Back to Journals » International Journal of Women's Health » Volume 14
An Investigation of Exosome Concentration and Exosomal microRNA (miR-155 and miR-222) Expression in Pregnant Women with Gestational Hypertension and Preeclampsia
Received 18 July 2022
Accepted for publication 4 November 2022
Published 7 December 2022 Volume 2022:14 Pages 1681—1689
DOI https://doi.org/10.2147/IJWH.S382836
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Aviwe Ntsethe, Irene Mackraj
Department of Human Physiology, School of Laboratory Medicine and Medical Sciences, University of KwaZulu-Natal, Durban, South Africa
Correspondence: Irene Mackraj; Aviwe Ntsethe, Department of Human Physiology, University of KwaZulu-Natal, Durban, South Africa, Tel +27 31-260-7770 ; +27 31-260-7192, Email [email protected]; [email protected]
Introduction: Hypertensive disorders of pregnancy are characterized by widespread maternal endothelial dysfunction. Elevated secretion of exosomes has been associated with endothelial dysfunction. Exosomes play a role in cell–cell communication by transferring microRNAs. These microRNAs are associated with the pathogenesis of hypertensive disorders of pregnancy through the regulation of endothelial function. This study characterizes exosomes and determines exosomal miR-155 and miR-222 expression levels in women with gestational hypertension (GH) and preeclampsia (PE).
Methods: Exosomes were isolated and thereafter characterised using NTA, microscopy and ELISA. Results: Exosomes were elevated in the serum of pregnant women with GH and PE (P< 0.05). The circulating exosomes and placental exosomes were increased in both GH and PE (P< 0.0001). The exosomal miR-155 increased in PE but not in GH (P < 0.05). MiR-222 decreased in PE (P < 0.05).
Discussion: Elevated exosomes in pregnant women with GH and PE may be indicative of exosomes being potential biomarkers for both GH and PE. The difference in the exosomal miR-155 and miR-222 expression in PE and GH suggested that these two disorders have different pathological pathways.
Keywords: exosomes, gestational hypertension, MiR-155, MiR-222, preeclampsia
Introduction
Hypertensive disorders of pregnancy (HDP) are the leading cause of maternal and neonatal morbidity and mortality locally and globally1. Approximately 18% of the global maternal deaths are due to HPDs.2 In South Africa, approximately 14.8% of maternal deaths are due to HDP.1 It is characterized by an aberrant increase in blood pressure with or without proteinuria in urine after the 20th week of gestation.3 Gestational hypertension (GH) and Preeclampsia (PE) are the most diagnosed hypertensive conditions in pregnancy.4 Both PE and GH are characterized by the development of new hypertension (systolic blood pressure of ≥140 mm Hg and diastolic blood pressure of ≥90 mm Hg) after 20 weeks of gestation.5 However, the difference pertains to comorbidities; women with PE have placentae characterized by high syncytial knots and different distributions of fibrin deposits.6 PE is also characterized by an increase in proteinuria (≥300 mg) and an increase in circulating extracellular vesicles (exosomes).
The etiology of HPD is still not well understood due to the multifactorial nature of the disorder. However, syncytiotrophoblast microparticles (STBM) directly released from the placenta into the maternal circulation were recently identified as factors that are antiangiogenic, pro-coagulant, and involved in endothelial dysfunction,7 a key pathological feature of HDP. Exosomes are a critical essential constituent of STBM. They are the smallest membrane vesicles (30–150 nm)8 released by exocytosis from multivesicular bodies of the endosome. Exosomes are secreted by multiple cell types and can be found in different body fluids such as saliva, plasma and urine.9 They have emerged as critical biological signalling entities because their cargo contains important biological molecules such as proteins, mRNA, and miRNA to induce cell-to-cell communication and signalling throughout the body. Therefore, it may play a vital role in the pathogenesis of diseases.
The biogenesis, characterization, and function of exosomes are new areas of study that has sparked considerable interest in the last decade. Exosomes (along with other types of extracellular vesicles) play a vital role in the regulation of a wide range of physiological and pathological cellular processes. They may be utilized for therapeutic purposes and act as candidate biomarkers in various diseases.10 Recently, exosomes have been linked to the pathogenesis of PE,7 but little is known about GH.
The release of exosomes is dependent on their microenvironment and is influenced by several factors, including oxygen tension and glucose concentrations.11 However, the role of exosomes in the pathophysiology of PE and GH is not well understood. It has been reported that the total and placental exosomes with no known associated pathologies play a pivotal role in the regulation of endothelial cell migration.12 Under normal conditions, maternal circulating placental exosomes increase with gestational age12,this inversely correlates to endothelial cell migration. Conversely, it has been demonstrated that placental exosomes could play a pathological role in PE.7 Reports also indicate that exosomes regulate immune tolerance during normal and complicated pregnancies.13,14 In addition, exosomal miRNAs can be incorporated by the recipient cells, where they modulate functional effects by changing host gene expression.15
MicroRNAs have been associated with a wide range of physiological processes, including proliferation, differentiation, apoptosis, inflammation, and angiogenesis.16 These processes are disrupted in HDP, suggesting that miRNAs play a crucial role in HDP pathogenesis. MiR-155 are essential regulators of eNOS expression, and inhibition of miR-155 restores eNOS expression and improves endothelial dysfunction.17 The majority of NO in the circulation is synthesized from the endothelium, aberrant expression of eNOS may reduce VEGF expression, causing endothelial dysfunction18. MiR-222 is one of the miRNAs that play a role in the pathogenesis of GH and PE.19 Upregulation of miRNA-222 is associated with the increased estradiol secretion and the early phases of pregnancy development are thought to depend on an elevation of oestradiol secretion.20
There is a lack of information regarding the role of exosomes in GH and PE. These disorders are classified as part of HDP and are characterized by widespread endothelial dysfunction.21 In addition, some women with GH are reported to develop PE in their third trimester of the subsequent pregnancy.22 Therefore, there is a possible link between these two disorders. Hence, the present study investigated the association of exosomes in the pathogenesis of GH and PE. The study aimed to determine the role of exosomes and exosomal miRNAs (miR-155 and miR-222) expression in GH and PE.
Methods and Materials
Regulatory Permissions
Ethical approval was received from the University of KwaZulu-Natal Biomedical Research Ethics Committee (BE229/16), South Africa. Health authority permission was also obtained, and all research participants provided written informed consent. The research was conducted ethically according to the principles of the Declaration of Helsinki.
Study Population
This is a cross-sectional study, and participants were recruited at a regional hospital in Durban, South Africa. Participants included normotensives (NH) with no obstetrical or medical complications (n = 15), GH (n = 15) and PE (n = 15). All participants were aged between 17 and 45 years. Patients with chronic hypertension, previous history of PE, diabetes, and other cardiovascular disorders were excluded. GH was defined as a blood pressure of ≥140/90mmHg with no proteinuria.23 Late-onset PE was defined as a sustained blood pressure of ≥140/90 mmHg with proteinuria of at least 300 mg after 34 weeks of pregnancy.1
Sample Collection
Blood samples were collected [BD Vacutainer Tubes (EDTA), Becton Dickinson and Company, South Africa], and the serum samples were stored at −80◦C for analyses.
Isolation and Purification of Exosomes from Maternal Circulation
Exosomes were isolated according to the method described previously in our laboratory.7 Serum (1 mL) was diluted with an equal volume of phosphate buffered saline (PBS; pH 7.4). Exosomes were isolated and purified by differential ultracentrifugation using a 30% sucrose cushion. In brief, to remove cells, centrifugation was initially performed at 2000 g at 4°C for 30 min, followed by 12,000 g at 4°C for 45 min. To remove the remaining debris, the supernatant was transferred to other centrifuge tubes and centrifuged at 110 000 g at 4°C for 120 min (Optima™ MAX-XP Ultracentrifuge, fixed angle MLA-55 rotor, Beckman Coulter Inc., Brea, CA, USA). To remove particles that were larger than 200 nm, the pellet was suspended in 2 mL of PBS and filtered through a 0.20 μm pore filter (Cellulose acetate, GVS™, Europe). The filtrate was centrifuged at 110 000 g at 4°C for 70 min following which the pellet was re-suspended in 2 mL PBS (pH 7.4) and centrifuged at 110 000 g for 70 min at 4°C. To purify the exosomes, the exosome pellet was suspended in 2 mL of PBS and subsequently purified using a 30% sucrose cushion and centrifuged at 110 000 g at 4°C for 75 min. The final pellet was re-suspended in 300 μL of PBS and stored at −80°C.
Nanoparticle Tracking Analysis
The size distribution of exosomes and their concentration were determined using the NS500 equipped with a 405nm laser and sCMOS camera (NanoSight NTA 3.0 Nanoparticle Tracking and Analysis Release, Version Build 0069). Serum samples were diluted with PBS (1:100) prior to analysis in order to obtain particle distribution of 10 and 100 particles per image (optimal, 50 particles per image) before the analysis with NTA system. Samples were introduced into the sample chamber using the following script: PUMPLOAD, REPEATSTART, PRIME, DELAY 10, CAPTURE 60 and REPEAT 5. Videos were recorded at a camera level of 10, camera shutter speed of 20ms and camera gain of 600, these settings were kept constant between samples. Each video was then analysed to give the mean particle size together with the concentration of particles. The size of the exosomes was represented as the mean particle size ± SEM.
Transmission Electron Microscopy
Exosomes were applied to a continuous carbon grid and negatively stained with 2% uranyl acetate. The size and morphology of the particles were examined using a JEOL 1010 transmission electron microscope (JEOL, Peabody, MA, USA) at the Microscopy and Microanalysis Unit, University of KwaZulu-Natal.
Quantification of the Circulating Exosomes
The concentration of total exosomes in the circulation was determined by the quantification of total immune-reactive CD63 enzyme-linked immune absorbency assay (ExoELISA™, System Biosciences, Mountain View, CA), as described by.7,11 CD63 is not an exosome specific marker but is commonly bound to the exosomal membrane and hence the method employed used isolated and purified exosomes, which contain the CD63 marker. The kit used, consists of an exosome specific primary CD63 antibody developed by the manufacturer. Briefly, exosomes were immobilized on microtiter plates for overnight at 37°C using exosome binding buffer supplied the manufacturer (System Biosciences). Plates were washed and incubated at room temperature for 1h with exosome specific primary antibody (CD63), followed by a wash step and incubation with secondary antibody (1:5000) at RT for 1h with agitation. Plates were thereafter washed and incubated with Super-sensitive TMB ELISA substrate at RT for 45 min with agitation. The reaction was terminated using Stop Buffer solution. Absorbance was measured at 450nm. The number of exosomes/mL were obtained using an exosomal CD63 standard curve that was generated using the calibrated exosome standard that was supplied.
Quantification of Placental Derived Exosomes
The relative concentration of placental-derived exosomes was determined by the quantification of human placental alkaline phosphatase in the exosomal fraction using a commercial ELISA kit (Elabscience, E-EL-H1976, Wuhan, P.R.C), as described by.7,11 Briefly, exosomes were allowed to bind to the primary PLAP specific antibody coated plates by incubation at 37°C for 90 min. Plates were washed and 50 µL of HRP-conjugate was added to each well and incubated at 37°C for 20 min. Plates were washed and incubated with 50 µL of substrate A and 50 µL of substrate B at 37°C for 15 min. The incubation was terminated using 50 µL of stop solution at RT for 2 min under agitation. Absorbance was measured at 450 nm. Exosomal PLAP was expressed as pg/mL serum. The quantification of PLAP in the exosomal fraction indicates the relative concentration of placental-derived exosomes (PLAP exosomes) in maternal circulation.
RNA Isolation
RNA was isolated from 100μL exosome sample using the PureLink RNA isolation Kit according to the manufacturer’s instructions (ThermoFisher Scientific) and was eluted in 50μL of nuclease free water.
Quantitative Real-Time Polymerase Chain Reaction Analysis for miRNAs
Total RNA from each sample was reverse transcribed into cDNA using iScript cDNA Synthesis Kit according to manufacturer’s protocol (Bio-Rad Laboratories, Hercules, CA, USA). Quantification of miRNAs was done using SYBR-Green 1 master mix with the light cycler 96 (Roche). 2μL of cDNA was used for qRT-PCR. The master mix contained 10μL SYBER- Green, 2μL forward respectively and 4μL RNase-free water for a final volume of 20μL. All samples were run in duplicates. The 3-step amplification procedure was performed at 95°C for 15 min, 60°C for 30 secs, and 72°C for 30 sec. The U6 small nuclear RNA (RNU6) was used to determine relative miRNA expression following a protocol by.24 The miRNAs of interest were miR-222 and miR-155 (Table 1).
 |
Table 1 MicroRNA Primer Sequence |
Statistical Analysis
GraphPad Prism 5 was used for statistical analysis. Statistical analysis was performed using one-way analysis of variance (ANOVA). Results were expressed as mean ± SEM from 15 patients in each group. P values ≤0.05 were considered as significant.
Results
Exosome Isolation and Characterization
Figure 1 shows the quantity of total exosome concentration in serum of normotensive, GH and PE groups obtained from the nanoparticle tracking analysis (NTA). There was a significant increase in the total exosome concentration of GH (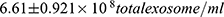 ) and PE group (
) and PE group (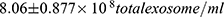 ) as compared to normotensive group (
) as compared to normotensive group (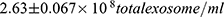 , P<0.05). Particles of ̴ 100 nm diameter were obtained from the TEM (Figure 2). In addition, CD63 ELISA demonstrated a significant increase in CD63 protein of both GH group (
, P<0.05). Particles of ̴ 100 nm diameter were obtained from the TEM (Figure 2). In addition, CD63 ELISA demonstrated a significant increase in CD63 protein of both GH group (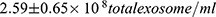 ) and of PE group (
) and of PE group (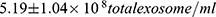 ) compared to normotensive (
) compared to normotensive (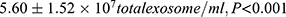 ) (Figure 3).
) (Figure 3).
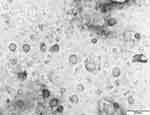 |
Figure 2 Electron micrograph of isolated exosomes, scale bar 200 nm. |
Quantification and Contribution of Placental-Derived Exosomes to Total Exosomes from Maternal Serum
To determine the relative contribution of placental exosomes to total exosomes present in maternal serum, the PLAP content per exosome was determined. The concentration of PLAP+ exosomes in maternal serum is represented in Figure 4. There was a significant increase in PLAP+ exosome concentration in both GH group (174.8 ± 20.66 pg/mL) and PE group (255.4 ± 16.76 pg/mL) as compared with normotensive (28.48 ± 6.19 pg/mL, P < 0.001).
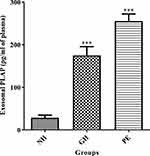 |
Figure 4 The concentration of placental derived exosomes (PLAP+ exosomes) in maternal serum. ***NH vs GH and ***NH vs PE, (P<0.001). ***Shows the level of significance between groups. |
MiRNA Expression in GH, PE and Normotensives
Figures 5 and 6 show exosomal miRNAs expression in normotensive (NH), PE and GH sample groups. There was a significant increase of exosomal miR-155 expression in PE as compared to normotensive exosomes (P<0.05) (Figure 5). There was a significant decrease in miR-222 expression in PE as compared with normotensive exosomes (P<0.05) (Figure 6).
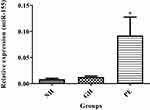 |
Figure 5 Exosomal miR-155 expression levels in normotensive (NH), PE and GH patient groups. *NH vs PE, (P<0.05). *Shows the level of significance between groups. |
 |
Figure 6 Exosomal miR-222 expression levels in normotensive (NH), PE and GH patient groups. **NH vs PE, (P<0.01). **Shows the level of significance between groups. |
Discussion
In the present study, the levels of total circulating exosomes in the serum of GH and PE were elevated (vs normotensive patients) (Figure 1). This study is in accordance with our previous findings of an increase in the total exosomes in PE (vs normotensives).7 The current findings add new information supporting exosomes as potential biomarkers of GH. The similarity in findings is not surprising given the linked pathologies involving endothelial dysfunction.21 This cellular stress in the form of endothelial dysfunction may stimulate secretion of exosomes25 and the exosomes themselves may cause endothelial dysfunction.
Characterization of the isolated particles using TEM was positive for exosomes as it showed particle size range of exosomes (75nm – 107nm) (Figure 2). A significant increase in CD63 in GH and PE as compared to normotensive exosomes was observed (Figure 3), confirming the presence of exosomes in the serum. Furthermore, placental exosome levels were significantly increased in GH and PE as compared to normotensives (Figure 4). Thus, the study showed that exosomes are not only potential biomarkers of PE but also of GH.
Altered expression of microRNAs may play a role in the pathogenesis of PE.19,26 Importantly, it has been reported that majority of miRNAs in serum are detectable in exosomes27 and exosome function as intercellular communicators via transfer of the biological information such as miRNAs. Leukemia cells communicate to endothelial cells via exosomal miRNAs which enhances endothelial cell migration27 thus exosomal miRNAs are very important for endothelial cell communication.
We found significantly increased levels of circulating exosomal miR-155 expression in PE (Figure 5). These findings correlate with previous study that found increased expression of miR-155 in placentae of PE patients17 and suppression of eNOS expression in trophoblastic cells, possibly impacting on their migratory behaviour. However, further work need to check if these were of placental origin. It is well known that nitric oxide is a potent vasodilator and decreased bioavailability leads to endothelial dysfunction. The eNOS expression is modulated via the modification of eNOS mRNA stability by affecting the binding of some cytoplasmic proteins with eNOS mRNA 3′ untranslated regions. MiR-155 inhibits eNOS mRNA stability by binding 3’UTR thus downregulating eNOS expression.18 The increased exosomal miR-155 in PE may play a role in the endothelial cells’ eNOS suppression, thus leading to endothelial dysfunction. In contrast, we found no significant difference between miR-155 expressions in GH vs normotensives. This may be due to the differential in pathological pathways of these two disorders, particularly the etiology.
We also found a significant decrease in miR-222 expression in PE compared to normotensive exosomes (Figure 6) but no significant difference between GH and normotensive exosomes. Upregulation of miR-222 increases oestradiol secretion.20 It has been shown that miR-222 is downregulated in the placental syncytiotrophoblast vesicles (STBMs) of preeclamptic patients.28
The MiR-221 and miR222 belong to the same family group and target common genes. They regulate endothelial cell migration and angiogenesis by targeting stem cell factor (SCF) receptor. SCF plays a vital role in promoting the survival and migration of endothelial cells. Thus, downregulation of miR-222 affects SCF and may lead to poor endothelial migration.29 In addition, miR-222 has also been found to induce the production of endothelial nitric oxide.30 Therefore, downregulation of miR-222 reduces nitric oxide bioavailability. Thus, this miRNA may play a pivotal role in the pathogenesis of PE.
Limitations
The eNOS expression in the exosomes was not measured.
Conclusion
Our preliminary studies support the role of exosomes as potential biomarkers of both PE and GH. Exosomal miRNA was altered in PE, keeping with the known pathology of the disease. In GH, we found no alteration of the investigated miRNAs. The differential findings are in keeping with the fact that the pathology is different with some common features. The clinical utility is based on the fact it will aid in diagnostics.
Ethics
Ethical approval for this study was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee on 27/07/16. The BREC reference number allocated to this study is BE229/16.
Acknowledgments
The authors would like to acknowledge Prof. M. Singh for the use of the NanoSight500 (Biochemistry Department, UKZN), and (Microscopy and Microanalysis Unit, UKZN). We would like to acknowledge also UKZN College of Health Science scholarship (CHS), National Research Foundation (NRF) for funding this project. We would also like to thank Dr K. Moodley, Mr. S. Eche, Dr O Khaliq and Ms. Zinhle Mkhize for their assistance.
Funding
The authors would like to thank the College of Health Science (UKZN) and National Research Foundation (NRF) for funding this project.
Disclosure
The authors of this study declare that there are no conflicts of interest.
References
1. Moodley J. Maternal deaths due to hypertensive disorders of pregnancy: data from the 2014–2016 Saving Mothers’ Report. In: Obstetrics and Gynaecology Forum. House Publications; 2018:28–32.
2. Muti M, Tshimanga M, Notion GT, Bangure D, Chonzi P. Prevalence of pregnancy induced hypertension and pregnancy outcomes among women seeking maternity services in Harare, Zimbabwe. BMC Cardiovasc Disord. 2015;15:111. doi:10.1186/s12872-015-0110-5
3. Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc. 2018;7(17):e009382. doi:10.1161/JAHA.118.009382
4. Savitz DA, Danilack VA, Engel SM, Elston B, Lipkind HS. Descriptive epidemiology of chronic hypertension, gestational hypertension, and preeclampsia in New York State, 1995–2004. Matern Child Health J. 2013;18:829–838. doi:10.1007/s10995-013-1307-9
5. Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998;105(11):1177–1184. doi:10.1111/j.1471-0528.1998.tb09971.x
6. Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–975. doi:10.1161/hypertensionaha.107.107607
7. Pillay P, Maharaj N, Moodley J, Mackraj I. Placental exosomes and pre-eclampsia: maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta. 2016;46:18–25. doi:10.1016/j.placenta.2016.08.078
8. Mincheva-Nilsson L, Baranov V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol. 2014;72(5):440–457. doi:10.1111/aji.12311
9. Jin J, Menon R. Placental exosomes: a proxy to understand pregnancy complications. Am J Reprod Immunol. 2018;79(5):e12788. doi:10.1111/aji.12788
10. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi:10.1016/j.jconrel.2015.07.030
11. Salomon C, Yee S, Mitchell MD, Rice GE. The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions. Biomed Res Int. 2014;2014:1–10. doi:10.1155/2014/693157
12. Salomon C, Torres MJ, Kobayashi M, et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One. 2014;9(6):e98667. doi:10.1371/journal.pone.0098667
13. Sabapatha A, Gerçel-Taylor Ç, Taylor DD. Specific isolation of placenta‐derived exosomes from the circulation of pregnant women and their immunoregulatory consequences 1. Am J Reprod Immunol. 2006;56:345–355. doi:10.1111/j.1600-0897.2006.00435.x
14. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi:10.1038/nri3622
15. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi:10.1038/ncb1596
16. Yan T, Liu Y, Cui K, Hu B, Wang F, Zou -L-L. MicroRNA‐126 regulates EPCs function: implications for a role of miR‐126 in preeclampsia. J Cell Biochem. 2013;114:2148–2159.
17. Li X, Li C, Dong X, Gou W. MicroRNA-155 inhibits migration of trophoblast cells and contributes to the pathogenesis of severe preeclampsia by regulating endothelial nitric oxide synthase. Mol Med Rep. 2014;10(1):550–554. doi:10.3892/mmr.2014.2214
18. Sun H-X, Zeng D-Y, Li RT, et al. Essential role of MicroRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60:1407–1414. doi:10.1161/HYPERTENSIONAHA.112.197301
19. Khaliq OP, Murugesan S, Moodley J, Mackraj I. Differential expression of miRNAs are associated with the insulin signaling pathway in preeclampsia and gestational hypertension. Clin Exp Hypertens. 2018;40:744–751. doi:10.1080/10641963.2018.1431257
20. Sang Q, Yao Z, Wang H, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013;98(7):3068–3079. doi:10.1210/jc.2013-1715
21. Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122:478–487. doi:10.1161/CIRCULATIONAHA.109.895458
22. Mudjari NS, Samsu N. Management of hypertension in pregnancy. Acta Med Indones. 2015;47(1):78–86.
23. Umegbolu E, Ogamba J. Incidence of gestational hypertension among pregnant women (2006–2015) in Enugu State, Southeast Nigeria: a retrospective study. International J Commun Med Public Health. 2017;4(2):357. doi:10.18203/2394-6040.ijcmph20170255
24. Kintiraki E, Papakatsika S, Kotronis G, Goulis DG, Kotsis V. Pregnancy-Induced hypertension. Hormones. 2015;14(2):211–223. doi:10.14310/horm.2002.1582
25. de Jong OG, Verhaar MC, Chen Y, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesic. 2012;1:18396. doi:10.3402/jev.v1i0.18396
26. Pillar N, Yoffe L, Hod M, Shomron N. The possible involvement of microRNAs in preeclampsia and gestational diabetes mellitus. Best Pract Res Clin Obstet Gynaecol. 2015;29(2):176–182. doi:10.1016/j.bpobgyn.2014.04.021
27. Gallo A, Tandon M, Alevizos I, Illei GG. The majority of MicroRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7(3):e30679. doi:10.1371/journal.pone.0030679
28. Cronqvist T, Saljé K, Familari M, et al. Syncytiotrophoblast vesicles show altered micro-RNA and haemoglobin content after ex-vivo perfusion of placentas with haemoglobin to mimic preeclampsia. PLoS One. 2014;9(2):e90020. doi:10.1371/journal.pone.0090020
29. Wu F, Yang Z, Li G. Role of specific microRNAs for endothelial function and angiogenesis. Biochem Biophys Res Commun. 2009;386(4):549–553. doi:10.1016/j.bbrc.2009.06.075
30. Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100(8):1164–1173. doi:10.1161/01.Res.0000265065.26744.17
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


