Back to Journals » Orthopedic Research and Reviews » Volume 13
An Extremely Rare Case of Upper Thoracic Salmonella Infection
Authors Librianto D , Suwarto S, Imran D, Pramukti H, Saleh I , Ipang F , Srie Utami W, Aprilya D
Received 11 May 2021
Accepted for publication 22 July 2021
Published 7 August 2021 Volume 2021:13 Pages 107—112
DOI https://doi.org/10.2147/ORR.S319616
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Didik Librianto,1,2 Suhendro Suwarto,3 Darma Imran,4 Hikmat Pramukti,3 Ifran Saleh,1 Fachrisal Ipang,1,2 Widyastuti Srie Utami,2 Dina Aprilya1
1Department of Orthopedic and Traumatology, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; 2Jakarta Spine Center, Pondok Indah Hospital, Jakarta, Indonesia; 3Department of Internal Medicine, Pondok Indah Hospital, Jakarta, Indonesia; 4Department of Neurology, Pondok Indah Hospital, Jakarta, Indonesia
Correspondence: Dina Aprilya
Department of Orthopedic and Traumatology, Faculty of Medicine Universitas Indonesia, Prof. Soelarto Building, 1st Floor, RS Fatmawati Street, Jakarta, 12430, Indonesia
Tel +6289655106136
Fax +6221-7660616
Email [email protected]
Background: Vertebral osteomyelitis is rare. Finding the right etiological agent is important to administer antibiotic regimen accordingly. The occurrence of this disease in endemic countries raises the susceptibility of a more common infection such as tuberculosis and pyogenic bacteria. Salmonella spp. infection is also common in endemic countries; however, extra-intestinal manifestation is very rare.
Methods: We present an extremely rare case of salmonella vertebral osteomyelitis (SVO) in the upper thoracic vertebrae of a 64-year-old patient with history of cardiac surgery and other pre-existing comorbidities. SVO was treated by antibiotics, surgical debridement and spinal stabilization.
Results: Three weeks after surgery and intravenous antibiotics, the patient recovered and was discharged without fever and back pain, with excellent motoric improvement.
Conclusion: Salmonella infection must be considered to be one of possible etiological agents in patients with suggestive spondylitis in emerging countries, especially in those with comorbidities.
Keywords: osteomyelitis, spondylitis, thoracic spine, salmonella
Introduction
Vertebral osteomyelitis is uncommon, with an incidence of 1 case per 100,000–250,000 population per year in the United States, with Staphylococcus aureus as the most common pathogen. In endemic countries, Mycobacterium tuberculosis must be considered as one of the causative agents. Spondylitis tuberculosis contributes to around 5% of extrapulmonary tuberculosis (EPTB), which is low at 3% of all TB cases. Salmonella rarely causes osteomyelitis (only about 0.5% to 2% of all cases). The salmonella vertebral infection is even rarer with no exact reported incidence worldwide.1–3
We presented a case of a 64-year-old male with upper thoracic salmonella vertebral osteomyelitis (SVO) with severe neurological deficit. A blood culture showed no bacterial colonization. The patient was treated with decompression laminectomy and posterior stabilization along with antibiotic ciprofloxacin in accordance with the sensitivity profile of the Salmonella spp that grew from vertebral abscess and tissue culture. This work has been reported in line with the CARE guidelines.4
Case Presentation
The patient was hospitalized due to a remittent high fever 2 weeks before admission. No gastro-intestinal symptoms were noted. The patient had mild back pain over the mid back area but still could walk independently. The blood culture showed Salmonella enterica infection which was sensitive to Ampicillin/Sulbactam, Doripenem, Doxycycline, Imipenem, Amoxicillin/Clavulanic acid, Ampicillin, Cefepime, Cefotaxime, Ceftazidime, Ceftriaxone, Meropenem, Ertapenem, and Piperacillin/Tazobactam. The patient received meropenem 1g daily for 7 days. The patient had a past history of coronary artery bypass grafting surgery due to atherosclerosis three years before admission and 10 years of steroid-induced diabetes mellitus which was also complicated with chronic kidney disease.
Two weeks afterwards, the patient was readmitted to the emergency ward with hypotension and a persistently high fever (39.4°C). The patient also had severe back pain and paraplegia 1 week before admission. The pain was centered in the upper back and radiating to the chest wall with Visual Analogue Scale (VAS) 7. There was weakness of both lower extremities with sensory loss below the chest. There was no history of recent trauma. The patient had lost 1 kg of weight in the past one month.
A blood sample for hematology and coagulation profile and blood culture was taken along with a urinary sample to rule out the source of infection. There was no leukocytosis, however there was an increase in neutrophils, decreased in lymphocytes, C-Reactive Protein was high (111.08 mg/L) and procalcitonin was 0.27 ng/mL. The blood culture was negative. Mantoux test and IGRA (Interferon Gamma Release Assays) for Tuberculosis was negative. HIV testing was negative with CD4 cell count of 504 cells/mm3. The patient was referred to the intensive care unit due to septic shock with unknown origin and was given a high dose meropenem injection 1 gram every 8 hours. The renal function was normal (upper limit) throughout the antibiotic administration and showed a maximum level of 37 mg/dL for ureum and 1.23 mg/dL for creatinine.
The critical condition subsided five days after hospital admission. After that, the patient was re-assessed for the neurological deficit and consulted an orthopedic spine surgeon. From physical examination, there was a midline tenderness over the upper thoracic spine region. We suspected a spinal cord compression with signs of upper motor neuron involvement with a decreased sensation from Th5 dermatome and Medical Research Council Scale for muscle power of 0/5 from L2-S1 on both lower extremities (American Spinal Injury Association [ASIA] impairment scale B).
Magnetic resonance imaging (MRI) of the spine revealed wedging on the 5th and 6th thoracal with intrathecal infiltration of hyperintensity mass in T2 weighted images which increased our suspicion of granulomatous infection from Mycobacterium tuberculosis with metastatic bone disease as the main differential diagnosis (Figure 1).
A week after hospital admission, we performed a posterior stabilization with the pedicle screws and rods system on 4th, 5th, 7th and 8th thoracic spines and transpedicular biopsy on 6th vertebra (Figure 2). A 3 cc of purulent discharge was oozing out from the 6th thoracal body while performing the biopsy. Tissue samples for culture and pathological exam were obtained and macroscopically similar to granulation tissue from tuberculous infection. Debridement was further attempted. Decompression was achieved by laminectomy and flavectomy on the 5th and 6th thoracal level. A similar granulation tissue was found underneath the laminae and yellow ligament. Postoperatively, the patient was sent back to ICU for general health stabilization.
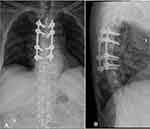 |
Figure 2 Post-operative X-ray showed pedicle screw and rod stabilization of upper thoracal spine: (A) antero-posterior view and (B) lateral view. |
The pathological exam revealed chronic inflammation without tuberculoma and caseous necrotic formation and no malignant cells were found. The tissue culture revealed Salmonella enterica infection which has the same sensitivity profile to the previous blood culture. The bacteria were sensitive to ciprofloxacin with minimum inhibitory concentration (MIC) below 0.06. We changed the antibiotic regimen to ciprofloxacin 400 mg IV twice daily for two weeks, followed by oral ciprofloxacin 500 mg twice daily for 6 weeks, with a total of 8 weeks therapeutic antibiotic. Three weeks after surgery, the patient was discharged with a significant improvement of the neurological condition with excellent motoric strength (ASIA E) and free of fever. There was slight discomfort over the surgical scar but the pain had significantly subsided. The patient was independently ambulated with a spinal brace. At the 3 month follow-up, the patient was symptom-free and had begun exercises to strengthen the back.
Discussion
Salmonella sp. can cause a broad spectrum of human diseases from gastroenteritis, typhoid fever and bacteremia with or without seeding to remote organs by either hematogenous or contiguous spread. Salmonella infection is a food-borne infection and primarily infects the gastro-intestinal system. It is a self-limiting disease with the course of about 4 weeks without antibiotic therapy. Signs and symptoms usually appear for 1–2 weeks. In the early course of the disease, the patient had a remittent fever and approximately in the second week the fever becomes persistently high, as experienced by our patient. On the other hand, infection from pyogenic bacteria usually gives a sudden rise of temperature whereas in tuberculosis, the signs and symptoms are somewhat less dramatic.2–4
Bacteremia in patients with low immunity can cause extra-intestinal infection although non-typhoidal salmonellosis is not always present with gastro-intestinal symptoms particularly in patients with low immunity. Salmonella osteomyelitis is an uncommon complication of salmonella infection (less than 1%). In sickle cell patients, salmonella was the major cause of osteomyelitis (70%). In non-sickle cell patients, salmonella-associated infection accounts for only 0.5% of all cases of osteomyelitis, and involvement of the spine is approximately quarter of all these cases.5,6
Bony manifestation of salmonella infection was firstly described by Sir Paget in 1876 in long bones. In 1899 Gibney presented four cases of typhoid spine following episodes of typhoid fever.2 Santos and Sapico2 study showed that most of salmonella osteomyelitis (54%) had predisposing factors such as immunocompromised patient, sickle cell disease, atherosclerosis, diabetes, collagen diseases, liver cirrhosis, systemic lupus erythematosus, lymphoma, liver diseases, previous surgery or trauma, elderly, steroid use and achlorhydria.5,7 This patient was older with several comorbidities: history of coronary artery bypass grafting surgery due to atherosclerosis and steroid-induced diabetes mellitus which was also complicated with chronic kidney disease. These comorbidities made the patient more susceptible to a complicated infection, while HIV infection as the most common cause of an immunocompromised state had been excluded.
Patients with immunodeficiencies state are predisposed to Non-typhoidal Salmonella (NTS) infection and symptoms not necessarily preceded with diarrhea or other gastro-intestinal symptoms because diarrhea is a defense mechanism that depends on a normal immune system.8 NTS has been shown to have a predilection for the endovascular environment.9 A report from Hibbert et al10 showed there is a tendency of developing endovascular infection in NTS. This patient had a positive NTS and a history of CABG. There is a strong suspicion of extra-intestinal colonization whether in the endovascular system or in the vascular graft that further seed into the vertebral vascular system and develop vertebral osteomyelitis. However, this patient did not have cardiac symptoms and signs so we did not proceed to further cardiac diagnostic evaluations.
Osteomyelitis is rarely developed in acute phase of salmonellosis but may arise during convalescence or even months and years after initial infection.11 Spinal salmonella infection is an extremely rare condition and the majority of cases involve lumbar vertebrae (50%), thoracic vertebrae (20%) and the rest occurs with multiple bone and joint involvement.7 The involvement of thoracic vertebrae itself usually occur in lower thoracic.5 Cohen et al12 reported the incidence of vertebral osteomyelitis in the past 80 years still appears to be uncommon.2,12 However, no literatures on SVO affecting upper thoracic vertebra were found during a literature search on several web-based search engines (PubMed, Science Direct and Google Scholars). It reflects an extreme rarity in our case presentation.
Pathogens can reach the spine by several routes: hematogenous spread from a distant site or focus of infection, direct inoculation from trauma or spinal surgery, contiguous spread from adjacent soft tissue infection, or as a result of vascular insufficiency. Hematogenous spread through arteries or veins (via Batson’s plexus) is the major mechanism in vertebral osteomyelitis and our patient probably developed typhoid osteomyelitis during typhoid bacteremia with additional susceptibility of stagnant blood flow due to pre-existing vascular insufficiency caused by atherosclerosis.3,5,11,13
Fever and back pain are the main presenting symptoms of any vertebral infection. Higher frequency of fever that accompanies any back symptoms should lead to early suspicion of infection thus prompting the appropriate workup. Laboratory studies usually reveal anemia and leukocytosis or leukopenia, along with raised erythrocyte sedimentation rate, but are non-specific.14 The patient had a sub-acute onset of persistent high fever, worsening back pain with severe neurological deficit and continue elevation of CRP and PCT which were acute phase markers despite meropenem therapy and negative blood culture.
In this patient, the radiological appearance of destructed discs and vertebral bodies including vertebral and paravertebral abscess brought a high index suspicion for an ongoing spinal infection. However, it is hard to distinguish pathological changes due to salmonella from other infectious agents solely from imaging findings. The gold standard diagnosis depends on isolation of the organism from the affected site. In spinal infection cases, the organism grew from vertebrae (either needle biopsy or intraoperative specimen), aspirate of adjacent fluid collection/ abscess, or blood culture from patients with clinical features of vertebral osteomyelitis. Gram staining was also routinely done to identify aerobic infection such as Staphylococcus aureus which is the main cause of pyogenic vertebral infection.2,3
In this patient, tissue culture grew Salmonella sp. which had exactly the same sensitivity profile as the previous blood culture during bacteremia. However, meropenem medication failed to prevent the occurrence of distance infection in this patient despite the sensitivity profile remained the same. This suggests the existence of persisters that are responsible for latent infection, extra-intestinal infection and post-treatment relapse. Hence, we re-assess drug sensitivity and adjust the treatment regimen by giving ciprofloxacin despite in the latest tissue culture also showed sensitivity to meropenem.14,15
Ciprofloxacin has been the drug of choice for the treatment of salmonella infection since 1990. However, there was therapeutic failure associated with strains showing MIC in the range 0.12 to 1 μg/mL which means that there is a decreased ciprofloxacin susceptibility. Considering this condition, in 2012 the Clinical and Laboratory Standards Institute (CLSI) revised the criteria of ciprofloxacin susceptibility with MIC ≤0.06 μg/mL as susceptible. In this patient, the consideration of changing treatment regimens was after evaluating the ciprofloxacin susceptibility profile. Furthermore, ciprofloxacin has a success rate often greater than 80% in treating gram negative vertebral osteomyelitis with or without regiment combination. It also has been proven that ciprofloxacin is the most effective treatment of gram-negative vertebral osteomyelitis with a total period of administration varying from 6–8 weeks (pure parenteral or combination with oral).2,6,16,17
The negative conversion of blood culture with a high suspicion of an emerging infectious disease is possible. The bacteria will be unavailable in the blood stream anymore after passing the bacteremia phase. The main consideration of surgical debridement in this patient was an acute and severe neurological deterioration with development of instability due to vertebral bodies destruction which contributed to debilitating pain in this patient, and further narrowing the canal which was already fully occupied with abscess. Furthermore, tissue culture and biopsy were gold standard procedures to confirm the diagnosis.2,3,5,6
Early recognition can lead to an early and appropriate antibiotic therapy. Although the disease is food-borne and more-likely to be self-limiting in nature, a patient with an immunocompromised state is at risk of bacteremia and have extra-intestinal infection. The clean and thorough debridement followed by giving the suitable intravenous antibiotic has brought a successful infection control in this patient.
Conclusion
Clinicians should be aware of the possibility of salmonella infection as an etiological agent in patients with suggestive vertebral osteomyelitis in emerging countries, especially in those with comorbidities such as immunocompromised state and cardiac surgery. No site predilection of NTS vertebral infection has been demonstrated. In abscess formation and evidence of vertebral destruction, surgical intervention is needed in addition to antibiotic therapy to control source of infection and prevent further damage to the spinal cord.
Disclaimer
No patient or author details are included in the figures.
Ethical Approval
None. Because this paper is reporting a case which is consisted only one patient and not considered as human research. Thus, it does not typically require IRB review and approval.
Consent for Publication
The patient provided informed consent for the case details and accompanying images to be published.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rajasekaran S, Soundararajan DCR, Shetty AP, Kanna RM. Spinal tuberculosis: current concepts. Glob Spine J. 2018;8(4_suppl):96S–108S. doi:10.1177/2192568218769053
2. Santos EM, Sapico FL. Vertebral osteomyelitis due to salmonellae: report of two cases and review. Clin Infect Dis. 1998;27(2):287–295. doi:10.1086/514668
3. Graeber A, Cecava ND. Vertebral osteomyelitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; April 4, 2021. PMID: 30335289.
4. D’Souza CR, Hopp PG, Kilam S. Osteomyelitis of the spine due to Salmonella: case report, review of clinical aspects, pathogenesis and treatment. Can J Surg. 1993;36:311–314.
5. Huang DB, DuPont HL. Problem pathogens: extra-intestinal complications of Salmonella enterica serotype Typhi infection. Lancet Infect Dis. 2005;5(6):341–348. doi:10.1016/S1473-3099(05)70138-9
6. Acharya S, Bhatnagar P. Salmonella spinal osteomyelitis: a case report and review of literature. Neurol India. 2004;52(4):499–500.
7. Khoo HW, Chua YY, Chen JLT. Salmonella typhi vertebral osteomyelitis and epidural abscess. Case Rep Orthop. 2016;2016:1–3. doi:10.1155/2016/6798157
8. Ortiz D, Siegal EM, Kramer C, Khandheria BK, Brauer E. Nontyphoidal cardiac salmonellosis: two case reports and a review of the literature. Texas Heart Inst J. 2014;41(4):401–406. doi:10.14503/THIJ-13-3722
9. Dohogne BJ, Srinivasan S, Arif-Tiwari H, Potharaju A. Nontyphoidal Salmonella as a cause of mediastinal abscess in a patient with extensive cardiac surgery. Cureus. 2020;12(8):8–14. doi:10.7759/cureus.9924
10. Hibbert B, Costiniuk C, Hibbert R, et al. Cardiovascular complications of Salmonella enteritidis infection. Can J Cardiol. 2010;26(8):323–325. doi:10.1016/S0828-282X(10)70444-X
11. Khan FY, El-Hiday AH, Kamel HA. Typhoid osteomyelitis of the lumbar spine. Hong Kong Med J. 2006;12(5):391–393.
12. Cohen PS, O’Brien TF, Schoenbaum SC, Medeiros AA. The risk of endothelial infection in adults with Salmonella bacteremia. Ann Intern Med. 1978;89(6):931. doi:10.7326/0003-4819-89-6-931
13. Skaf GS, Domloj NT, Fehlings MG, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3(1):5–16. doi:10.1016/j.jiph.2010.01.001
14. Shrestha P, Mohan S, Roy S. Bug on the back: vertebral osteomyelitis secondary to fluoroquinolone resistant Salmonella typhi in an immunocompetent patient. BMJ Case Rep. 2015;2015:10–12. doi:10.1136/bcr-2015-212503
15. Rohilla R, Bhatia M, Gupta P, Singh A, Shankar R, Omar BJ. Salmonella osteomyelitis: a rare extraintestinal manifestation of an endemic pathogen. J Lab Physicians. 2019;11(02):164–170. doi:10.4103/jlp.jlp_165_18
16. Gupta V, Pal K, Bhagat A, Goel A, Chander J. Quinolone susceptibility in salmonella isolates based on minimum inhibitory concentration determination. J Lab Physicians. 2020;12:263–267. doi:10.1055/s-0040-1721163
17. Shrestha KL, Pant ND, Bhandari R, Khatri S, Shrestha B, Lekhak B. Re-emergence of the susceptibility of the Salmonella spp. isolated from blood samples to conventional first line antibiotics. Antimicrob Resist Infect Control. 2016;5(1). doi:10.1186/s13756-016-0121-8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

