Z, Xiao T. The efficacy of 3D printing-assisted surgery for traumatic fracture: a meta-analysis. Postgr Med J. 2019;95:414–419. doi:10.1136/postgradmedj-2019-136482
39. Morgan C, Khatri C, Hanna SA, Ashrafian H, Sarraf KM. Use of three-dimensional printing in preoperative planning in orthopaedic trauma surgery: a systematic review and meta-analysis. World J Orthop. 2020;11(1):57–67. doi:10.5312/wjo.v11.i1.57
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.