Back to Journals » International Journal of General Medicine » Volume 16
An Energy-Efficient Test and Predictive Model for Recurrence After Radiotherapy in Localized Intermediate and Advanced Cervical Cancer Were Created Using Thymidine Kinase 1 in Conjunction with Inflammatory Markers and Tumor Markers
Received 28 September 2023
Accepted for publication 20 November 2023
Published 8 December 2023 Volume 2023:16 Pages 5789—5797
DOI https://doi.org/10.2147/IJGM.S442389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yuanyuan Luo,* Xiaojie Ma*
Oncology; Affiliated Hospital of North Sichuan Medical College; Nanchong, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaojie Ma, Email [email protected]
Purpose: Construction of a nomogram model based on Thymidine kinase 1 (TK1) in combination with inflammatory indicators and tumor markers to predict the probability of recurrence in mid- to late-stage cervical cancer.
Methods: One hundred fourteen instances of intermediate and advanced cervical cancer admitted to our hospital’s radiotherapy department between June 2017 and January 2023 were retrospectively studied. Logistic regression analysis includes variables relevant for univariate analysis. Meaningful indications from multifactor analysis were included in the nomogram model, the model’s correctness was evaluated using the C-index, and the model’s effectiveness was assessed using calibration curves, clinical decision curves, and clinical impact curves.
Results: A nomogram model was created due to the logit regression analysis that revealed the squamous cell carcinoma antigen (SCC) and TK1 as independent recurrence predictors following cervical cancer radiation (P< 0.05). The C index and Area Under the Curve (AUC) were 0.79 (95% CI 0.67– 0.91). The AUC and C-index were both more extraordinary than those of TNM staging alone (C-index 0.57, 95% CI 0.43– 0.71) and SCC alone (C-index 0.67, 95% CI 0.51– 0.82). Calibration curves, Decision Curve Analysis (DCA), and clinical impact curves (CIC) indicate that the model predicts probabilities more accurately.
Conclusion: The nomogram model based on TK1 combined with inflammatory markers and tumor markers is more reliable than the TNM staging and SCC systems alone for forecasting recurrence after radiotherapy in intermediate- and advanced-stage cervical cancer. It is also a cheap, practical, and simple-to-obtain model that can supplement the TNM staging system for forecasting prognosis and significantly enhances clinicians’ decision-making.
Keywords: cervical cancer, thymidine kinase 1, inflammatory factors, prognosis, nomogram
Despite increasing immunization and cervical cancer(CC) screening, cervical cancer is still the fourth most frequent malignancy in women globally.1 The principal method of treatment for cervical cancer that is intermediate or advanced is concurrent chemoradiation according to the most recent recommendations.2 Furthermore, 20–30% of women with intermediate or advanced cervical cancer will experience recurrence within five years, even with standard treatment, and the 5-year survival rate for those with recurrence is just under 10%. The gold standard for determining a patient’s prognosis for cervical cancer is the 2018FIGO TNM staging.3,4 Even cervical cancer patients with the same TNM stage and undergoing the same treatment have varying prognoses. This shows that without considering other pathologic variables or serum biomarkers, the present staging systems cannot reliably predict the prognosis of cervical cancer. Therefore, the primary difficulties for clinical diagnostic and therapeutic study in this sector are providing early prediction of recurring patients, widening the therapy window, and mechanistically understanding the heterogeneity of cervical cancer evolution. In order to enhance patient outcomes and survival, it is necessary to develop simple models that can be used to predict the likelihood of recurrence of middle- and late-stage cervical cancer in advance. These models can also be used to modify the treatment plans of patients.
According to studies, systemic signs of inflammation have been linked to the emergence of several tumor types.5–8 Inflammatory indices like the Systemic Immunoinflammatory Index (SII) and the Platelet-to-Lymphocyte Ratio (PLR), Neutrophil-to-Lymphocyte Ratio (NLR), Lymphocyte-to-Lymphocyte Ratio (LMR). SII have all been linked to poor prognosis in a variety of tumors. Although those indicators have been linked to poor prognosis in several tumor types, it is questionable if they are both helpful in predicting the prognosis of cervical cancer. A tumor-specific squamous cell carcinoma antigen (SCC) tracks and monitors numerous squamous cell cancers. One of the essential enzymes for DNA synthesis in tumor cells is thymidine kinase 1 (TK1), which is strongly associated with cell proliferation and has thus far been crucial in determining the effectiveness of many malignancies.9–12 Less research has been done on serum TK1 to determine whether cervical cancer that is intermediate or advanced will return following radiotherapy. To predict the recurrence of cervical cancer after radiotherapy, this study developed an innovative nomogram model based on inflammatory factors, serum TK1, and tumor markers and compared its predictive power to that of the TNM staging system and SCC. The goal was a precise prognosis prediction for patients with intermediate and advanced cervical cancer following radiation.
Information and Methods
Subjects of the Study
A retrospective analysis was done on the clinical information of 114 instances of medium- and advanced cervical cancer patients admitted to the radiotherapy department of our hospital between June 2017 and January 2023. The following requirements must be met: 1) Magnetic Resonance Imaging (MRI) stage II to IVA (2018 FIGO staging); 2) pathological confirmation of cervical cancer diagnosis; and 3) no prior history of radiation. Acute hepatitis or cirrhosis are not included because of radiotherapy contraindications, secondary malignant tumors of other organs present, systemic infections, coagulation issues, autoimmune diseases, or imperfect clinical data.
Collection of Clinicopathological Data
Through the electronic medical record system, clinicopathological information on the study participants was gathered, including the age, gender, tumor diameter, and TNM stage of the patients. Before radiation, patients’ laboratory results were obtained, including neutrophil count, lymphocyte count, monocyte count, white blood cell count, platelet count, hemoglobin count, SCC and TK1.Medical Ethics Committee of the Affiliated Hospital of North Sichuan Medical College blessed the study (2023ER85-1).And written informed consent was obtained from all patients.
Treatment
A Siemens AG, Germany, simulated positioning CT was used for scanning, a thermoplastic body mold was used to establish the position, the radiotherapist marked the target region, and a Synergy gas pedal was used for irradiation.PTV 50Gy/25Fx, five times per week; afterloading therapy, six Gy/dose, once weekly for four weeks. Additionally, cisplatin 30mg/m2 synchronized treatment was given once per week.
Calculation of Indicators of Inflammation
NLR = neutrophil count/lymphocyte count, PLR = platelet count/lymphocyte count, LMR = lymphocyte count/monocyte count, SII = neutrophil count × platelet count/lymphocyte count.
Serum TK1 Collection
All cervical cancer patients had five milliliters of peripheral venous blood drawn while fasting within the week before radiotherapy. A chemiluminescent enzyme immunoassay was used to measure the amount of serum TK1 after the serum was centrifuged at 3000 rpm for 10 min (Baumann et al, Shanghai, China).
Statistical Methods
GraphPad Prism 9 and SPSS 25.0 were used to analyze and create graphs. The measurement data were first checked for normality (x±s) before the one-way analysis of variance. The Fisher’s exact test and χ2 test were utilized for count data. Logistic regression analysis was used to do multifactorial analysis. The cut-off value for continuous variable data was calculated using the Area Under the Curve (AUC) from the subject’s work characteristic curve. The R language and the RMS package created the nomogram model. The performance of the nomogram was evaluated using the C-index, AUC, Calibration curve, Decision Curve Analysis (DCA), and clinical impact curve (CIC) and then compared to the TNM staging system and SCC. The difference has a p<0.05 significance level.
Results
Patient General Characteristics and Inflammatory Indicators and TK1 Critical Values Were Determined
The patients were (54.30 + 11.13) years old on average. Sixteen patients relapsed. Ninety-eight individuals had no relapses. Patients with recurrence were, on average, (62±15.88) years old, while those without recurrence were, on average, (54±10.16) years old. Receiver Operating Characteristic (ROC) analysis showed that the AUC for diagnosing radiotherapy recurrence at the levels of NLR, PLR, LMR, SII, and TK1 were 0.60, 0.59, 0.43, 0.77, and 0.73, with the optimal cut-off values of 3.66 (95% CI 0.43–0.76), 105.177 (95% CI 0.45–0.72), 7.08 (95% CI of 0.25–0.60), 2105.23 (95% CI of 0.64–0.90), and 1.23 (95% CI of 0.59–0.86) (see Figure 1).
Unifactorial and Multifactorial Analysis of Prognosis of Radiotherapy for Cervical Cancer
Univariate analysis linked SCC, TK1, and SII with recurrent cases. SCC and TK1 were identified as independent risk factors for recurrence following analysis of the unifactorial practical factors into a multifactorial logistic regression model (see Table 1).
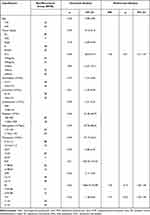 |
Table 1 Results of Univariate and Multivariate Analysis |
Prognostic Model Construction for Recurrence Columnogram Prognosis After Radiotherapy for Cervical Cancer
SCC and TK1 were incorporated into the nomogram prediction model of recurrence after radiotherapy for cervical cancer based on the outcomes of multifactorial logistics regression (Figure 2). The C-index and AUC were both 0.79 (95% 0.67–0.91) (Figure 3). TNM staging (C-index 0.57, 95% CI 0.43–0.71) and SCC system (C-index 0.67, 95% CI 0.51–0.82) were lower than the nomogram. The model’s overall predictive rate was 86.84% (see Table 2). The calibration curves demonstrated that the predicted curves and the actual curves fit each other well (Figure 4). Comparing the nomogram model to the TMN staging and SCC systems, DCA revealed that the nomogram model had a larger range of threshold probabilities in prediction (Figure 5). The CIC shows that the model has a better range of clinical applications (Figure 6).
 |
Table 2 Probability of Recurrence Predicted by the Recurrence Model After Radiotherapy for Cervical Cancer |
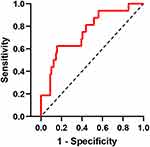 |
Figure 3 ROC curve of radiotherapy recurrence prediction model for cervical cancer. |
 |
Figure 4 Calibration curve of the recurrence prediction model after radiotherapy for cervical cancer. |
 |
Figure 5 Cervical cancer radiotherapy recurrence prediction model and DCA curve for TNM and SCC prediction. |
 |
Figure 6 CIC curve of radiotherapy recurrence prediction model for cervical cancer. |
Discussion
Cervical cancer(CC) is the most common disease in women worldwide and has significant death and morbidity rates.13,14 To improve the survival rate of patients with middle- and late-stage cervical cancer, it is urgent to predict the likelihood of recurrence in advance and modify the treatment plan. Recurrence is an essential factor impeding cervical cancer survival, and the 5-year survival rate of patients with recurrence is not optimistic.
The prognosis of cervical cancer has been predicted by several earlier research. Cibula et al15 developed a predictive model for general clinical characteristics of cervical cancer but left out SII inflammatory variables, NLR, PLR, and LMR. It has been demonstrated that many cancers’ onset, growth, and progression are correlated with systemic inflammation. So, in our prediction model, we took inflammatory factors into account. Additionally, it has been demonstrated that TK1 is linked to the growth of tumor cells. TK1 was not included in the prognostic model for the efficacy prediction of cervical cancer, despite Chen16 et al’s suggestion that it was related to the prognosis of cervical cancer.In order to predict recurrence following radiotherapy for cervical cancer, we creatively integrated TK1 with inflammatory variables and tumor markers in the model in this work. A nomogram model was used to predict the recurrence risk of patients with advanced cervical cancer after radiation. Establishing a prediction model and thoroughly evaluating it using predictors can help to determine prognosis more correctly as tumor treatment becomes more precise and personalized.
We found and incorporated two independent prognostic variables, namely TK1 and SCC, based on multivariate analysis findings. The nomogram demonstrated that whereas SCC contributed very little to determining the prognosis of patients with cervical cancer, TK1 considerably predicted patients’ prognoses.SCC has been widely utilized as a marker for squamous cell carcinoma of the head and neck, lung, and esophagus,17,18 and TK1 is a DNA proliferation marker whose increase signals aberrant cell proliferation.
Numerous studies have demonstrated that a range of molecular markers, such as P16, Ki-67, and CK1, are related to the prognosis of cervical cancer.19 Immunohistochemical assays, however, are pricy and not frequently used. As a result, the nomogram created in this study is an easy-to-use, low-cost tool that can effectively predict recurrence in cervical cancer patients.
Comparing the nomogram prediction model to the TNM staging and SCC systems demonstrates good prediction capabilities. The C-index of the nomogram model was 0.79, which was greater than the C-indices of the SCC system (0.67) and TNM staging alone (0.57). Compared to the conventional TNM staging system and SCC, DCA and CIC show that the nomogram model created in this work has a higher overall net benefit over a wide range of threshold probabilities. In light of the findings, as mentioned earlier, it can be concluded that the nomogram model developed for this study is an accurate method for predicting the return of cervical cancer.
A bias in case selection is possible because this investigation was retrospective. Additionally, this study lacked a broadly relevant external validation cohort and was conducted in a single center with few patients. It is recommended to carry out a multicenter study with a bigger sample size and external validation.
Conclusion
In summary, this study developed a nomogram model of recurrence in cervical cancer patients treated with radiotherapy. Compared with the traditional TNM staging system and SCC, this nomogram is more accurate in predicting prognosis. It is also simple, inexpensive and easy to obtain, which greatly improves clinicians’ decision-making ability and prognosis. However, the sample size of this study is small, and the conclusions obtained still need to be validated by prospective studies with large samples.
Ethics Approval and Informed Consent
This study was approved by the Medical Ethics Committee of the Affiliated Hospital of North Sichuan Medical College blessed the study (2023ER85-1) and written informed consent was obtained from all patients and the study complied with the Declaration of Helsinki.
Acknowledgments
The authors wish to thank Xiaojie Ma for help of data analysis.
Author Contributions
The work reported is significantly enhanced by the contributions of all authors, who took part in the conception, study design, execution, data acquisition, analysis, and interpretation, or in all of these areas; who drafted, revised, or critically reviewed the article; who approved the final version to be published; who decided which journal to submit the article to; and who agreed to take responsibility for all aspects of the work. Yuanyuan Luo, Xiaojie Ma, contributed to this work equally and shared the first authorship.
Funding
The Clinical Research of Sichuan Anti-Cancer Association (Qilu) provided funding for this project. [XH2022-XXX].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet. 2019;393(10167):169–182. doi:10.1016/S0140-6736(18)32470-X
2. Abu-Rustum NR, Yashar CM, Bean S, et al. NCCN Guidelines Insights: cervical Cancer, Version 1.2020. J Natl Compr Canc Netw. 2020;18(6):660–666. doi:10.6004/jnccn.2020.0027
3. Bhatla N, Singhal S, Dhamija E, et al. Implications of the revised cervical cancer FIGO staging system. Indian J Med Res. 2021;154(2):273–283. doi:10.4103/ijmr.IJMR_4225_20
4. Wright JD, Matsuo K, Huang Y, et al. Prognostic Performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet Gynecol. 2019;134(1):49–57. doi:10.1097/AOG.0000000000003311
5. Cursano MC, Kopf B, Scarpi E, et al. Prognostic Role of Systemic Inflammatory Indexes in Germ Cell Tumors Treated With High-Dose Chemotherapy. Front Oncol. 2020;10:1325. doi:10.3389/fonc.2020.01325
6. Lee BM, Chung SY, Chang JS, et al. The Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio Are Prognostic Factors in Patients with Locally Advanced Pancreatic Cancer Treated with Chemoradiotherapy. Gut Liver. 2018;12(3):342–352. doi:10.5009/gnl17216
7. Gao Y, Guo W, Cai S, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma. J Cancer. 2019;10(14):3188–3196. doi:10.7150/jca.30281
8. Li N, Zhang Y, Qu W, et al. Analysis of systemic inflammatory and coagulation biomarkers in advanced cervical cancer: prognostic and predictive significance. Int J Biol Markers. 2023;38(2):133–138. doi:10.1177/03936155231163599
9. He X, Wang M, Zhong WL. Application Value of Serum TK1 and PCDGF, CYFRA21-1, NSE, and CEA plus Enhanced CT Scan in the Diagnosis of Nonsmall Cell Lung Cancer and Chemotherapy Monitoring. J Oncol. 2022;2022:1–6. doi:10.1155/2022/8800787
10. Bergqvist M, Nordmark A, Williams A, et al. Thymidine kinase activity levels in serum can identify HR+ metastatic breast cancer patients with a low risk of early progression (SWOG S0226). Biomarkers. 2023;28(3):313–322. doi:10.1080/1354750X.2023.2168063
11. Tribukait B, Lundgren PO, Kjellman A, et al. Prediction of Overall Survival by Thymidine Kinase 1 Combined with Prostate-Specific Antigen in Men with Prostate Cancer. Int J Mol Sci. 2023;24(6):5160. doi:10.3390/ijms24065160
12. Costa Svedman F, Jalsenius M, Grozman V, et al. Dynamics of plasma thymidine kinase activity in metastatic melanoma reflects immune checkpoint inhibitor efficacy. Acta Oncol. 2022;61(9):1116–1120. doi:10.1080/0284186X.2022.2121615
13. Buskwofie A, David-West G, Clare CA. A Review of Cervical Cancer: incidence and Disparities. J Natl Med Assoc. 2020;112(2):229–232. doi:10.1016/j.jnma.2020.03.002
14. Vu M, Yu J, Awolude OA, et al. Cervical cancer worldwide. Curr Probl Cancer. 2018;42(5):457–465. doi:10.1016/j.currproblcancer.2018.06.003
15. Cibula D, Dostálek L, Jarkovsky J, et al. Post-recurrence survival in patients with cervical cancer. Gynecol Oncol. 2022;164(2):362–369. doi:10.1016/j.ygyno.2021.12.018
16. Chen G, He C, Li L, et al. Nuclear TK1 expression is an independent prognostic factor for survival in pre-malignant and malignant lesions of the cervix. BMC Cancer. 2013;13(1):249. doi:10.1186/1471-2407-13-249
17. Zhu H. Squamous Cell Carcinoma Antigen: clinical Application and Research Status. Diagnostics. 2022;12(5):1065. doi:10.3390/diagnostics12051065
18. Schepens EJA, Al-Mamgani A, Karssemakers LHE, van den Broek D, van den Brekel MWM, Lopez-Yurda M. Squamous Cell Carcinoma Antigen in the Follow-up of Patients With Head and Neck Cancer. Otolaryngol Head Neck Surg. 2023. doi:10.1002/ohn.510
19. Volkova LV, Pashov AI, Omelchuk NN. Cervical Carcinoma: oncobiology and Biomarkers. Int J Mol Sci. 2021;22(22):12571. doi:10.3390/ijms222212571
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


