Back to Journals » Drug Design, Development and Therapy » Volume 13
An investigation of the stability of meropenem in elastomeric infusion devices
Authors Foy F, Luna G, Martinez J, Nizich Z, Seet J, Lie K, Sunderland B , Czarniak P
Received 12 April 2019
Accepted for publication 12 July 2019
Published 1 August 2019 Volume 2019:13 Pages 2655—2665
DOI https://doi.org/10.2147/DDDT.S212052
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Finbarr Foy,1 Giuseppe Luna,1 Jorge Martinez,1 Zach Nizich,2 Jason Seet,2 Katie Lie,2 Bruce Sunderland,1 Petra Czarniak1
1School of Pharmacy and Biomedical Sciences, Faculty of Health Sciences, Curtin University, Bentley, WA, Australia; 2Sir Charles Gairdner Hospital, Department of Pharmacy, Nedlands, WA, Australia
Purpose: To evaluate the stability of meropenem trihydrate in elastomeric infusion devices at a range of selected concentrations (6, 12, 20 and 25 mg/mL) at ambient, refrigeration and freezing temperatures.
Methods: Meropenem Ranbaxy® (meropenem trihydrate equivalent to anhydrous meropenem 1 g) vials for injection were reconstituted with 0.9% sodium chloride and adjusted to pH 6.5 using 1 M hydrochloric acid. Following preparation, solutions were stored for 7 days at either 6.7°C in elastomeric infusion devices or at −19°C in glass vials; samples of each concentration were removed from the infusion devices at specific time-points and stored for 24 hrs at 22.5°C. All solutions were assayed at specific time-points using high-performance liquid chromatography. Forced degradation in hydrochloric acid, sodium hydroxide and hydrogen peroxide was carried out at 40°C.
Results: The lowest concentration of meropenem (6 mg/mL) displayed the highest stability. It maintained >90% of its initial concentration for up to 144 hrs when stored at 6.7°C and 72 hrs following 24 hrs storage at 22.5°C, having been initially refrigerated for 48 hrs. Meropenem 20 mg/mL required immediate administration following preparation under ambient temperatures, whilst meropenem 25 mg/mL did not remain stable following 24 hrs storage at ambient temperatures. Frozen meropenem solutions displayed good stability in all concentrations but were physically unstable due to the formation of a precipitate.
Conclusion: At lower concentrations, meropenem showed suitable stability for storage and administration in elastomeric infusion devices, at refrigerated temperatures. To enhance the stability of lower concentration solutions when exposed to ambient temperatures by ambulatory patients, a more adept method of maintaining lower temperatures that reflect refrigerated conditions for elastomeric infusion devices should be devised.
Keywords: meropenem, stability, HPLC, elastomeric infusion device, OPAT
Plain Language Summary
Currently, there is a lack of stability data on meropenem at various concentrations in elastomeric infusion devices for use in outpatient parenteral antimicrobial therapy (OPAT). The use of OPAT to deliver meropenem as a continuous infusion in the “hospital in the home” setting has many advantages. Further, prolonged storage of reconstituted meropenem in elastomeric infusion devices may be required in rural or remote administration sites where several days’ supply may be needed. Therefore, this study aimed to evaluate the stability of meropenem (as the trihydrate) in elastomeric infusion devices at a range of selected concentrations (6, 12, 20 and 25 mg/mL) at ambient, refrigeration and freezing temperatures.
This study found that at lower concentrations of 6 mg/mL, meropenem showed suitable stability for storage and administration in elastomeric infusion devices, maintaining >90% of its initial concentration for up to 144 hours when stored at 6.7°C and 72 hours following 24 hours storage at 22.5°C (having been initially refrigerated for 48 hours). Meropenem 25 mg/mL displayed the least stability. At these higher concentrations of meropenem, methods should be devised to maintain the temperature at refrigerated temperatures if an elastomeric diffusion device is used during 24-hour continuous infusion.
Introduction
Meropenem is a broad spectrum carbapenem antibiotic with antimicrobial activity against a wide range of Gram-negative and Gram-positive bacteria, including anaerobes.1 It exhibits a time-dependent antibacterial effect with antibacterial activity related to the time for which the free concentration is maintained above the minimum inhibitory concentration (MIC) during a dosing interval.1,2 Administering meropenem via continuous infusion (CI) maintains serum drug concentrations above the MIC for susceptible bacteria3,4 thereby optimising pharmacodynamic targets in plasma, especially against less susceptible bacteria, such as Pseudomonas aeruginosa.5 Comparative studies between meropenem administered via CI and intermittent infusion have shown that CI improves infection eradication and requires a shorter duration of treatment.2
Outpatient parenteral antimicrobial therapy (OPAT), which allows certain antibiotics to be administered in the patients home over an extended period via an elastomeric infusion device, is gaining momentum in many countries as a beneficial treatment option.6–8 It reduces inpatient hospital stay, thereby freeing hospital beds and reducing healthcare system costs.7 OPAT offers a high degree of patient acceptability,9 allowing patients to resume some degree of normal activity, and is associated with low-levels of superinfection or other health-related complications.7 Elastomeric infusion devices can be used for meropenem OPAT to deliver the antibiotic via CI,10 therefore optimizing the pharmacokinetic-pharmacodynamic profile in the treatment of susceptible bacterial infections.
Several studies have investigated the stability of meropenem in solution at various concentrations, temperatures and pH values.10–17 Improved meropenem stability was reported when sodium chloride 0.9% was used to prepare intravenous (IV) solutions, compared to other diluents such as glucose 5% or 10%.14 Ambient temperature and meropenem concentration have a significant impact on meropenem stability. At relatively high concentrations of 20 mg/mL,10 40 mg/mL11 or 64 mg/mL,13 at various temperatures (4–40°C), meropenem displayed unfavorable stability over 24 hrs. At lower concentrations of 5 mg/mL, meropenem stability was maintained for up to 24 hrs at 25°C and for up to 8 hrs at 35°C.12 At a higher concentration of 64 mg/mL, meropenem retained above 90% of the initial concentration following 24 hrs storage at 4°C.13 According to the European Pharmacopeia, the stability of a solution for infusion is maintained when the drug concentration remains above 90% of the initial concentration, throughout the infusion interval.10,12
In a recent study which investigated the stability of meropenem CIs in ambulatory care, researchers reported improved stability over a 24 hr period at a lower concentration of 10 mg/mL.10 When ice bricks were used to maintain cooler conditions, 10 mg/mL and 20 mg/mL solutions in elastomeric infusion devices showed reduced degradation, in comparison to infusers that were not cooled, when exposed to ambient temperatures.10
Few studies have investigated meropenem stability in elastomeric infusion devices in prospect of OPAT.10,18 Further, data on the outcomes on the long-term stability of pH adjusted commercial, reconstituted meropenem solutions are not available. Previous degradation studies which investigated the degradation rate constant of meropenem at a range of pH values (4.0–12.0), reported pH 6.0–6.5 to be an optimum range for stability.16
The aim of this study was to determine the stability of reconstituted meropenem in elastomeric infusion devices at selected concentrations, reconstituted at optimum pH and under varying temperature conditions including refrigeration (2–8°C), ambient temperatures and freezing conditions (−19°C), over seven days.
Materials and methods
Materials
Analytical grade meropenem as the trihydrate of 71.8% certified meropenem purity (lot LRAA8715, expiry 03/2020; Sigma-Aldrich, USA) and commercially available meropenem powder for injection (batch number 2962309, expiry 12/2020, Meropenem Ranbaxy; Ranbaxy Pty Ltd, NSW, Australia) were used throughout this investigation. Commercially available meropenem powder for injection was available as meropenem trihydrate and included sodium carbonate.
All assays conducted used acetonitrile (lot 180372; Fisher Chemical, USA) of High Performance Liquid Chromatography (HPLC) grade, sodium dihydrogen orthophosphate monohydrate (batch number 16256; Merck Pty Ltd, VIC, Australia) and orthophosphoric acid 85% (batch number 1076916; Thermo Fisher Scientific, WA, Australia). Water was accessed through a MilliQ Ultrapure Water System (Merck, VIC, Australia) consisting of a four-bowl ultrapure cartridge kit with a conductivity of 0.05 μS; this was used throughout the duration of the investigation to prepare all buffer, standard and sample solutions.
Analyses of standard and sample solutions used HPLC. A Shimadzu Prominence LC-20AT HPLC was linked to a Shimadzu Prominence SIL-20ACHT Auto Sampler and a Shimadzu Prominence SPD20A UV Wavelength Detector (Shimadzu Corp, Kyoto, Japan); the mobile phase was de-gassed using a Shimadzu DGU-20AC5R Degassing Unit. An Apollo C18 reverse phase column (150 X 4.6 mm, 5 µ particle size; lot 50629864) and Lab Solutions® Version 5.85 software (Shimadzu Corp, Kyoto, Japan) were used for the HPLC analysis and data processing.
Forced degradation studies used hydrogen peroxide 30% analytical grade (batch number 16082255558; Ajax Finechem Pty Ltd, NSW, Australia), sodium hydroxide pellets (Chem Supply Pty Ltd, SA, Australia) and hydrochloric acid 32% analytical reagent (batch number 140410006; Ajax Finechem Pty Ltd, NSW, Australia). A Werke EH4.2 water bath (IKA®, Germany) maintained the solutions at a constant temperature of 40°C (±0.2°C), measured using a Brannan 76 mm immersion thermometer (Brannan, UK).
A Baxter LV10 elastomeric infusion device (Baxter Healthcare, NSW, Australia) modified to include a Testo 175 T2 temperature logger (±0.1°C) (Testo, VIC, Australia), with the probe located next to the infuser balloon, was used to record the average temperature of the infusion device worn over 24 hr periods. Six frozen ice-bricks (Medichill Cool Cubes; Medichill, WA, Australia) were placed at 8 hr intervals in a hip-bag with the infusion device during this time-period. The same logger device removed from the infusion device measured the refrigeration temperature.
In determining the stability of commercial meropenem in elastomeric infusion devices, separate Baxter LV10 devices were used and solutions were prepared using commercially available meropenem, sodium chloride 0.9% intravenous infusion BP (batch number S16F1, expiry 6/2019; Baxter Healthcare, NSW, Australia) and hydrochloric acid 32%. A CyberScan 510 pH meter (Thermo Fisher Scientific, WA, Australia) attached to an Ionode pH electrode (model IJ44C; QLD, Australia) was calibrated and used to record the pH of solutions throughout the study.
Assay methodology
The HPLC method was modified from Mendez et al15 to develop a stability-indicating, HPLC analysis of meropenem. The mobile phase was 10% acetonitrile and 90% sodium dihydrogen orthophosphate monohydrate 30 mM, which was adjusted to pH 3.0 using orthophosphoric acid 85%. The phosphate buffer was prepared and filtered using a 0.45 μm nylon filter membrane (lot 020415; Altech Chemical Ltd, WA, Australia). The HPLC used a 1.0 mL/min flow rate and an injection volume of 20 μL, with a run time of 12 mins. The UV wavelength detector was set to 298 nm. This method used isocratic elution.
The precision of the method was based on the United States Pharmacopeia’s precision specification as replicate injections of an analyte providing data with a relative standard deviation (RSD) of less than 2%.17 A 0.5 mg/mL standard solution of meropenem was prepared using the analytical grade sample and was analyzed six times. A second standard solution was prepared and the intraday assay was repeated the following day. The results from the two consecutive days were compared to provide interday-variation data, to thus determine the precision of the method. A five-point calibration curve was produced using analytical grade meropenem standard solutions, with concentrations of 0.05, 0.1, 0.25, 0.5 and 1 mg/mL, to evaluate linearity between chromatographic peak area and concentration.
Forced degradation assays used 6 mg/mL and 25 mg/mL solutions of commercial meropenem, in hydrogen peroxide 3%, sodium hydroxide 0.1 M or hydrochloric acid 0.1 M. Solutions were sealed in 10 mL volumetric flasks and immersed in the water bath at 40°C. Each solution was assayed for concentration at selected time points (0, 0.5, 1.0, 1.5, 2.0, and 4.0 hrs). A calculated volume of hydrochloric acid 1 M was initially added to the solutions containing hydrochloric acid 0.1 M to neutralize the sodium carbonate in the commercial meropenem powder, to thus achieve the acidic conditions required.
Ambient temperature calculation
During the summer month of January, on three separate days with a different investigator on each day, the elastomeric infusion device equipped to a temperature logger was worn in a hip-bag, on the waist, for 24 hrs. During this time, the temperature was recorded every ten minutes. A separate group of investigators conducted the same experiment, over two days, during the previous winter month of July. The average kinetic temperature, TK, recorded over 24 hrs, for each investigator, was calculated using Equation (1). A Challenge 700 stability chamber (Angelantoni Industries, Italy) was used and set to a temperature based on the data acquired.
(1)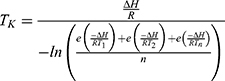
where 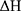 is the activation energy of meropenem (61.3 kJ/mol16),
is the activation energy of meropenem (61.3 kJ/mol16), 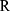 is the gas constant (8.314 J/K/mol),
is the gas constant (8.314 J/K/mol), 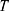 is the temperature in Kelvin and
is the temperature in Kelvin and  is the number of temperature sample points.
is the number of temperature sample points.
Stability samples of meropenem
Commercial meropenem was used to prepare solutions containing meropenem 6 mg/mL, 12 mg/mL, 20 mg/mL and 25 mg/mL. The solutions were made up to the nominal volume of the elastomeric infusion devices, 240 mL, using sodium chloride 0.9%14 intravenous infusion BP; the solutions were adjusted to approximately pH 6.516 using hydrochloric acid. Following preparation, each solution was transferred to an elastomeric infusion device. The luer-lock of each device was cut off, with a clamp used to control the flow of solution from the devices. Approximately 45 mL of solution was withdrawn from each infuser before storage in a refrigerator (2–8°C). On Days 2, 3 and 4 approximately 10 mL of solution was withdrawn from the infusers and 30 mL on Day 7. For the purposes of this investigation, Day 1 corresponded to time-point (t) 0 hrs, and Day 8 to t=168 hrs.
All HPLC analyses were performed in triplicate; a mean peak area (± SD) was quantified. The concentration of meropenem remaining at each time-point, for each meropenem concentration, was expressed as the mean percentage and standard deviation (SD) of the initial concentration at t=0 hrs; the initial concentration was expressed as 100%.
The concentration of meropenem in each solution stored in the refrigerator was determined at t=0, 24, 48, 72 and 144 hrs; the pH of each solution was recorded at t=0 and 144 hrs. For each time-point meropenem concentration was measured, the infusion devices were removed from the refrigerator (except t=0 hrs) and the required amount of solution withdrawn, before returning to the refrigerator. The solutions withdrawn from the refrigerator were allowed to equilibrate to room temperature, prior to analysis.
At t=0, 24, 48 and 144 hrs, a portion of the solution withdrawn from each device was stored separately in HPLC glass vials and transferred to the stability chamber. Following 24 hrs in the stability chamber, these solutions were assayed (t=24, 48, 72 and 168 hrs) and measured for pH (t=24 and 168 hrs). On Day 4, infuser solutions were not placed in the stability chamber.
Of the solutions withdrawn from the infusion devices at t=0 hrs, approximately 15 mL of each was transferred to separate HPLC glass vials, which were then placed in a freezer (−19°C). The solutions were removed from the freezer at t=144 hrs (Day 7) and thawed to room temperature before pH measurement. The frozen samples were filtered before undergoing dilution for HPLC analysis using a 0.2 μm GHP Acrodisc® syringe filter (Sigma-Aldrich, USA). The data for concentration and pH of the solutions withdrawn from the infusion devices at t=0 hrs served as data for the frozen solutions, at t=0 hrs.
Solutions for HPLC analysis were first diluted: 6 mg/mL by a factor of 10, 12 mg/mL by a factor of 20, 20 mg/mL by a factor of 25 and 25 mg/mL by a factor of 50. These dilution factors were maintained throughout the study for each assay. Each dilution was then analyzed by HPLC in triplicate.
Results
Assay methodology
The HPLC assay developed for meropenem analysis showed a retention time of 6.6 mins (Figure 1). Over the study period, the retention time in repeated assays varied from 5–7 mins.
 |
Figure 1 (A) Showing the HPLC spectrum of the blank; (B) Showing the HPLC assay of 0.5 mg/mL analytical grade meropenem using the developed HPLC assay. |
A standard curve was prepared using analytical grade solutions, which established a linear relationship between meropenem peak area and concentration (y=3.18 X107x +72425.8; R2=0.9999).
RSD values obtained for intraday precision for meropenem peak areas were 0.11% and 0.084% respectively. Interday data prepared by compiling the intraday data from both days provided an RSD of 0.81%. All values were below 2%, thus demonstrating adequate overall precision.
In the forced degradation experiments, meropenem showed almost complete degradation at one hour in hydrogen peroxide 3% for both 6 mg/mL and 25 mg/mL samples; no interfering degradation peaks were formed at the retention time-point, with degradation peaks distinguishably separated from meropenem’s retention time (Figure 2A). The solution containing 6 mg/mL meropenem in sodium hydroxide 0.1 M was assayed up to time-point 1.5 hrs, at which meropenem was not detectable, nor were interfering degradation peaks evident (Figure 2B). The 25 mg/mL solution containing sodium hydroxide 0.1 M was assayed up to time-point 4 hrs; similarly, no interfering degradation peaks were present. For both sodium hydroxide 0.1 M solutions, degradation peaks were noticeably separated from the meropenem peak (Figure 2B). The 6 mg/mL and 25 mg/mL solutions in hydrochloric acid 0.1 M showed a degradation peak with a retention time close to that of meropenem (Figure 2C). This peak disappeared by time-point 4.0 hrs in the 6 mg/mL meropenem solution, which suggested it was an intermediate compound. For meropenem 25 mg/mL the degradation peak appeared as a broad-shaped peak at 0.5 hrs, with it only showing significant interference with the meropenem peak area at 1.5 hrs. At 0.5 hrs of forced degradation, less than 20% of the initial meropenem concentration was retained. At 4.0 hrs the degradation peak was still present, at which time the meropenem peak area and corresponding concentration were negligible (Figure 2C).
The limit of detection (LOD) and limit of quantitation (LOQ) were estimated from the baseline noise. The values were both lower than 0.1 µg/mL.
Ambient temperature determination
When the elastomeric infusion device was worn for 24 hrs during the summer months, the average kinetic temperature obtained was 28.4°C, whereas when worn over the winter months it was 17.4°C. In consideration of seasonal variation in temperature, the stability chamber was set to a relative midpoint of these data, at 22.5 °C.
Stability studies
The concentration of meropenem remaining in the refrigerated elastomeric infusion devices at each time point is shown in Table 1. The average refrigerator temperature was 6.7°C (Range: 5.6–6.9°C). The concentrations of meropenem remaining in each solution following an additional 24 hrs incubation at 22.5 °C are also shown (Table 2).
 |
Table 1 Percentage of meropenem remaining in solutions in refrigerated (6.7°C) elastomeric infusion devices, over 7 days, at different time points; t=hours |
An assay anomaly occurred in the data for peak area in meropenem 20 mg/mL at t=0 hrs. The data available at t=24, 48, 72 and 144 hrs of the refrigerated elastomeric infusion device was plotted as the natural logarithm of peak area against time; a line-of-best-fit (R2=0.999) was generated from the plotted data. Meropenem degradation displays first-order kinetics.18 Therefore, extrapolation to the y-intercept of the line-of-best-fit provided the peak area at t=0 hrs.
For the solutions frozen at t=0 hrs and analyzed for concentration at t=144 hrs, the concentration remaining was expressed as the mean (SD) of the initial concentation. Meropenem 6 mg/mL retained 96.75% (0.18%) of its initial concentration and meropenem 12 mg/mL retained 94.05% (0.29%) of its initial concentration, at t=144 hrs; meropenem 20 mg/mL and 25 mg/mL exhibited 94.55% (0.07%) and 88.87% (0.12%), respectively, of their initial concentrations.
At t=144 hrs, precipitate existed in each frozen solution when thawed to room temperature. Attempts to dissolve the precipitate using a Bransonic® ultrasonic cleaner (model 2510E-DTH; Branson Ultrasonics, USA) followed by vigorous shaking, were unsuccessful. The fine sediment remaining was filtered prior to dilution for HPLC analysis.
The pH values of each solution, at the specified time points, for solutions refrigerated in elastomeric infusion devices, stored for 24 hrs at 22.5°C and frozen for 7 days, are shown in Table 3. Only small changes in pH were evident over the study period.
Discussion
This study has provided important practical chemical stability data for commercial meropenem as the trihydrate in continuous infusion devices commonly used by outpatients requiring IV administration. Findings from previous investigations have shown meropenem degradation is concentration, time and temperature dependant,10–13 with intermolecular aminolysis giving rise to the concentration effect.19 Our findings from this investigation are consistent with those factors. The lowest concentration of meropenem examined (6 mg/mL), displayed the highest stability as it maintained above 90% of its initial concentration in the elastomeric infusion device, under refrigeration, for the seven day study period. The 6 mg/mL solution also retained above 90% of its initial concentration up to t=72 hrs following 24 hrs at 22.5°C, having been initially refrigerated for 48 hrs. Hence the expiry period for use of this concentration is 72 hrs.
Of the remaining concentrations, only 12 mg/mL retained greater than 90% of the initial meropenem concentration after initial refrigeration for 24 hrs and subsequent administration. The meropenem sample with concentration of 25 mg/mL demonstrated greater than 10% degradation within the first 24 hrs.
It is clear that exposure to summer temperatures in Western Australia had a marked effect on the stability of meropenem. To slightly improve its use under these conditions, two separate 12 hr infusors may give improved stability over a 24 hr administration period. Other possible approaches of using two 12 hrs infusors per day could assist with overall stability and delivery of higher meropenem doses, such as 25 mg/mL, at 24 hrs. A major factor in improving stability would be developing a carrier system that maintained a much lower temperature, for example <5°C. Additionally storage in a refrigerator close to 2°C would also be beneficial since the reported activation energy of 61.3 KJ/mol. causes a large temperature effect so lowering storage temperature will markedly improve stability.16 In a comparable study by Manning et al,10 following 24 hrs under ambient conditions and whereby ice-bricks were rotated every 8 hrs to achieve cooler conditions, meropenem 20 mg/mL did not maintain stability at 24 hrs (87.0% retention).
In a study using a different elastomeric infusion device and a more adept method (equivalent to refrigeration) of maintaining lower infuser temperatures (5°C), 20 mg/mL and 30 mg/mL solutions of meropenem demonstrated favorable stability for OPAT settings, with 98.6% and 99.1%, respectively, of the initial drug concentrations being maintained following 24 hrs.20
The setting of this investigation was in the south-west of Western Australia, where the average, maximum monthly temperature ranges from 31.6°C in the summer, to 18.4°C in the winter.21 Investigations attempting to determine meropenem stability at higher temperatures, such as 30°C,12,13 35°C13 or 37°C,11,13 have reported unreliable stability, with one study suggesting against meropenem’s use in CI, as a result.13 The average temperature of the elastomeric infusion device, over 24 hrs, recorded by investigators during the summer month of January was above room temperature, at 28.4°C. During the 24 hr period over which the temperature was logged every 10 mins, there were notable fluctuations in the temperature, particularly when the ice-bricks were changed every 8 hrs; it was noted that the temperature declined to approximately 20–25°C upon changing the ice-bricks, to then rise considerably to approximately 30°C following two to three hours. The Medichill ice-bricks used were small, therefore possessing a large surface-area-to-volume ratio. It would therefore be expected that they gained heat relatively fast; based on these data, the extent to which they maintain cooled conditions under warmer climates is evidently problematic, despite the relative frequency of rotating every 8 hrs. This is in contrast to studies that wedged a meropenem solution (stored in an elastomeric infusion device cassette; intended for administration in an OPAT setting) between two ice-bricks, which were changed every 8 hrs, yet recorded an average infuser solution temperature of 5°C, over 24 hrs.20 Room temperature was recorded as ranging from 20°C to 25°C throughout that period.
Despite our data for the recorded temperatures during summer having a higher maximum value (32.9°C), the data presented by Grant et al20 is promising in providing an alternate method of achieving lower solution temperatures, which may see higher concentrations, such as 20 mg/mL or 25 mg/mL, maintain stability for 24 hrs under ambient conditions, following removal from refrigeration. This is justified further in our data, where meropenem 20 mg/mL retained above 90% of the initial concentration for three days at temperatures of 6.7°C, and meropenem 25 mg/mL for two days. These factors require addressing from a systematic and economical view-point on behalf of the ambulatory care provider, as the pump-based elastomeric infusion devices have become a favorable option due to ease of use for the patient, as well as their relatively low cost to supply. However, storing the solution for infusion in a cassette may provide greater contact between the ice-bricks used for cooling and the solution, in comparison to that provided by the pump-based infusers, where a gap exists between the balloon containing the solution, and the wall of the container, where the ice-bricks would make contact. In using a cassette, the meropenem solution may withstand the lower temperatures (2–8°C) required to maintain the long-term stability desired in OPAT.
Despite our data reporting good long-term stability for meropenem solutions following freezing for seven days at concentrations of 6 mg/mL, 12 mg/mL and 20 mg/mL, it was found that freezing the solutions was impractical. Upon thawing, a distinct precipitate, comprised of large crystals, existed in each solution; based on solubility, this precipitate was concluded to be sodium carbonate.22 The precipitate proved too difficult to dissolve and the current commercial formulation requires further evaluation before freezing storage could be implemented.
At a low meropenem concentration of 6 mg/mL, the data showed promising stability over a seven-day period (when stored at 6.7°C), which correlates to promising stability in an OPAT setting. However, the pharmacokinetic parameters, such as steady-state concentration, must be considered, and how these compare to the infecting pathogen’s MIC. Manning et al considered the pharmacokinetic issues.10 To achieve optimum pharmacodynamic activity and to optimize the time-dependent killing of meropenem, the steady-state concentration should be at least three to four fold the MIC for a majority, if not all, of the dosing interval.1,2,4,23 At a meropenem concentration of 10 mg/mL (intended for CI; equivalent to a daily dose of 2.4 g), a population pharmacokinetic model determined the median, 2.5th centile and 97.5th centile steady-state meropenem plasma concentrations, which were compared to breakpoints for Enterobacteriaceae and Pseudomonas aeruginosa.10 The 2.5th centile, steady-state plasma concentration fell below the breakpoint for Pseudomonas aeruginosa, suggesting administering meropenem at 6 mg/mL, most of the population will achieve ineffective steady-state concentrations against Pseudomonas aeruginosa, which is a common pathogen meropenem is used to treat, particularly in cystic fibrosis.10,23,24 The administration of meropenem via CI at 12.5 mg/mL or 25 mg/mL over 24 hrs gave predicted steady-state meropenem plasma concentrations that are adequately above the MIC for Enterobacteriaceae, throughout the entire dosing interval.23
Previous studies investigating the stability of reconstituted meropenem have not made pH adjustments to the solutions containing commercial-sample drug. For this study the pH of the commercial meropenem solutions prepared were adjusted to pH 6.5, which was previously determined to show the lowest rate of degradation,16 particularly in comparison to the expected pH of the commercial meropenem solutions (pH 7.3–8.3), as specified by the manufacturer.25 From the initial pH measurement following solution preparation, to the final time-point at which pH measurement occurred, the pH values of the solutions remained relatively consistent. If the relatively long-term stability exhibited by some meropenem concentrations in this study was partially attributed to the pH adjustment is unclear. A control, unadjusted solution was not available for comparison. As previously mentioned, Manning et al10 used very similar investigation settings to those of this study. At a concentration of 20 mg/mL, these investigators found the meropenem had 87.0% retention following 24 hrs under ambient temperatures.10 Our data shows slightly improved stability at similar ambient temperatures following 24 hrs, with meropenem 20 mg/mL having 90.7% retention. It is unknown if this improved stability was associated with the pH adjustment. Other investigators reported good meropenem stability at concentrations at 20 mg/mL after 24 hrs (98.6% retention), with no pH adjustments.20 However, these solutions were maintained at 5°C which is much lower than the ambient temperatures used in this investigation (22.5°C).20
The model rate equation for meropenem over the pH range of 4.0–10.0 is taken from Takasu et al16 (Equation (2)).
(2)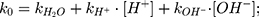
where 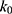 is the observed rate constant,
is the observed rate constant, 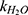 is the first-order rate constant for the spontaneous water-catalyzed degradation reaction,
is the first-order rate constant for the spontaneous water-catalyzed degradation reaction, 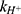 and
and 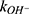 are the second-order rate constants for the hydrogen-ion-catalyzed degradation and the hydroxide-ion-catalyzed reaction, respectively.
are the second-order rate constants for the hydrogen-ion-catalyzed degradation and the hydroxide-ion-catalyzed reaction, respectively.
At pH 6.0, 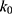 = 1.32×10-3 h-1; at pH 6.5,
= 1.32×10-3 h-1; at pH 6.5, 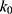 = 1.28×10-3 h-1; at pH 8.0,
= 1.28×10-3 h-1; at pH 8.0, 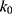 = 4.17×10-3 h-1. Hence, at pH 6.5 meropenem displays the lowest rate of degradation, which was the average recorded pH of the meropenem solutions at t=0 hrs. At pH 8.0, the estimated degradation rate is 3.25-fold the degradation rate at pH 6.5. This is suggestive that the higher retention in meropenem 20 mg/mL in this study, in comparison to Manning et al,10 is potentially due to the pH adjustment. However, it suggests that the high-degree of stability reported by Grant et al20 was probably achieved with the low temperature maintained. It would be advantageous for further investigations to compare the stability of meropenem solutions when pH adjustments are made (to pH 6.5) with an unadjusted control.
= 4.17×10-3 h-1. Hence, at pH 6.5 meropenem displays the lowest rate of degradation, which was the average recorded pH of the meropenem solutions at t=0 hrs. At pH 8.0, the estimated degradation rate is 3.25-fold the degradation rate at pH 6.5. This is suggestive that the higher retention in meropenem 20 mg/mL in this study, in comparison to Manning et al,10 is potentially due to the pH adjustment. However, it suggests that the high-degree of stability reported by Grant et al20 was probably achieved with the low temperature maintained. It would be advantageous for further investigations to compare the stability of meropenem solutions when pH adjustments are made (to pH 6.5) with an unadjusted control.
A limitation was the limited time points at which the meropenem solutions were assayed for meropenem concentration. Additional time points may have provided data that indicated longer stability times than could be applied to this study which were based on measured concentrations of meropenem.
A limitation of this study was that a bulk solution to fill enough infusers for a week’s supply was not prepared under sterile conditions. However, we consider that hydrochloric acid could be prepared sterile by filtration or purchased sterile in ampoules, and a predetermined amount added (from previous experimentation) that would be known to adjust the pH to about 6.5. This could also be considered for a hospital pharmacy asepsis suite with a sample of the bulk preparation tested for pH externally, with any required adjustment made prior to filling the infusers.
Conclusion
Meropenem as the trihydrate in elastomeric administration devices displays reasonable stability at lower concentrations (6 mg/mL and 12 mg/mL), under refrigerated conditions (6.7°C). Higher concentrations of meropenem (20 mg/mL and 25 mg/mL) require methods to be devised to maintain the temperature at refrigerated temperatures whilst the infusion device is used during the 24 hr CI administration. Further methods need to be devised to improve the stability of the 20 mg/mL and 25 mg/mL concentrations before they would be suitable for use in infusor devices. Changes to the infusion devices used, and the materials used to keep the infusers cool, may lead to a functional meropenem stability following 24 hr CI, when exposed to variable environmental temperatures, thus rendering these higher concentrations investigated as appropriate for use in ambulatory care. Although the stability of meropenem is retained for prolonged durations through freezing, the subsequent formation of insoluble precipitates in commercial meropenem solutions containing sodium carbonate is considered inappropriate until further investigations have been conducted.
Abbreviations
CI, continuous infusion; HPLC, high performance liquid chromatography; MIC, minimum inhibitory concentration; OPAT, outpatient parenteral antimicrobial therapy; RSD, relative standard deviation; SD, standard deviation.
Acknowledgement
The authors acknowledge the assistance of Elaheh Ghotbaldini and Meera Patel in the determination of the average kinetic temperature by wearing the elastomeric infusion device equipped to a temperature logger during the winter months.
Disclosure
The authors declare that they have no conflicts of interest to disclose in this work.
References
1. Lee YR, Baker NT. Meropenem-vaborbactam: a carbapenem and beta-lactamase inhibitor with activity against carbapenem-resistant Enterobacteriaceae. Eur J Clin Microbiol Infect Dis. 2018;37(8):1411–1419. doi:10.1007/s10096-018-3260-4
2. Zhao H, Gu J, Lyu J, et al. Pharmacokinetic and pharmacodynamic efficacies of continuous versus intermittent administration of meropenem in patients with severe sepsis and septic shock: a prospective randomized pilot study. Chin Med J. 2017;130(10):1139–1145. doi:10.4103/0366-6999.205859
3. Jaruratanasirikul S, Sriwiriyajan S. Comparison of the pharmacodynamics of meropenem in healthy volunteers following administration by intermittent infusion or bolus injection. J Antimicrob Chemother. 2003;52(3):518–521. doi:10.1093/jac/dkg486
4. Keil S, Wiedemann B. Antimicrobial effects of continuous versus intermittent administration of carbapenem antibiotics in an in vitro dynamic model. Antimicrob Agents Chemother. 1997;41(6):1215–1219. doi:10.1128/AAC.41.6.1215
5. Roberts JA, Kirkpatrick CMJ, Roberts MS, et al. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother. 2009;64(1):142–150. doi:10.1093/jac/dkp139
6. Saillen L, Arensdorff L, Moulin E, et al. Patient satisfaction in an outpatient parenteral antimicrobial therapy (OPAT) unit practicing predominantly self-administration of antibiotics with elastomeric pumps. Eur J Clin Microbiol Infect Dis. 2017;36(8):1387–1392. doi:10.1007/s10096-017-2944-5
7. Chapman ALN. Outpatient parenteral antimicrobial therapy. BMJ. 2013;346:f1585. doi:10.1136/bmj.f1164
8. Chapman ALN, Seaton RA, Cooper MA, et al. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: a consensus statement. J Antimicrob Chemother. 2012;67(5):1053–1062. doi:10.1093/jac/dks003
9. Mitchell ED, Czoski Murray C, Meads D, et al. Clinical and cost-effectiveness, safety and acceptability of community intravenous antibiotic service models: CIVAS systematic review. BMJ. 2017;7(4):e013560.
10. Manning L, Wright C, Ingram PR, et al. Continuous infusion of meropenem in ambulatory care: clinical efficacy, safety and stability. PLoS One. 2014;9(7):
11. Berthoin K, Le Duff CS, Marchand-Brynaert J, Carryn S, Tulkens PM. Stability of meropenem and doripenem solutions for administration by continuous infusion. J Antimicrob Chemother. 2010;65(5):1073–1075. doi:10.1093/jac/dkq044
12. Franceschi L, Cojutti P, Baraldo M, Pea F. Stability of generic meropenem solutions for administration by continuous infusion at normal and elevated temperatures. Ther Drug Monit. 2014;36(5):674–676. doi:10.1097/FTD.0000000000000054
13. Viaene E, Chanteux H, Servais H, Mingeot-Leclercq M, Tulkens PM. Comparative stability studies of antipseudomonal beta-lactams for potential administration through portable elastomeric pumps (home therapy for cystic fibrosis patients) and motor-operated syringes (intensive care units). Antimicrob Agents Chemother. 2002;46(8):2327–2332. doi:10.1128/AAC.46.8.2327-2332.2002
14. Patel PR, Cook SE. Stability of meropenem in intravenous solutions. Am J Health Syst Pharm. 1997;54(4):412–421. doi:10.1093/ajhp/54.2.171
15. Mendez ASL, Steppe M, Schapoval EES. Validation of HPLC and UV spectrophotometric methods for the determination of meropenem in pharmaceutical dosage form. J Pharm Biomed Anal. 2003;33(5):947–954. doi:10.1016/S0731-7085(03)00366-2
16. Takasu Y, Yoshida M, Tange M, Asahara K, Uchida T. Prediction of the stability of meropenem in intravenous mixtures. Pharm Bull. 2015;63(4):248–254. doi:10.1248/cpb.c14-00516
17. United Stats Pharmacopeia and National Formulary (USP41-NF36) [internet].
18. Mendez ASL, Dalomo J, Steppe M, Schapoval EES. Stability and degradation kinetics of meropenem in powder for injection and reconstituted sample. J Pharm Biomed Anal. 2006;41(4):1363–1366. doi:10.1016/j.jpba.2006.02.017
19. Mendez A, Chagastelles P, Palma E, Nardi N, Schapoval E. Thermal and alkaline stability of meropenem: degradation products and cytotoxicity. Int J Pharm. 2008;350(1–2):95–102. doi:10.1016/j.ijpharm.2007.08.023
20. Grant EM, Zhong MK, Ambrose PG, Nicolau DP, Nightingale CH, Quintiliani R. Stability of meropenem in a portable infusion device in a cold pouch. Am J Health Syst Pharm. 2000;57(10):992–995. doi:10.1093/ajhp/57.suppl_4.S10
21. Bureau of meteorology. Climate statistics for Australian locations [Internet]. 2019. Available from: http://www.bom.gov.au/climate/averages/tables/cw_009225.shtml.
22. British Pharmacopoeia [Internet]. Vol. 1. London: Medicines and Healthcare products Regulatory Agency; 2019. Sodium Carbonate. Available from: https://www-pharmacopoeia-com.dbgw.lis.curtin.edu.au/bp-2019/monographs/sodium-carbonate.html?date=2019-01-01&text=sodium+carbonate.
23. Kuti JL, Nightingale CH, Knauft RF, Nicolau DP. Pharmacokinetic properties and stability of continuous-infusion meropenem in adults with cystic fibrosis. Clin Ther. 2004;26(4):493–501. doi:10.1016/S0149-2918(04)90051-3
24. Prescott WA, Gentile AE, Nagel JL, Pettit RS. Continuous-infusion antipseudomonal Beta-lactam therapy in patients with cystic fibrosis. P & T. 2011;36(11):723–763.
25. Meropenem Ranbaxy. In MIMS Online Database [Internet]. MIMS Australia; 2014. Available from: https://www-mimsonline-com-au.dbgw.lis.curtin.edu.au/Search/FullPI.aspx?ModuleName=Product%20Info&searchKeyword=meromerom&PreviousPage=~/Search/QuickSearch.aspx&SearchType=&ID=93950001_2.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



