Back to Journals » OncoTargets and Therapy » Volume 15
An Effective Hormonal Therapy for a Patient with Estrogen Receptor 1 (ESR1)-Amplified Metastatic Ovarian Cancer: A Case Report
Authors Wang Y, Tan S, Pan E, Ma Y , Wu X, Yu Z, Jiang K
Received 15 March 2022
Accepted for publication 27 May 2022
Published 9 June 2022 Volume 2022:15 Pages 643—649
DOI https://doi.org/10.2147/OTT.S363856
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Yue Wang,1,* Shuang Tan,2,* Evenki Pan,3 Yutong Ma,3 Xue Wu,3 Zhe Yu,1 Kui Jiang1
1Department of Medical Oncology, the Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, People’s Republic of China; 2Department of Gynecology, the Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, People’s Republic of China; 3Geneseeq Research Institute, Nanjing Geneseeq Technology Inc, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kui Jiang; Zhe Yu, Department of Medical Oncology, the Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, People’s Republic of China, Tel +8617709873696 ; +8618641103913, Email [email protected]; [email protected]
Abstract: Hormonal therapy is an important treatment option for estrogen receptor (ER)-positive patients with advanced ovarian cancer. Although ER overexpression has been previously used as an indicator for hormonal therapy, the clinical outcomes of advanced ovarian cancer patients receiving hormonal therapy remain unsatisfactory. Additional biomarkers for screening patients are needed to improve its efficacy. In this study, we reported a metastatic ovarian cancer case with estrogen receptor 1 (ESR1) gene amplification and protein overexpression, which showed sustained partial response to hormonal therapy, including letrozole and tamoxifen, and displayed an overall survival of 47 months. The response to the therapy was evaluated by imageological examinations, cancer antigen-125 (CA-125) tests, and circulating tumor DNA (ctDNA) sequencing using capture-based hybrid next-generation sequencing. Our clinical data suggested that ESR1 amplification might be a potential predictor of response to hormonal therapy in ovarian cancer. The combination of tumor detection techniques including imaging, CA-125 and ctDNA would enable confirmation of tumor response with high confidence.
Keywords: ESR1 amplification, ovarian cancer, hormonal therapy, letrozole, tamoxifen, circulating tumor DNA
Background
Ovarian cancer is the second leading cause of gynecologic cancer death.1 It accounts for 3.6% and 4.4% of female new cancer cases and deaths per year worldwide.2 In China, its incidence and mortality among all female cancer cases are 2.9% and 2.2%, slightly lower than the global level.3
At present, tumor cytoreductive surgery combined with platinum-based chemotherapy is the primary treatment for advanced ovarian cancer.4 Although the surgery in combination with chemotherapy is effective for most patients, its efficacy for ovarian cancer is not satisfactory and the recurrent rate of ovarian cancer remains high. Hormonal therapy is one of the treatment options for patients with estrogen receptor (ER)-positive advanced ovarian cancer. Studies have shown that the objective response rate and disease stability duration of high-grade serous ovarian cancer patients receiving hormonal therapy were ~15% and 9.6 months, respectively.5 The insufficient efficacy of hormonal therapy in ER-positive patient cohort limits its clinical application in advanced ovarian cancer and the identification of additional biomarkers for rational patient selection to improve its efficacy is paramount.
There are two subtypes of ER proteins, ERα and ERβ, which are encoded by estrogen receptor 1 (ESR1) and ESR2 genes, respectively. It has been reported that 25%–86% of ovarian cancers have ER expression.6 The success of hormonal therapy in breast cancer and the high frequency of ER expression in ovarian cancer have spurred great effort in evaluating hormonal therapy’s efficacy in ovarian cancer. Albeit its low frequency in ovarian cancer, ERS1 gene amplification has been shown 100% associated with ER expression in ovarian cancer,6 indicating it is one of the mechanisms for upregulating ER expression. To our knowledge, we reported the first case of ESR1-amplified metastatic ovarian cancer that showed a durable response to both tamoxifen- and letrozole-based hormonal therapy. Our case report encouraged further exploration of hormonal therapy biomarkers for precision medicine in ovarian cancer.
Case Presentation
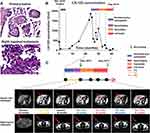
In March 2016, a 78-year-old female patient diagnosed with stage IIIC ovarian cancer underwent cytoreductive surgery. Tumor nodules were found in the right ovary, right fallopian tube, left ovary, appendix, omentum, and umbilicus. Postoperative pathology of tumor samples from the ovary revealed high-grade serous adenocarcinoma (Figure 1A). Cancer antigen 125 (CA-125) decreased from 441.82 U/mL to 71.01 U/mL after cytoreductive surgery (Figure 1B). Subsequently, she was treated with six cycles of docetaxel plus carboplatin chemotherapy. After postoperative chemotherapy, regular follow-up with monitoring of CA-125 was recommended and performed.
The information of systematic therapies is summarized in Table 1, and as shown in Figure 1B, the level of CA-125 remained at a relatively low level within nine months after surgery. While an enlarged lymph node in the right-groin area was noted after ten months of surgery, the size of which increased at the next follow-up with an elevated CA-125 level. At that time, the patient refused the recommended chemotherapy and continued the regular follow-ups. Chemotherapy was recommended to the patient; however, she refused the treatment. Unfortunately, multiple metastatic lesions were found by abdominal enhanced computed tomography (CT) in the hepatic hilum, intraperitoneal cavity, and right cardiophrenic angle (36*47 mm) after 24 months of surgery with a dramatic increase in CA-125 level (1862.18 U/mL). Eastern Cooperative Oncology Group (ECOG) performance status of the patient at the time was grade 1. Four cycles of docetaxel plus carboplatin were administered as the second-line chemotherapy, which led to a stable disease (SD) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria (Figure 1C). Side effects of grade 2 white blood cell decrease and grade 1 platelet decrease were reported. Then, she received two cycles of gemcitabine, which only resulted in a temporary decrease in CA-125 level.
 |
Table 1 Systemic Therapy Received by the Patient |
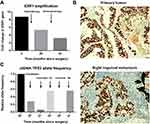
In September 2018, the level CA-125 increased to 1113.17 U/mL and an aspiration biopsy was performed on the right inguinal lymph node metastasis. The hematoxylin and eosin stains of the biopsy sample confirmed high-grade serous adenocarcinoma (Figure 1A). Both primary and metastatic tumor specimens underwent capture-based hybrid next-generation sequencing (NGS) by targeting 425 cancer-related genes, which revealed 8.7- and 5.3-fold ESR1 gene amplification in the primary tumor and metastatic lesion, respectively (Figure 2A). Immunohistochemical (IHC) staining (VENTANA anti-ER (SP1) Rabbit Monoclonal Primary Antibody, Roche) results showed a positive expression of ER in both primary tumor (>80%) and metastatic inguinal lymph node (>90%) (Figure 2B). Based on the NGS-detected ESR1 amplification and ER overexpression, the patient received tamoxifen (20 mg oral daily), a hormonal therapy targeting the ER, starting in September 2018. After three months of tamoxifen treatment, both the right groin and the hepatic hilar tumor nodules significantly shrank (Figure 1C), which reached a partial response (PR) according to RECIST 1.1, and the level of CA-125 decreased to 186.38 U/mL. However, the size of hepatic hilar lymph nodes significantly increased (Figure 1C) after another two months of tamoxifen treatment, suggesting a progressive disease (PD). Aromatase inhibitor letrozole (2.5 mg oral daily) was subsequently given, which led to the shrinkage of metastatic hepatic hilar and right-groin nodules as well as the decrease in CA-125 (Figure 1C). However, after five months of letrozole treatment, CA-125 increased and hepatic hilar lymph node metastasis enlarged again. A re-biopsy of the right inguinal lymph node metastasis was performed for NGS testing, and a 3.2-fold amplification of ESR1 was detected (Figure 2A). The patient then tried hormonal and targeted therapy and chemotherapy but responded poorly and died in February 2020.
Discussion and Conclusions
Although estrogen has a well-defined clinical value in breast cancer,7 its role in ovarian cancer is less well established. Significant research interest has been seen in evaluating the clinical value of ER expression in ovarian cancer, especially regarding the sensitivity to hormonal therapy.8–12 However, mixed clinical outcomes of hormonal therapy were reported in several clinical trials assessing their correlation with ER expression in ovarian cancer. Such diverse results might result from the different methods used to determine ER positivity and the lack of additional predictive biomarkers. In our study, we incorporated targeted NGS with IHC examination, which demonstrated the correlation between ESR1 gene amplification and positive ER expression.
Tamoxifen is one of the first anti-estrogens to enter clinical trials before 2000. As early as 1981, Myers et al reported a favorable response to tamoxifen in three ovarian cancer patients.13 A systematic review of 648 ovarian cancer patients treated with tamoxifen in clinical trials reported an overall response rate of 13% and a disease control rate of 38%.14 Since 2002, aromatase inhibitors have been evaluated in a number of clinical trials for ovarian cancer treatment.8,15,16 Letrozole is one of the aromatase inhibitors that can block the conversion of androgen to estrogen by inhibiting the key enzyme aromatase in the estrogen synthesis pathway.17 Two early studies of letrozole have shown that the CA-125 response rate of ovarian cancer patients was improved by restricting ER expression status.8,10 A recent clinical study showed that letrozole as a maintenance therapy for patients with advanced ER-positive serous ovarian cancer significantly increased the 24-month relapse-free survival rate from 38.5% to 60%.18
In this case report, we presented a patient who was sensitive to hormonal therapy and obtained an overall survival of 47 months. The 18-month platinum-free interval after the first-line chemotherapy prompted the use of second-line platinum-based chemotherapy in the hope of a good tumor response. However, the side effect, suboptimal response (stable disease) and the rapid increase in CA-125 level during the second-line chemotherapy promoted a search for alternative therapies. The targeted NGS and IHC tests were performed for primary and metastatic tumor samples, which showed an ESR1 gene amplification and ER protein overexpression, respectively. These findings led to the subsequent use of hormonal therapies, including tamoxifen and letrozole. The best response to tamoxifen and letrozole was PR with a five-month progression-free survival. However, this single ESR1-amplified case with optimal response to hormonal therapy is not sufficient for concluding the predictive role of ESR1 gene amplification; thus, more comprehensive studies in larger cohorts are needed. A Phase III trial of chemotherapy with or without bevacizumab in patients with platinum-resistant recurrent ovarian cancer has shown that patients’ median overall survival is 16.6 months and 13.3 months, respectively.19 However, the presented patient obtained a survival of 47 months (from Mar. 2016 to Feb. 2020) by the combination of cytoreductive surgery, chemotherapy and hormonal therapy, which was better than the median overall survival of 33.6 months for the platinum-sensitive recurrent ovarian cancer treated with chemotherapy plus bevacizumab in another phase III clinical trial.20
Circulating tumor DNA (ctDNA) sequencing with liquid biopsy has become a reliable tool for comprehensive genomic assessment and resistance mechanism exploration during treatment. In this case, the changes in ctDNA levels, represented by the allele frequency of TP53 R273C mutation, were associated with CA-125 levels and treatment efficacy (Figure 2C). The R273C mutation of TP53 was observed in several cancer types and related to cancer cell proliferation and invasion.21 Previous studies also showed that TP53 mutation in breast cancer could inhibit ER expression and increase tamoxifen resistance.22 Thus, the accumulation of TP53 mutation detected by NGS might explain the progression upon hormonal therapies.
In conclusion, we reported a metastatic ovarian cancer case with high level of ESR1 gene amplification, which was associated with strong ER protein expression and prolonged the favorable response to hormonal therapies including tamoxifen and letrozole. Our study promoted the discovery of additional biomarkers for hormonal therapy in ovarian cancer and NGS-detected ESR1 gene amplification might be a strong candidate in addition to IHC-validated ER expression.
Abbreviations
ER, estrogen receptor; ESR1, estrogen receptor 1; CA-125, cancer antigen 125; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; NGS, next-generation sequencing; IHC, immunohistochemical; PD, progressive disease; ctDNA, circulating tumor DNA.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable requests.
Ethics Approval and Consent to Participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee of the Second Affiliated Hospital of Dalian Medical University (reference number 2020059) and with the Helsinki Declaration (as revised in 2013).
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Acknowledgments
We sincerely thank the patient for supporting our work. We thank Dr. Qiuxiang Ou for helping interpret the genetic test results.
Funding
This study did not receive any specific grant.
Disclosure
Evenki Pan, Yutong Ma, and Xue Wu are employees of Nanjing Geneseeq Technology Inc., China. The other authors declare no conflicts of interest related to this case report.
References
1. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280–304. doi:10.3322/caac.21559
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN Guidelines Insights: ovarian Cancer, Version 1.2019. J Natl Compr Canc Netw. 2019;17(8):896–909. doi:10.6004/jnccn.2019.0039
5. George A, McLachlan J, Tunariu N, et al. The role of hormonal therapy in patients with relapsed high-grade ovarian carcinoma: a retrospective series of tamoxifen and letrozole. BMC Cancer. 2017;17(1):456. doi:10.1186/s12885-017-3440-0
6. Issa RM, Lebeau A, Grob T, et al. Estrogen receptor gene amplification occurs rarely in ovarian cancer. Mod Pathol. 2009;22(2):191–196. doi:10.1038/modpathol.2008.130
7. Yue W, Wang JP, Li Y, et al. Effects of estrogen on breast cancer development: role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127(8):1748–1757. doi:10.1002/ijc.25207
8. Smyth JF, Gourley C, Walker G, et al. Antiestrogen therapy is active in selected ovarian cancer cases: the use of letrozole in estrogen receptor-positive patients. Clin Cancer Res. 2007;13(12):3617–3622. doi:10.1158/1078-0432.CCR-06-2878
9. Stanley B, Hollis RL, Nunes H, et al. Endocrine treatment of high grade serous ovarian carcinoma; quantification of efficacy and identification of response predictors. Gynecol Oncol. 2019;152(2):278–285. doi:10.1016/j.ygyno.2018.11.030
10. Bowman A, Gabra H, Langdon SP, et al. CA125 response is associated with estrogen receptor expression in a Phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin Cancer Res. 2002;8(7):2233–2239.
11. Hollis RL, Stanley B, Iida Y, et al. Hormone receptor expression patterns define clinically meaningful subgroups of endometrioid ovarian carcinoma. Gynecol Oncol. 2019;155(2):318–323. doi:10.1016/j.ygyno.2019.09.001
12. Paleari L, Gandini S, Provinciali N, Puntoni M, Colombo N, DeCensi A. Clinical benefit and risk of death with endocrine therapy in ovarian cancer: a comprehensive review and meta-analysis. Gynecol Oncol. 2017;146(3):504–513. doi:10.1016/j.ygyno.2017.06.036
13. Myers AM, Moore GE, Major FJ. Advanced ovarian carcinoma: response to antiestrogen therapy. Cancer. 1981;48(11):2368–2370. doi:10.1002/1097-0142(19811201)48:11<2368::
14. Perez-Gracia JL, Carrasco EM. Tamoxifen therapy for ovarian cancer in the adjuvant and advanced settings: systematic review of the literature and implications for future research. Gynecol Oncol. 2002;84(2):201–209. doi:10.1006/gyno.2001.6489
15. Ramirez PT, Schmeler KM, Milam MR, et al. Efficacy of letrozole in the treatment of recurrent platinum- and taxane-resistant high-grade cancer of the ovary or peritoneum. Gynecol Oncol. 2008;110(1):56–59. doi:10.1016/j.ygyno.2008.03.014
16. Papadimitriou CA, Markaki S, Siapkaras J, et al. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer. Long-term results of a phase II study. Oncology. 2004;66(2):112–117. doi:10.1159/000077436
17. Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol. 2001;12(11):1527–1532. doi:10.1023/a:
18. Heinzelmann-Schwarz V, Knipprath Meszaros A, Stadlmann S, et al. Letrozole may be a valuable maintenance treatment in high-grade serous ovarian cancer patients. Gynecol Oncol. 2018;148(1):79–85. doi:10.1016/j.ygyno.2017.10.036
19. Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–1308. doi:10.1200/JCO.2013.51.4489
20. Aghajanian C, Goff B, Nycum LR, Wang YV, Husain A, Blank SV. Final overall survival and safety analysis of OCEANS, a Phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2015;139(1):10–16. doi:10.1016/j.ygyno.2015.08.004
21. Li J, Yang L, Gaur S, et al. Mutants TP53 p.R273H and p.R273C but not p.R273G enhance cancer cell malignancy. Hum Mutat. 2014;35(5):575–584. doi:10.1002/humu.22528
22. Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8(5):8921–8946. doi:10.18632/oncotarget.13475
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.