Back to Journals » Infection and Drug Resistance » Volume 15
An Exploration of the Safety of “Pneumonia Prevention No. 1” in Healthy Populations
Authors Meng ZH, Ma WY, Wang Y, Li YR, Zhang J, Liu YF, Zhao XK, Da CH
Received 9 June 2022
Accepted for publication 19 September 2022
Published 23 November 2022 Volume 2022:15 Pages 6695—6701
DOI https://doi.org/10.2147/IDR.S377974
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Zuo-Huan Meng,1,* Wen-Yuan Ma,1,* Ying Wang,2 Ya-Rong Li,2 Jing Zhang,3 Yin-Fang Liu,4 Xin-Ke Zhao,5,6 Chun-He Da6
1Institute of Traditional Chinese Medicine, The Third Affiliated Hospital of Gansu University of Chinese Medicine, Baiyin, People’s Republic of China; 2Department of Pharmacy, The Third Affiliated Hospital of Gansu University of Chinese Medicine, Baiyin, People’s Republic of China; 3Nutrition Department, The Third Affiliated Hospital of Gansu University of Chinese Medicine, Baiyin, People’s Republic of China; 4Respiratory Medicine, The Third Affiliated Hospital of Gansu University of Chinese Medicine, Baiyin, People’s Republic of China; 5Gansu University of Chinese Medicine, Lanzhou, People’s Republic of China; 6The Third Affiliated Hospital of Gansu University of Chinese Medicine, Baiyin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zuo-Huan Meng, Institute of Traditional Chinese Medicine, The Third Affiliated Hospital of Gansu University of Chinese Medicine, Baiyin, 730900, People’s Republic of China, Tel +8609438813373, Email [email protected] Chun-He Da, The Third Affiliated Hospital of Gansu University of Chinese Medicine, Baiyin, 730900, People’s Republic of China, Tel +8609438810612, Email [email protected]
Background: “Pneumonia Prevention No.1” belongs to ‘traditional Chinese medicine prescription for prevention of viral pneumonia and influenza’ was urgently formulated by Notice on Printing the Novel Coronavirus Diagnosis and Treatment Scheme for COVID-19 (Trial Version 3) and Traditional Chinese Medicine Prevention and Treatment Scheme for COVID-19 in Hubei Province (Trial). Because the prescription drug has the bidirectional regulation function of human immune function, moderate improvement of immune function can effectively resist virus invasion, while excessive immune function will produce immune overresponse. Excessive immune response will aggravate the condition of patients with COVID-19, resulting in the death of severe patients.
Methods: Twenty medical workers aged 20– 60 years old, who had no immune disease, no current disease and healthy physical examination, were selected as participants. The participants took Hubei “Pneumonia Prevention No.1” decoction, one dosage each day, twice a day, for 7 consecutive days. With the before-after control method, blood samples were collected from the median cubital veins before and after medication. Immunoglobulin IgA, IgG and IgM were measured by immunoturbidimetry, and T lymphocyte subsets CD3, CD4, CD8 and CD4/CD8 were measured by flow cytometry. The changes of indexes before and after medication were compared with SPPS 13.0 statistical software. The data were expressed by (mean ± standard deviation). T-test was adopted, and P < 0.05 was considered statistically significant (P < 0.05).
Results: The results of this study show that in healthy participants, the immunoglobulin and T lymphocyte subsets did not differ significantly before and after drug administration (P > 0.05).
Conclusion: Under normal drug administration circumstances, “Pneumonia Prevention No. 1” had no significant regulating effect on the immune system in a healthy population and did not increase the immune system capacity beyond a reasonable range. It is safe to be used as a prophylactic measure in healthy populations.
Keywords: “Pneumonia Prevention No. 1”, healthy population, immunoglobulin, T lymphocyte, safe medication
Introduction
SARS-CoV-2 is the seventh coronavirus known to infect humans. Andersen and his collaborators, based on genome sequencing analysis, clearly show that SARS-CoV-2 is not a laboratory construct or a purposefully manipulated virus. They propose two scenarios that could plausibly explain the origins of SARS-CoV-2: 1) Natural selection in an animal host before zoonotic transfer, and 2) Natural selection in humans following zoonotic transfer. There is also the question of whether selection during passage could have given rise to SARS-CoV-2.1 The origins were in Europe, North America, and Asia.2 However, it was in Wuhan, Hubei Province, where it was not treated as an electronic cigarette pneumonia and influenza for the first time.3–5
The virus called COVID-19 is an acute infectious pneumonia, and its pathogen is the novel beta coronavirus.6 Infected people show different degrees of symptoms. Although most human coronavirus infections are mild, causing a slight fever and cough, some can develop into pneumonia, and some infections can cause severe acute respiratory syndrome and death.7–9 Due to its great potential for harm, high transmissibility, and confirmed cases in many countries, on January 30, 2020, World Health Organization announced that pneumonia infected by SARS CoV-2 constituted a “public health emergency with international concern”; on February 11, it was named “COVID-19”; on March 11, COVID-19 was assessed as a pandemic.10–12 The basic reproduction number R0 of COVID-19 is between 2.2 and 5.7,6,13,14 which is near the infection rate of poliomyelitis, rubella, and smallpox.15
Since November 13, 2020, over 50 million cases have been confirmed worldwide, making this pandemic one of the most severe in modern times, causing great impacts on the future social order, politics, economics, and culture. Under such serious circumstances, global public health is facing unprecedented challenges. The development of vaccines and specific drugs is a long process. Acquired immune deficiency syndrome (AIDS) still has no usable vaccine, and even if the development of a drug or vaccine for AIDS was successful, it would still be a long process to meet the needs of such a large population. In countries and regions where the pandemic is severe, adequate personal protection is needed to prevent disability and death from infection before a drug or vaccine is developed.
The COVID-19 virus is highly contagious, and those with low immunity are more susceptible to infection.16 It has been confirmed that the severity of COVID-19 is related to the decreased number and function of T cells, and patients with a lower T cell count are likely to need earlier and more urgent treatments to avoid a poor outcome.17,18 Traditional Chinese medicine (TCM) values preventing diseases, and at present, “Pneumonia Prevention No. 1” (a decoction containing Saposhnikovia divaricata, Astragalus membranaceus, Atractylodes macrocephala Koidz, Cyrtomium fortunei J. Sm, Lonicera japonica Thumb, Citrus reticulata Blanco, and Eupatorium fortunei Turcz.) was widely used in the first stages of the pandemic in China and had positive effects on medical workers. The herbal decoction formula is from Hubei Provincial Hospital of TCM, COVID-19 Pneumonia Traditional Chinese Medicine Prevention Formula (First Edition). After usage, dosage, and the applicable population was determined, the herbal decoction was generally accepted to have a beneficial regulatory effect on patients, including sub-healthy, tense, and tired people.19,20 The decoction is made from adding and subtracting constituents from Jade Windscreen powder, which has been proven by modern pharmacology can enhance the immune function of lung-qi deficiency model rats, which is mainly reflected in the significant increase of CD3+, CD4+ proportion and CD4+/CD8+ ratio, and the significant decrease of CD8+ ratio in the peripheral blood T lymphocyte (P < 0.01).21 Clinical trials confirmed that the patients in the experimental group with Yupingfeng Powder were superior to the control group in the improvement of IgA, IgG and IgM.22 The question of whether it can have negative effects on a healthy population and cause overregulation of immunocompetence remains. Because moderate immune response is very important for pathogenic microorganism clearance and host survival, excessive immune response will cause “cytokine storm”, which is an uncontrolled inflammatory response, thus resulting in lung injury, severe pneumonia and host death.
This study was conducted according to the Declaration of Helsinki,23 Guidelines for Good Clinical Practice (ICH–GCP),24 Approval and Management Standard of the Clinical Test of Drugs,25 and the Guidelines for General Considerations of the Clinical Test of Drugs.26 It was proven that this decoction works well in a healthy population and has a two-way regulatory protective effect on an unhealthy population.27
Materials and Methods
Objects of Study
Twenty medical workers between the ages of 20 and 60 years of age and assessed to be healthy and without immune or other diseases were chosen.
Research Method
Flow Chart Diagram of the Study (See Figure 1), 20 medical workers aged 20–60 years old, who had no immune disease, no current disease and healthy physical examination, were selected as participants. The participants took Hubei “Pneumonia Prevention No.1” decoction, one dosage each day, twice a day, for 7 consecutive days. With the before-after control method, blood samples were collected from the median cubital veins before and after medication. Immunoglobulin IgA, IgG and IgM were measured by immunoturbidimetry, and T lymphocyte subsets CD3, CD4, CD8 and CD4/CD8 were measured by flow cytometry. The changes of indexes before and after medication were compared with SPPS 13.0 statistical software. The data were expressed by 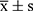 (mean ± standard deviation). T-test was adopted, and P < 0.05 was considered statistically significant (P < 0.05). Sample testing was conducted by the Gansu Provincial Jinyu Medical Laboratory Science Co., Ltd.
(mean ± standard deviation). T-test was adopted, and P < 0.05 was considered statistically significant (P < 0.05). Sample testing was conducted by the Gansu Provincial Jinyu Medical Laboratory Science Co., Ltd.
Decoction composition: Astragalus membranaceus 15g, roasted Atractylodes macrocephala Koidz. 10g, Saposhnikovia divaricate 10g, Cyrtomium fortunei J. Sm. 6g, Lonicera japonica Thunb. 10g, Citrus reticulata Blanco 6g, Eupatorium fortunei Turcz. 10g.
Course of treatment: Chinese herbal compound decoction, one formula per day, boiled with water and taken orally in 2 doses for a 7-day course.
The Chinese herbal compound decoction was provided by the pharmacy department of the 1st People’s Hospital of Baiyin.
Testing of Immune Indices
1. A total of 10mL of venous blood was sampled before and after drug administration from the median cubital vein. Serum was extracted and stored at −30℃ for unified testing, and immunoglobulin IgA, IgM, and IgG levels were determined through immune turbidimetry.
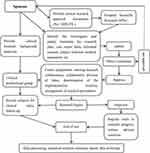 |
Figure 1 Flow chart diagram of the study. |
2. A total of 5mL of median cubital venous blood was extracted before and after drug administration, and EDTA was used for anticoagulation. CD3, CD4, CD8, and CD4/CD8 levels were determined by flow cytometry.
Statistical Analysis of Data Indicators
SPSS 13.0 software was used for analysis and processing. Data before and after the drug administration were presented as mean ± standard deviation (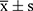 ), and a t-test was used, with P < 0.05 considered statistically significant.
), and a t-test was used, with P < 0.05 considered statistically significant.
Results
Changes in Serum Immunoglobulin Levels
After 7 days of taking the drug, the results showed that the IgA, IgG, and IgM levels of the participants showed no significant changes before and after drug administration, and the P values were all greater than 0.05, as shown in Table 1.
 |
Table 1 Comparison of IgA, IgG and IgM Levels Before and After Drug Administration |
Changes in T Lymphocyte Subsets
After 7 days of taking the drug, the results showed no significant changes between CD3, CD4, CD8, and CD4/CD8 levels before and after taking the drug, and the P values were all greater than 0.05, as shown in Table 2.
 |
Table 2 Comparison of CD3, CD4, CD8 and CD4/CD8 Levels Before and After Drug Administration |
There were no significant differences in changes of serum immunoglobulin and T lymphocyte subsets before and after medication (P>0.05), which indicates that Hubei “Pneumonia Prevention No.1” decoction can not cause excessive immune response in healthy population. This study conformed to the experimental expectation, and it was confirmed for the first time that Hubei “Pneumonia Prevention No.1” decoction had no damage to the immune function of healthy population who took the medicine reasonably when preventing COVID-19 infection.
Discussion
In the process of viral infection, the virus is usually prevented from spreading in the body through humoral immunity. If the virus has infected the host cells, it is through cellular immunity, which is the combining of effector T cells with target cells. This changes the permeability and osmotic pressure of target cells and finally leads to cell rupture and death. Humoral immunity is a specific immune response mediated by B lymphocytes. The B cells recognize corresponding antigens and are activated under the synergistic effects of T lymphocytes and macrophages. The B cells are then differentiated into plasma cells, which produce antibodies. These antibodies eliminate pathogens through the antigen-antibody reaction and maintain the homeostasis of our internal environment.28 Among them, IgA, IgG, and IgM are important immunoglobulins that perform the humoral immune function in the body and are also early effectors in the body’s defense against viral infection. When the levels of the three Ig immunoglobulins are significantly low, humoral immune deficiency or a low humoral immune function is possible. Cellular immunity is a specific immune response mediated by T lymphocytes. CD3, CD4, and CD8 molecules are lymphocyte surface antigens, which have antiviral functions and can help regulate our immune system.
COVID-19 is an infectious respiratory disease with high morbidity and mortality. Some patients had mild condition in the early stage, and sudden aggravation in the later stage. Eventually, they died of multiple organ failure, which was aggravated mainly by cytokine storm caused by immune disorder.7
The main causes of cytokine storm are excessive immune response caused by infection, disease and iatrogenic tumor.29 Cytokine storm in severe pneumonia is caused by the rapid recruitment and activation of phagocytes such as monocytes and neutrophils and complement system, which eliminates a mass of chemokines and inflammatory cytokines caused by pathogenic microorganisms entering the lungs.30
Infection with SARS CoV-2 can stimulate inflammatory immune response, in which the production of reactive oxygen species (ROS) mediated by epithelial cells can result in cell death. ROS can also stimulate the synthesis of NF-κB and NLRP3, which is beneficial to increase the level of cytokines, thus resulting in cytokine storm and clinical related diseases such as MODS (Multiple Organ Dysfunction Syndrome), septicemia and ARDS (Acute Respiratory Distress Syndrome).31 Acute pulmonary inflammatory response can damage the integrity of pulmonary endothelial cells, and phagocytes, red blood cells and serum will enter alveolar cavity, resulting in pulmonary edema. Neutrophils enter alveolar cavity and promote the release of reactive oxygen species, protease and cytokines.32 In addition, the number of CD8 + T cells increased significantly, while the number of CD4 + T cells decreased significantly, which caused the decrease of CD4/CD8 ratio, and the patients appeared over-activation of immune defense mechanism, which resulted in severe lung injury.33 Lung injury can lead to obstruction of oxygen exchange, acute respiratory distress syndrome and eventually systemic multiple organ failure.34 Therefore, moderate immune response is characterized by balance. Immune response can not only effectively eliminate pathogens, but also limit pathological damage to an acceptable range, which is most conducive to the survival of patients.
The results of this experiment showed that there was no significant change in immunoglobulin index and T lymphocyte subsets in healthy participants before and after medication. For example, cyclic nucleotides (cAMP, cGMP) are important active substances affecting immune function. Yupingfeng Powder has a bidirectional regulatory effect on cAMP, and the change of cAMP is positively correlated with the change of cGMP, while the change of cAMP is inversely correlated with the change of cAMP/cGMP. Therefore, it can be established that Yupingfeng Powder has a bidirectional regulatory immunopharmacological activity.35 In addition, the prescription can also inhibit the excessive release of cytokines and reduce the storm function of cytokines. For example, studies by Min et al36 showed that the inhibition of Yupingfeng powder on delayed type hypersensitivity may be related to the inhibition of IFN-γ and IL-4 secretion. Qinghe et al37 showed that Yupingfeng Powder can down-regulate TNF-α, significantly increase IL-10 expression, and effectively inhibit inflammatory response. Fangjun et al38 found that the traditional Chinese medicine components contained in Yupingfeng Powder have the functions such as scavenging free radicals, inhibiting lipid peroxidation, stabilizing cell membrane, enhancing antioxidant capacity and reducing the release of inflammatory cytokines.
The cause of COVID-19 was “wet poison”, its pathogenesis was “wet, poison, heat, stasis, close, syncope, and yang collapse.” Hence the basic treatment strategy was to declare lung and remove dampness.39 During the COVID-19 outbreak, the Chinese herbal compound decoction “Pneumonia Prevention No. 1” was taken as a preventive prescription in many provinces and regions, including our hospital, and has been shown to be safe and effective.
Jade Windscreen powder is composed of Saposhnikovia divaricate, Astragalus membranaceus, and Atractylodes macrocephala Koidz, which benefits qi and strengthens the protective function of the body. Cyrtomium fortunei J. Sm. and Lonicera japonica Thunb. have heat-clearing and detoxification functions,40,41 and Citrus reticulata Blanco42,43 and Eupatorium fortunei Turcz.44 can clear damp turbidity. The combined use of these drugs can achieve heat-clearing and detoxification, drying dampness, resolving phlegm, and adjusting immunity effects.
Conclusion
Hubei “Pneumonia Prevention No.1” has no obvious humoral and cellular immune regulation function for healthy population, but it has no damage to the immune function of healthy population with normal and rational drug use, with certain safety. Meanwhile, it is speculated that this prescription has certain functions in maintaining stability and inhibiting cytokine storm on immune function.
Highlights
- “Pneumonia Prevention No. 1” is an emergency prescription for the outbreak of SARS-CoV-2. Many Chinese medicine prescriptions are used in clinical practice without safety verification.
- Reasonable use of “Pneumonia Prevention No. 1” will not cause disorders of the immune system in humans.
- The number of people infected with SARS-CoV-2 is still increasing, and “Pneumonia Prevention No. 1” is still worth promoting.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of the Third Affiliated Hospital of Gansu University of Chinese Medicine (YL-KY-2020-026). Written informed consent was obtained from all participants.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding
This study was supported by the Baiyin Science and Technology Planning Project (grant No 2020-ZX), Science and Technology Public Relations Project of the First People’s Hospital of Baiyin (grant No 2020YK-09) and the National Key Research and Development Program of China (Grant no 2020YFC2005504).
Disclosure
The authors declare that they have no competing interests.
References
1. Andersen G, Andrew Rambaut W, Lipkin I, et al. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–452. doi:10.1038/s41591-020-0820-9
2. Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci USA. 2020;117(17):9241–9243. doi:10.1073/pnas.2004999117
3. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi:10.1016/S0140-6736(20)30185-9
4. Zhu N, Zhang D, Wang W, et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017
5. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi:10.1002/jmv.25681
6. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi:10.1056/NEJMoa2001316
7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
8. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769–777. doi:10.1093/cid/ciaa272
9. Guan W-J, Zheng-yi N, Yu H, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi:10.1056/NEJMoa2002032
10. The Xinhua News Agency. WHO director-general’s statement on IHR emergency committee on novel coronavirus; 2020.
11. World Health Organization. Address by the Director-General of WHO at the Media Briefing on the 2019 New Coronavirus. Geneva: World Health Organization; 2020.
12. World Health Organization, WHO Director-General’s opening remarks at the media briefing on COVID-19; 2020.
13. Yang Y, Lu Q, Liu M, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 2020;2020:1.
14. Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470–1477. doi:10.3201/eid2607.200282
15. Yifan C. The largest number of cases so far, the new coronary pneumonia study shows that the previous basic infection number of 2.2 may be underestimated. Economic Observer Network; 2020.
16. Chen N, Min Z, Xuan D, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
17. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. doi:10.3389/fimmu.2020.00827
18. Godeau D, Petit A, Richard I, Roquelaure Y, Descatha A. Return-to-work, disabilities and occupational health in the age of COVID-19. Scand J Work Environ Health. 2021;47(5):408–409. doi:10.5271/sjweh.3960
19. Lin L, Xia Z, Yuejun L, et al. Effect of Hunan prevention new coronary pneumonia No.1 prescription on human cellular immunity and humoral immunity. Guid J Trad Chin Med Pharm. 2020;26(10):8–11.
20. Congmin Z, Jinkai W, Xiaojing Z, et al. Research progress on immunopharmacological effects of Yupingfeng granules. Modern J Integr Tradit West Med. 2020;29(20):2274–2277.
21. Sheng Y, Zhonglin Z, Mingyong Y, Lingli Z. Effect of Yupingfeng powder on the immune function of rats with lung-qi deficiency syndrome. J China Pharm. 2016;27(22):3041–3044.
22. Lina G, Zheng X. Research progress of clinical application and pharmacology of Yupingfeng powder. Shandong J Tradit Chin Med. 2020;39(12):1369–1374.
23. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Jahrbuch für Wissenschaft und ethik; 2014.
24. ICH. E6(R2) Guideline for good clinical practice; 2019.
25. State Food and Drug Administration, National Health Commission. ”Good clinical practice for drugs”; 2020.
26. Food and Drug Administration. Guidelines for general considerations in drug clinical trials [EB/OL]; 2017.
27. Xuan-Xuan XU, Ling-Ru LI, Yan-Ling WU, et al. Study on dual immunoregulatory mechanism of yupingfeng powder from aspects of external asthenia and latent pathogens. J Anhui Univ Chin Med. 2015;34(2):1–3.
28. Fahao C. Preparation of Compound Ginsenoside Nanoemulsion and Its Immune Enhancement Effect. Xianyang: Northwest Sci-Tech University of Agriculture and Forestry; 2012. Chinese.
29. Lili H, Puyang G, Yue F, Wei Z, Enlong W, Jian G. Analysis on application of Chinese materia medica in treatment of COVID-19 by suppressing cytokine storm. China Tradit Herb Drugs. 2020;51(6):1.
30. Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, et al. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi:10.1146/annurev-pathol-011110-130327
31. Wang W, Chen J, Yu X, Lan HY. Signaling mechanisms of SARS-CoV-2 Nucleocapsid protein in viral infection, cell death and inflammation. Int J Biol Sci. 2022;18(12):4704–4713. doi:10.7150/ijbs.72663
32. Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct Target Ther. 2021;6(1). doi:10.1038/s41392-021-00679-0
33. Quinton LJ, Mizgerd JP. Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling. Annu Rev Pathol. 2015;77:407–430.
34. Jiao L, Min F. Severe pulmonary infections and immune disorders: problems and prospects. Chin J Pract Intern Med. 2022;42(03):196–201.
35. Ruiqing G, Xiangben M. Pharmacological and clinical research progress of Yupingfeng powder in respiratory diseases. Zhejiang J Tradit Chin Med. 2003;2003(7):44–45.
36. Min H, Liang W, Jie Z, et al. The characteristics of different extracts of Yupingfeng powder in inhibiting delayed-type hypersensitivity reactions. Chin Med Pharmaco Clinic. 2010;26(2):4.
37. Qinghe P, Shengrong C, Shu Z, et al. Effect of jade screen powder combined with six gentlemen decoction of. J Liaoning Univ Tradit Chin Med. 2013;15(10):95.
38. Fangjun C. Protective Effects of Total Polysaccharides Fromyu-Ping-Feng Powder on Acute Liver Injury and Study on Its Mechanism. Hefei: Anhui Medical University; 2008.
39. Liu LT, Li J, Peng GY, et al. Analysis of similarities and differences between coronavirus disease 2019 and severe acute respiratory syndrome. World J Tradit Chin Med. 2020;6:145–151. doi:10.4103/wjtcm.wjtcm_21_20
40. Jia L, Baofei Y, Mingyue Z, et al. Potential mechanism of couplet medicines baical skullcap root-honeysuckle for coronavirus disease 2019 based on network pharmacology. WJTCM. 2020;15(4):502–511.
41. Yuexi C, Hegang L. The advance of the research on cyrtomium fortunei. Mod Chin Med. 2014;16(12):1043–1048. Chinese.
42. Zhihao X, Jian Q, Xiaobing P, et al. Favorable outcome of adjunctive traditional Chinese medicine therapy in liver cirrhosis: a large cohort study in Southwest China. Complement Ther Med. 2020;51:1–8.
43. Changya W. Discussion on the pharmacological effects of dried tangerine peel. Electronic J Clin Med Lit. 2020;7(15):135.
44. Wenli W, Qiuling W. Application and research progress of eupatorium fortunei. Strait Pharm J. 2019;31(6):28–30. Chinese.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
