Back to Journals » Psoriasis: Targets and Therapy » Volume 12
An Open-Label, Randomized, Prospective, Comparative, Three-Arm Clinical Trial to Evaluate the Safety and Effectiveness of Apremilast with Three Different Titration Methods in Patients with Chronic Plaque Psoriasis in India
Authors Viswanath V , Joshi P, Lawate P, Tare D, Dhoot D , Mahadkar N, Barkate H
Received 14 January 2022
Accepted for publication 17 March 2022
Published 22 April 2022 Volume 2022:12 Pages 53—61
DOI https://doi.org/10.2147/PTT.S357184
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Uwe Wollina
Vishalakshi Viswanath,1 Pradnya Joshi,1 Prakash Lawate,1 Dakshata Tare,1 Dhiraj Dhoot,2 Namrata Mahadkar,2 Hanmant Barkate2
1Department of Dermatology, Rajiv Gandhi Medical College, Thane, Mumbai, India; 2Department of Global Medical Affairs, Glenmark Pharmaceuticals Ltd, Mumbai, Maharashtra, India
Correspondence: Dhiraj Dhoot, Department of Global Medical Affairs, Glenmark Pharmaceuticals Ltd, B D Sawant Marg, Andheri (E), Mumbai, Maharashtra, 400099, India, Tel +91 9619811219, Email [email protected]
Purpose: To minimize adverse effects (AEs), apremilast is recommended to titrate at the initiation of therapy. But still, many patients experience AEs, resulting in discontinuation of therapy. As a result, many dermatologists have adapted to further titrate apremilast in different ways. The present study was planned to evaluate the safety and effectiveness of apremilast in different dose titration methods as initiation therapy in the treatment of plaque psoriasis.
Patients and Methods: In this open-label, randomized, prospective, comparative, three-arm, single center study, 128 plaque psoriasis patients were included. Patients were randomized into three groups. Group I received standard titration for the first 6 days; Group II received all tablets in a starter pack as once a day (OD) total for 13 days; and Group III received two starter packs as 8 tablets each of apremilast 10 mg and 20 mg as OD and 10 tablets of 30 mg as OD, in total for 26 days. All groups received apremilast 30 mg as twice a day after initial titration. The total duration of apremilast therapy in all groups was 16 weeks.
Results: In safety assessment, AEs were reported in 50%, 41.3% and 25% in Groups I, II and III, respectively (p < 0.05) with nausea being the most common AE. In Group I, 10.53% of patients discontinued apremilast whereas 6.52% and 2.27% discontinued in Groups II and III respectively. Maximum number of AEs were seen in Group I in first week only (74.19%) compared with other groups. At week 16, on the Psoriasis Area and Severity Index, PASI 75 was achieved in 31.43%, 42.4% and 33.3% of patients in Groups I, II and III, respectively with no statistical difference between any groups.
Conclusion: It can be concluded that slower titration is a useful strategy for minimizing AEs while at the same time maintaining effectiveness of apremilast.
Keywords: apremilast, adverse events, titration, effectiveness
Introduction
Apremilast, a phosphodiesterase 4 (PDE-4) inhibitor, has demonstrated clinical benefits in the management of psoriasis not only in randomized controlled trials (RCTs), ESTEEM 1 and 2 but also in real-world studies.1,2 The inhibition of PDE-4 is associated with an increase in intracellular cAMP levels, and subsequently modulates inflammatory responses, and thus helps in maintaining a balanced immune system.3–5 While apremilast is proven to be safe and well tolerated, it may cause a few temporary and mild to moderate adverse events (AEs) such as gastrointestinal upset, nausea, muscle pain and headache during the initiation of the therapy.6 These side effects are linked to cAMP levels which are decreased in psoriasis. Because of higher incidence of AEs, dose titration of apremilast is recommended at the initiation of therapy over a period of one week. However, despite the initial dose titration many patients develop AEs, leading to discontinuation of therapy in real-world practice.7
Although several interventions are well demonstrated for the management of AEs, sometimes dose adjustment and discontinuation of the therapy may be required.7,8 Recently published Indian papers on apremilast also highlighted a slowdown of titration in the initial period.9,10 Owing to this, for reduction in AEs and better compliance by patients, many dermatologists in India further slow down titration therapy up to 2–4 weeks, but studies regarding the safety of apremilast in different titration methods are lacking. The objective of this clinical study is to evaluate the safety and effectiveness of different dose titration methods of apremilast as initiation therapy in the management of patients with plaque psoriasis.
Materials and Methods
Study Participants
Adult patients (≥18 years of age) of either gender and diagnosed with chronic plaque psoriasis were included in the study. Patients with history of anti-psoriatic medications in any form within 2 weeks and use of biologics within 12–24 weeks were excluded. Additionally, patients with any significant medical illness such as diabetes or cardiac disease, immunocompromised conditions, sexually transmitted diseases, etc. that would have prevented study participation, and female patients who were pregnant or lactating were excluded from the study as per the investigator’s discretion.
The study was conducted in compliance with the protocol approved by the Ethics Committee (Rajiv Gandhi Medical College and Chhatrapati Shivaji Maharaj Hospital, Thane). Written informed consent was obtained from all the patients prior to participation in the study. This study was registered with a clinical trial registry (CTRI/2020/04/024631). This study was performed in accordance with Good Clinical Practices and the Declaration of Helsinki 1996.
Study Design and Treatment
This was an open-label, randomized, prospective, comparative, three-arm, and single-center study. The objective of this study was to study the initial adverse effect profile associated with apremilast titration dose and its effectiveness in each group. Patients were randomized by simple randomization technique into three groups. Group I received apremilast 30 mg twice a day after standard titration for the first 6 days. Group II received all tablets in a starter pack as once a day (OD) for 13 days, followed by apremilast 30 mg twice a day. Group III received two starter packs as 8 tablets of apremilast 10 mg OD for 8 days, followed by 20 mg OD for the next 8 days, and 30 mg OD for the next 10 days; thus totaling 26 days followed by apremilast 30 mg twice a day (Figure 1). All the patients were prescribed moisturizer only along with apremilast with antihistamines if needed. The total duration of the therapy in all groups was 16 weeks.
 |
Figure 1 Apremilast titration methods. Abbreviation: mg; milligram. |
Outcome Assessments
The primary objective of the study was to compare the percentage of patients presenting with adverse events (AEs) and the number of patients discontinuing treatment due to AEs in each group. The secondary objective was to compare the effectiveness of apremilast in each group.
Analysis Set
A safety analysis was performed on a full analysis set (SAF), (i.e., those patients who have received at least one dose of apremilast and completed at least one post-baseline follow-up visit). The effectiveness analyses were performed on the intent-to-treat set (ITT), (i.e., those patients who have received at least one dose of apremilast and had at least one post baseline efficacy assessment).
Statistical Analysis
Results were presented as mean scores, and the groups were compared using one-way ANOVA with Tukey HSD test and Fisher’s exact test. The level of significance was set at p <0.05. The difference in the proportion of patients with a change in mean scores (based on improvement criteria), was analysed using the chi-square test. Data were analysed using the IBM SPSS (Statistical Package for Social Sciences) statistics version 20.
Results
Patient Disposition and Baseline Characteristics
Of the 128 patients enrolled in this study, all (100%) were included in the SAF, and 95 patients (74.22%) were included in the ITT. Thirty-three patients (25.78%) were lost to follow-up before the first effectiveness assessment visit (Figure 2).
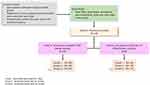 |
Figure 2 Study Design. |
There were 38 patients in Group I, 46 patients in Group II and 44 patients in Group III. Male predominance was seen in all groups with a mean (SD) age of 43.58 (9.78) in Group I, 42.53 (10.73) in Group II and 38.7 (12.5) years in Group III, respectively (Table 1). All the baseline characteristics under SAF are noted in Table 1. Patient distribution was homogeneous in all groups.
 |
Table 1 Baseline Demographic Characteristics for Full Analysis Set (SAF) |
Safety
Safety outcomes in all groups are summarized in Table 2. In Group I, 50% reported AEs, 41.3% reported AEs in Group II whereas 25% reported AEs in Group III. There was statistical difference (p <0.05) between Group I and III but no statistical difference was noted between Groups II and III. Nausea was the most common AE reported in all groups, followed by gastrointestinal upset and headache. Though all the groups experienced AEs, the maximum number of AEs were seen in Group I in first week only (74.19%) compared with other groups whereas in Groups II and III, 24.32% and 42.85% patients reported AEs in first week. In subsequent weeks, there was reduction in occurrence of AEs in all groups, as shown in Table 2. Most of the AEs occurred at the dose of apremilast 30 mg. As a result, 4 patients (10.53%) of the patients discontinued the therapy in Group I, 3 patients (6.52%) in Group II and 1 patient (2.27%) in Group III (Table 2). There was no significant statistical difference between any groups in terms of discontinuation of apremilast.
 |
Table 2 Details of Adverse Events in All Groups |
Treatment Response
All ITT patients were included in the treatment response group. There were 35 patients in Group I, 33 patients in Group II and 27 patients in Group III. All the baseline characteristics under ITT are noted in Table 3. Patient distribution was homogeneous in all groups.
 |
Table 3 Baseline Demographic Characteristics for Intention to Treat (ITT) |
Effectiveness
At week 10, in Group I, 10 patients (28.5%) and 5 patients (14.5%) achieved Psoriasis Area and Severity Index (PASI) 50 and 75 respectively while in Group II, 12 (36.3%) and 6 patients (18.1%) achieved PASI 50 and 75 respectively. In Group III, PASI 50 was achieved in 8 patients (29.6%) and PASI 75 in 2 patients (7.4%). At week 16, 14 (40%), 6 (18.1%) and 7 (26%) patients achieved PASI 50 and 11 (31.43%), 14 (42.4%), 9 patients (33.3%) achieved PASI 75 in Groups I, II and III respectively (Figure 3). On intergroup comparison, there was no statistically significant difference between any groups.
 |
Figure 3 Effectiveness evaluation in all groups for PASI. Abbreviation: PASI; Psoriasis area and severity index. |
Improvement in Mean Scores
There was a significant statistical difference between the baseline and the end of treatment in all groups in terms of improvement of all mean scores such as mean PASI, mean body surface area (BSA) and mean symptoms scores (p <0.05) as shown in Figures 4 and 5, but on intergroup comparison, there was no statistical significant difference, as shown in Table 4.
 |
Table 4 Clinical Response at Week 10 and Week 16 |
 |
Figure 4 Improvement in mean PASI and BSA scores at week 10 and week 16. Abbreviations: PASI; Psoriasis area and severity index, BSA; body surface area. |
 |
Figure 5 Improvement in mean scores at week 10 and week 16. |
Discussion
All the landmark clinical trials1,2 and real-world studies11 have examined the safety and effectiveness of apremilast for a complete duration of therapy but this study has examined safety during the titration of therapy. Apremilast is a PDE-4 inhibitor and the inhibition of PDE-4 is associated with an increase in intracellular cAMP levels, which subsequently modulate the inflammatory responses, and thus help in maintaining balance of the immune system.3–5 It has been found that the cAMP-specific PDE-4 is highly expressed in some specific organs such as the gastrointestinal tract, the musculoskeletal system, the brain and the skin,12–14 and hence adverse effects associated with these systems that are observed with apremilast are accredited to PDE-4 inhibition.13,15,16 Moreover, it has been found that patients with inflammatory diseases express higher levels of PDE-4 than healthy individuals.13,17 Hence, in such patients, more attention is required to minimize AEs with maintaining the balance of efficacy.
In earlier studies where apremilast dosing began at the full dose, more AEs were reported than Phase II studies, where apremilast titration was done over a period of 6 days. Hence initial dose titration is recommended to lower the AEs.18,19 But, despite titration, a high number of AEs were reported from landmark trials and real-world studies, leading to the discontinuation of the drug.11
In our study, 50%, 41.3% and 25% of the patients reported ≥1 AE in Groups I, II and III, respectively which was statistically significant. This is in line with recently published real-world studies on titration suggesting that a further slowdown of titration led to a lesser rate of AEs.10 Moreover, recently published papers on apremilast also pointed towards a slower titration in the initial period.9,20 Additionally, patients in Group I experienced the maximum number of AEs in the first week compared with other groups. The incidence of AEs in subsequent weeks was reduced in all groups. This suggests that further slowdown of titration helps in reducing the AEs associated with apremilast. Nausea was the most commonly reported AE in our study, unlike the other study on titration where GI upset was the most commonly reported. As reported by Giembycz et al., nausea and emesis were among the commonest adverse effects of PDE-4 inhibitors due to the expression of PDE-4 in the central nervous system.21
Additionally, the percentage of patients discontinuing apremilast was reported as lower compared with other studies, though this was not significant. This could be due to a smaller sample size. But the trend of lower AEs with a slowdown of titration was reported as significant. Additionally, though these AEs were mild to moderate in nature and resolved with the continuation of the drug, most of the AEs occurred in the initial 2 weeks of dosing.
In terms of effectiveness, 31.43%, 42.4% and 33.3% of the patients achieved PASI 75 at the end of 16 weeks in Groups I, II and III, respectively. This is in line with landmark clinical studies on apremilast.1,2 But as mentioned above, there was no statistical difference between any of the groups suggesting that even further slowdown of titration at the initiation of therapy did not affect the efficacy of apremilast. As per a recently published report from India, a minimum of 24 weeks of therapy was recommended by experts for better efficacy.9 Effectiveness was also statistically significant at week 16 from baseline in all groups in the mean score of PASI, BSA and symptoms score suggesting that all titration methods were equally effective.
To the best of our knowledge, the present study is the first prospective, randomized and comparative real‑world experience of safety and effectiveness of apremilast with three different titration methods. This study provides evidence that in patients who are intolerant to apremilast titration in the initial period, further slowdown of titration is an effective strategy. This strategy will not only help in reducing adverse effects but also help in the reduction of the discontinuation of apremilast therapy. Moreover, this strategy may help in improving compliance by patients. Additionally, clinical efficacy was also well documented with the further slowdown of titration. The clinical implication is that apremilast should be titrated gradually and tailored according to the patient’s tolerance, especially in patients who do not tolerate standard dose titration. There were some limitations such as small sample size and only one center being involved in the study.
Conclusion
In conclusion, the slow titration of apremilast is associated with lesser occurrence of AEs, and hence, fewer chances of discontinuation of the treatment. Additionally, in terms of effectiveness, there was no statistical difference between any groups in terms of PASI and BSA improvement. Hence, it can be concluded that slower titration is a useful strategy for reducing AEs but at the same time maintaining the efficacy of apremilast.
Data Sharing Statement
The datasets are available only on request due to privacy/ ethical restrictions, and can be requested from [email protected].
Disclosure
Dr Dhiraj Dhoot, Dr Namrata Mahadkar and Dr Hanmant Barkate are employees of Glenmark Pharmaceuticals Ltd. The other authors report no conflicts of interest in this work.
References
1. Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a Phase III, randomized, controlled trial (efficacy and safety trial evaluating the effects of apremilast in psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49. doi:10.1016/j.jaad.2015.03.049
2. Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–1399. doi:10.1111/bjd.14164
3. Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83(12):1583–1590. doi:10.1016/j.bcp.2012.01.001
4. Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–2029. doi:10.1016/j.cellsig.2014.05.014
5. Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC. Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov. 2014;13(4):290–314. doi:10.1038/nrd4228
6. Dattola A, Del Duca E, Saraceno R, Gramiccia T, Bianchi L. Safety evaluation of apremilast for the treatment of psoriasis. Expert Opin Drug Saf. 2017;16(3):381–385. doi:10.1080/14740338.2017.1288714
7. Daudén Tello E, Alonso Suárez J, Beltrán Catalán E, et al. Multidisciplinary management of the adverse effects of apremilast. Manejo de los efectos adversos de apremilast desde un abordaje multidisciplinar. Actas Dermosifiliogr. 2021;112(2):134–141. doi:10.1016/j.ad.2020.08.007
8. Langley A, Beecker J. Management of common side effects of apremilast. J Cutan Med Surg. 2018;22(4):415–421. doi:10.1177/1203475417748886
9. Rajagopalan M, Dogra S, Saraswat A, Varma S, Banodkar P. the use of apremilast in psoriasis: an Indian perspective on real-world scenarios. Psoriasis. 2021;11:109–122. doi:10.2147/PTT.S320810
10. De A, Sarda A, Dhoot D, Barkate H. Apremilast titration: real-world Indian experience. Clin Dermatol Rev. 2021;5(2):183–186.
11. Parasramani S, Thomas J, Budamakuntla L, Dhoot D, Barkate H. Real-world experience on the effectiveness and tolerability of apremilast in patients with plaque psoriasis in India. Indian J Drugs Dermatol. 2019;5(2):83–88.
12. Chiricozzi A, Caposiena D, Garofalo V, Cannizzaro MV, Chimenti S, Saraceno R. A new therapeutic for the treatment of moderate-to-severe plaque psoriasis: apremilast. Expert Rev Clin Immunol. 2016;12(3):237–249. doi:10.1586/1744666X.2016.1134319
13. Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/fphar.2018.01048
14. Halpin DM. ABCD of the phosphodiesterase family: interaction and differential activity in COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(4):543–561. doi:10.2147/COPD.S1761
15. Dyke HJ, Montana JG. Update on the therapeutic potential of PDE4 inhibitors. Expert Opin Investig Drugs. 2002;11(1):1–13. doi:10.1517/13543784.11.1.1
16. Dietsch GN, Dipalma CR, Eyre RJ, et al. Characterization of the inflammatory response to a highly selective PDE4 inhibitor in the rat and the identification of biomarkers that correlate with toxicity. Toxicol Pathol. 2006;34(1):39–51. doi:10.1080/01926230500385549
17. Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28(7):753–763. doi:10.1016/j.cellsig.2016.01.007
18. Busa S, Kavanaugh A. Drug safety evaluation of apremilast for treating psoriatic arthritis. Expert Opin Drug Saf. 2015;14(6):979–985. doi:10.1517/14740338.2015.1031743
19. Young M, Roebuck HL. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor: a novel treatment option for nurse practitioners treating patients with psoriatic disease. J Am Assoc Nurse Pract. 2016;28(12):683–695. doi:10.1002/2327-6924.12428
20. Carrascosa JM, Belinchón I, Rivera R, Ara M, Bustinduy M, Herranz P. The use of apremilast in psoriasis: a Delphi Study. Estudio Delphi para el uso de apremilast en la psoriasis. Actas Dermosifiliogr. 2020;111(2):115–134. doi:10.1016/j.ad.2019.07.005
21. Giembycz MA. Phosphodiesterase 4 inhibitors and the treatment of asthma: where are we now and where do we go from here? Drugs. 2000;59(2):193–212. doi:10.2165/00003495-200059020-00004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
