Back to Journals » Journal of Inflammation Research » Volume 16
AMPK Signalling Pathway: A Potential Strategy for the Treatment of Heart Failure with Chinese Medicine
Authors Liu C, Guo X, Zhou Y, Wang H
Received 11 October 2023
Accepted for publication 10 November 2023
Published 21 November 2023 Volume 2023:16 Pages 5451—5464
DOI https://doi.org/10.2147/JIR.S441597
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Changxing Liu,1,* Xinyi Guo,2,* Yabin Zhou,3 He Wang3
1First Clinical Medical School, Heilongjiang University of Chinese Medicine, Harbin, 150040, People’s Republic of China; 2Clinical Medical School, Chengdu University of Traditional Chinese Medicine, Chengdu, 610075, People’s Republic of China; 3Department of Cardiology, The First Hospital of Heilongjiang University of Chinese Medicine, Harbin, 150040, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yabin Zhou; He Wang, Email [email protected]; [email protected]
Abstract: Heart failure (HF) is a complex clinical syndrome that represents the advanced stage of cardiovascular disease, characterized by systolic and diastolic dysfunction of the heart. Despite continuous updates in HF treatment drugs, the morbidity and mortality rates remain high, necessitating ongoing exploration for new therapeutic targets. Adenosine monophosphate-activated protein kinase (AMPK) is the serine/threonine protein kinase which responds to adenosine monophosphate (AMP) levels.Activation of AMPK shifts cellular metabolic patterns from synthesis to catabolism, enhancing energy metabolism in pathological conditions such as inflammation, ischemia, obesity, and aging. Numerous studies have identified AMPK as a vital target for HF treatment, with herbal monomers/extracts and compounds affecting key signaling factors including rapamycin targeting protein (mTOR), silencing regulator protein 1 (SIRT1), nuclear transcription factor E2-related factor 2 (Nrf2), and nuclear transcription factor-κB (NF-κB) through regulation of the AMPK signaling pathway.This modulation can achieve the effects of improving metabolism, autophagy, reducing oxidative stress and inflammatory response in the treatment of heart failure, with the advantages of multi-targeting, comprehensive action and low toxicity.The modulation of the AMPK pathway by Traditional Chinese Medicine (TCM) has emerged as a crucial research direction for the prevention and treatment of HF, but a systematic summary and generalization in this field is lacking. This article provides an overview of the composition, regulation, and mechanism of the AMPK signaling pathway’s influence on HF, as well as a summary of current research on the regulation of the AMPK pathway by TCM for HF prevention and treatment. The aim is to serve as a reference for the diagnosis and treatment of HF using TCM and the development of new drugs.
Keywords: AMPK signalling pathway, Chinese medicine, heart failure, mechanism of action, review
Introduction
Heart failure (HF) is a complex clinical syndrome characterised by a decrease in the heart’s ability to pump and/or fill with blood.1 It is a progressive disease and is the endpoint of the vast majority of cardiovascular diseases. In recent years, the survival rate of HF has gradually increased due to population aging, advances in medical technology, and improved health care policies, but its morbidity and mortality rates have not declined, which indirectly leads to an increase in hospitalisation rates, and an increase in the cost of health care.2,3 HF continues to be a growing public health problem on a global scale. According to statistics, the prevalence of heart failure is expected to increase by 46% from 2012 to 2030, and the proportion of the total population with heart failure is projected to rise from 2.4% to 3.0%.4,5 Western medicine in the drug treatment of this disease mainly uses cardiotonic, diuretic, vasodilator, etc. In the acute or advanced stage of heart failure, there are many non-pharmacological treatments, including cardiac resynchronisation therapy, implantable cardiac defibrillator, cardiac transplantation, total artificial heart, gene and cell therapy.6–8 Although western medical treatment is perfect and can achieve certain efficacy and prolong the life span of patients to a certain extent, there are still some limitations in clinical treatment because of its many adverse reactions, poorer prognosis, and poorer results.9 Therefore, finding drugs that target key pathological aspects of HF, improving and optimising therapeutic regimens, reducing mortality and recurrence rates, and improving clinical outcomes are key to current research into the treatment of heart failure.
Adenosine monophosphate-activated protein kinase (AMPK) serves as an “energy sensor” regulating cellular metabolism.10,11 It is a highly conserved serine/threonine protein kinase that is widely expressed in eukaryotic organisms and various organs, allowing it to sense changes in the cellular ratio of adenosine monophosphate (AMP) to adenosine triphosphate (ATP). AMPK plays a crucial role in regulating multiple metabolic pathways and maintaining cellular ATP homeostasis, making it the “master metabolic switch” of the body.12–14 By restoring energy supply, regulating autophagy, and improving cardiac ventricular remodeling, AMPK contributes to the restoration of cardiac function and the slowing of disease progression in heart failure.15,16
In recent years, the role of Chinese medicines in the prevention and treatment of HF has become more and more prominent, and the number of studies exploring the targets of Chinese medicines in intervening in HF and their mechanisms of action based on AMPK signalling has also increased.17,18 Numerous pharmacological studies have shown that Chinese medicines have the advantages of good safety and precise efficacy in the prevention and treatment of HF, and the AMPK signalling pathway is one of the key pathways through which the active ingredients of Chinese medicines and Chinese medicinal extracts can play a role in improving the outcomes in HF.19–21 This article will review the existing pharmacological and experimental research results of TCM intervention in AMPK signalling pathway to improve HF in recent years.
AMPK Signaling Pathway Composition
AMPK is a key regulator of cellular metabolism in eukaryotes.22 It functions as an energy-sensitive heterotrimeric protein kinase complex consisting of catalytic (α) and regulatory (β, γ) subunits.12 This complex plays a crucial role in maintaining energy homeostasis by modulating ATP catabolism and anabolism.22,23 In mammals, there are 12 different heterotrimeric combinations of AMPK, depending on the subunit composition (α1/α2, β1/β2, γ1/γ2/γ3).23 These combinations determine the nucleotide activation and sensitivity to phosphorylation. The α-subunit contains the kinase structural domain and a crucial Thr172 residue that is phosphorylated by upstream kinases CAMKK2 and LKB1. The β-subunit contains a carbohydrate-binding module for glycogen binding and a C-terminal structural domain for binding the α- and γ-subunits. The γ-subunit comprises four tandem cystathionine-β-synthases (CBS) domains that directly bind ATP and adenosine diphosphate (ADP), enabling AMPK to respond to changes in the ATP-to-AMP ratio.24–26 (See Figure 1.)
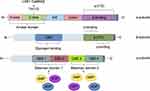 |
Figure 1 Subunit structure of AMPK. |
AMPK Signaling Transduction
AMPK signaling is activated in response to low energy levels, typically occurring under stressful conditions. The catalytically active α subunit of AMPK undergoes phosphorylation at threonine 172 (T172), which leads to the activation of AMPK signaling.27 Upstream mediators such as calcium/calmodulin-dependent protein kinase β (CaMKKβ), transforming growth factor β-activated kinase 1, and liver kinase B1 (LKB1) induce T172 phosphorylation in response to activated AMPK.24,28 Metabolic stress triggers AMPK signaling by increasing AMP levels and decreasing ATP levels. This process is intriguing because elevated levels of AMP and adenosine diphosphate during metabolic stress bind to the γ-subunit, leading to AMPK activation and T172 phosphorylation.29 It’s important to note that AMPK can also be activated independently of AMP levels. Calcium signaling and its accumulation in cells can induce T172 phosphorylation, resulting in CaMKKβ-dependent upregulation of AMPK.30 Additionally, there are endogenous inhibitors of AMPK signaling. Protein phosphatases such as protein phosphatase 2A, protein phosphatase 2Cα, and magnesium/manganese-dependent protein phosphatases can inhibit AMPK by dephosphorylating T172 and inhibiting its activation.31
Mechanisms Associated with AMPK Amelioration of Heart Failure
AMPK Improves Interstitial Fibrosis in Heart Failure
Myocardial fibrosis (MF) is a pathological process characterized by the excessive deposition of extracellular matrix proteins due to the transformation of cardiac fibroblasts into myofibroblasts. It plays a significant role in the development of heart failure.32,33 Among the isoforms of TGF-β, TGF-β1 is predominantly expressed in the heart and is a potent fibrogenic cytokine.34 It regulates cell proliferation, inflammation, collagen fiber deposition, and cardiac fibroblast activation.35 The TGF-β/Smad3 signaling pathway, with Smad2/3 as downstream effectors, is crucial in pressure overload-induced myocardial fibrosis.36 TGF-β1 additionally activates Smad-independent signaling pathways such as ERK, JNK, and p38 MAPK, all of which are implicated in fibroblast activation.37
AMPK activation has been shown to ameliorate myocardial fibrosis. In cardiac fibroblasts, AMPK activation inhibits the expression of hepatocyte nuclear factor (HNF-4), thereby regulating TGF-β transcription.38 It also inhibits ERK, an essential regulator of cardiac fibroblast growth and proliferation.36 Furthermore, AMPK inhibits JNK/NF-κB signaling, attenuating inflammation, reactive oxygen species (ROS), and cell death while protecting cardiomyocytes.39
Based on these findings, it can be inferred that AMPK attenuates myocardial fibrosis and exerts a beneficial effect on heart failure.
AMPK Improves Energy Metabolism and Maintains Mitochondrial Homeostasis in Heart Failure
The heart, as a highly energy-demanding organ, requires a substantial amount of energy to fulfill the needs of the body.40 In a physiological state, the heart obtains energy through the oxidation of various substrates such as fatty acids, glucose, and lactate. These substrates are metabolized to acetyl coenzyme A through processes like β-oxidation and glycolysis. Acetyl coenzyme A then enters the tricarboxylic acid cycle and oxidative phosphorylation in the mitochondria, effectively providing energy for the heart.41–43 However, when myocardial hypertrophy occurs, ATP production efficiency decreases, leading to a shift in the heart’s fuel preference from fatty acids to glucose and an increase in glycolysis, resulting in lactate accumulation.44
On the other hand, AMPK plays a crucial role in regulating enzymes involved in ATP production and promotes mitochondrial biogenesis, thereby enhancing energy supply in heart failure.45 Under pathological conditions, AMPK is activated in myocardial tissues through phosphorylation. This activation facilitates the transport of GLUT4 protein from the cytoplasm to the membrane, contributing to improved glucose uptake and utilization under pathological conditions, which provides some protection to the heart.46 Additionally, AMPK inhibits GLUT4 endocytosis, ensuring that GLUT4 remains in the active site.47 Moreover, AMPK increases fatty acid uptake and oxidation by promoting the membrane transport of fatty acid transporter protein CD36, facilitating fatty acid uptake by cardiomyocytes. CPT-1, a key enzyme that regulates β-oxidation, is activated by AMPK, which inhibits acetyl-coenzyme A carboxylase (ACC) and reduces the production of malonyl-coenzyme A. This, in turn, increases CPT-1 activity and promotes fatty acid oxidation.48,49
AMPK also directly influences mitochondrial biosynthesis by interacting with peroxisome proliferator-activated receptor (PPAR) γ coactivator-1α (PGC-1α), leading to increased mitochondrial synthesis or maintenance of mitochondrial function stability. Both AMPK and PGC-1α are considered potent activators of mitochondrial biosynthesis in the heart.50 PGC-1α activates estrogen-related receptor (ERR), which promotes transcription of genes associated with mitochondrial biosynthesis.51 Additionally, AMPK can stimulate mitochondrial biosynthesis directly by phosphorylating the serine of PGC-1α or indirectly by activating Sirtuin 1 (Sirt1).52,53 These combined actions ultimately result in increased ATP production, alleviating the energy supply-demand imbalance in heart failure and improving its condition.
AMPK Improves Oxidative Stress in Heart Failure
Excessive production of reactive oxygen species (ROS) during oxidative stress can cause damage to cardiomyocytes, leading to cardiac remodeling and ultimately heart dysfunction and heart failure (HF). In recent years, several pharmacological studies have shown that activating AMPK in the myocardium can protect the heart from oxidative stress.54 For example, resveratrol has been found to inhibit NADPH oxidase-mediated ROS production and enhance the activity of cardiac antioxidant enzymes through AMPK activation, thereby reducing cardiomyocyte damage caused by oxidative stress. Junlong et al55 demonstrated that selegiline can reduce mitochondrial superoxide production by activating the AMPK-endothelial nitric oxide synthase axis, thereby protecting endothelial cells from oxidative stress injury.
The beneficial effects of AMPK activation are mediated through mitochondria, particularly the inhibition of mitochondrial permeability transition pore (PTP) opening.56 Although PTP opening is a well-known phenomenon that occurs in response to oxidative stress, the molecular identity of the pore complex is not yet clear. It has been well established that cyclophilin D (CypD) plays an important role in regulating of PTP opening.57,58 It has been found that metformin-induced activation of AMPK has been shown to prevent acute oxidative stress in mitochondria by blocking the interaction between peroxisome proliferator-activated receptor-α (PPARα) and CypD, which ultimately prevents PTP formation. In addition, AMPK increases the expression of mitochondrial superoxide dismutase (SOD), thereby reducing ROS production.59 In conclusion, certain drugs can attenuate oxidative stress-induced cardiomyocyte injury by activating AMPK. However, the specific mechanism of the protective effect of AMPK against oxidative stress needs further investigation.
AMPK Improves Inflammation in Heart Failure
Inflammation resulting from myocardial ischemia and infection plays a crucial role in the development of heart failure (HF). Extensive research has demonstrated the significance of inflammation as a mechanism in HF development.60,61 Initially, low levels of inflammation act as an early response to ischemic injury, promoting healing and cardiac tissue remodeling. However, persistent or heightened inflammation is the primary cause of HF exacerbation.62 Previous studies have revealed that AMPK downregulates acetylation levels of targets such as nuclear factor ΚB (NF-ΚB), AP-1, and histones through SIRT1, thus inhibiting the expression of inflammation-related genes and attenuating the inflammatory response.63 Additionally, AMPK demonstrates a protective effect against TNF-α-induced cardiomyocyte necrosis and inflammatory cell infiltration in ischemic cardiomyopathy, as TNF-α acts as a pro-inflammatory cytokine that stimulates the cascade of inflammation in vivo.64 Empagliflozin, a sodium-glucose cotransporter protein 2 inhibitor, has been shown to activate AMPK and reduce the inflammatory response in macrophages treated with lipopolysaccharide.65 Moreover, liraglutide has been demonstrated to activate AMPK, protecting cardiomyocytes from metabolic disorders and mitochondrial dysfunction induced by interleukin 1β.66 Berberine, an important inflammatory regulator, can downregulate the expression of galactoglucan-3 through the regulation of NF-κB and AMPK signaling pathways, thus reducing macrophage activation induced by oxidized LDL and attenuating inflammation.67 Collectively, these findings indicate that AMPK’s participation in the attenuation of the inflammatory response contributes to delaying the progression of HF.
AMPK Regulates Autophagy in Heart Failure
AMPK activation can play a cardioprotective role by inducing autophagy in the heart.68 mTOR, known as an energy sensor, regulates the balance between nutritional status and cell growth. Normally, mTOR inhibits autophagy and promotes cell growth under conditions of adequate nutrition. However, during nutrient deficiency or disease, mTOR is inactivated, leading to increased autophagy.69 Conversely, inhibiting mTOR complex 1 (mTORC1) enhances autophagy, thereby protecting the heart and improving cell survival.70 Studies have demonstrated that AMPK attenuates pressure overload-induced cardiac hypertrophy by inhibiting mTORC1 and promoting autophagy.71 AMPK phosphorylates tuberous sclerosis complex 2 (TSC2), a negative regulator of mTORC1, leading to mTORC1 inhibition and subsequent autophagy induction through its downstream target, p70S6K.72 Autophagy, regulated by AMPK, plays a crucial role in maintaining cardiac function under stress conditions.73 Additionally, AMPK directly enhances autophagy by phosphorylating the Unc-51-like kinase 1 (ULK1) kinase complex.74 Both autophagy and cardiac hypertrophy influence the development of heart failure, suggesting that AMPK’s promotion of autophagy may ameliorate heart failure. However, it is important to note that autophagy’s effects on heart failure can be dual. While low levels of autophagy are cardioprotective under pressure overload, excessive autophagy can worsen heart failure by causing increased susceptibility to injury and dysfunction. Studies have shown that inhibiting the CaMKKβ-AMPK-mTOR pathway downregulates autophagy, exhibiting protective effects in hypertrophied hearts.75 Moreover, significantly increased autophagy levels impair cardiac function, indicating that prolonged autophagy activation may be detrimental to the heart.76 Therefore, these findings suggest that although autophagy can ameliorate heart failure and protect the heart, excessive autophagy can contribute to heart failure progression by degrading functional proteins and organelles and inducing cardiomyocyte death.
AMPK Improves Endoplasmic Reticulum Stress in Heart Failure
The endoplasmic reticulum is a multifunctional organelle that is essential for protein synthesis, folding, translocation and calcium homeostasis, and lipid synthesis. In the presence of hypoxia, elevated protein synthesis and calcium overload can lead to dysfunction and cause endoplasmic reticulum stress.77–79 In cardiomyocytes, if endoplasmic reticulum stress is induced for a prolonged period of time, the expression of C/EBP homologous protein (CHOP) can be upregulated, which can lead to apoptosis in cardiomyocytes during the transition from hypertrophy to heart failure.80 The activation of AMPK can influence endoplasmic reticulum stress. Studies have shown81 that activation of AMPK inhibits cardiac dysfunction and cardiomyocyte apoptosis induced by endoplasmic reticulum stress and increases BNP levels. Xu et al82 demonstrated that nickel-stripe protein-like METRNL attenuates apoptosis of cardiomyocytes by attenuating endoplasmic reticulum stress, and that its mechanism is related to the activation of AMPK-PAK2 signalling. Park et al83 confirmed that AMPK can reduce the accumulation of unfolded proteins by inhibiting mTOR activity, thereby reducing endoplasmic reticulum stress. However, the mechanism by which AMPK reduces endoplasmic reticulum stress in heart failure has not been fully elucidated.
AMPK Improves Ferroptosis in Heart Failure
Ferroptosis is a novel form of cell death characterized by iron overload and accumulation of lipid peroxides.84 It plays a significant role in the processes of cell proliferation, differentiation, aging, and death, and has been shown to be involved in heart failure (HF).85 The occurrence of ferroptosis is accompanied by a decrease in the levels of glutathione (GSH) and glutathione peroxidase 4 (GPX4). Studies have indicated that during myocardial infarction, interference with small interfering RNA (siRNA) or the ferroptosis inducer RSL3 inhibiting GPX4 may result in the accumulation of lipid peroxides, leading to the conclusion that GPX4 can prevent ferroptosis during acute myocardial infarction.86 Nrf2 is a transcription factor with antioxidant properties, and heme oxygenase-1 (HO-1) and GPX4 are downstream genes of Nrf2.87 When the body is under oxidative stress, Nrf2 is released from the Kelch-like ECH-associated protein 1 (Keap-1) binding site and rapidly translocates into the nucleus, where it interacts with antioxidant response element (ARE) in the promoter region of target genes.88 Research has shown that activation of the AMPK/glycogen synthase kinase-3β (GSK-3β)/Nrf2 pathway can upregulate the expression of GPX4 to inhibit lipid peroxidation and ferroptosis, thereby reducing myocardial ischemia-reperfusion injury (MIRI).89 Therefore, modulation of the AMPK pathway can suppress ferroptosis and protect cardiomyocytes in HF.
The pathogenesis of the specific AMPK signaling pathway in HF is shown in Figure 2.
 |
Figure 2 Pathogenesis of AMPK signaling pathway in HF. |
Chinese Medicine Intervenes in HF Through the AMPK Signalling Pathway
It is urgent to find effective drugs to treat HF, and studies have shown that the above pathophysiological processes play an important role in the treatment of HF. Due to its advantages of multi-components, multi-targets and few adverse effects, several studies have demonstrated that Chinese medicine combinations and active ingredients and extracts of Chinese medicine can protect cardiomyocytes and alleviate HF by up-regulating or inhibiting the expression of relevant target genes through regulating the AMPK signalling pathway.
Herbal Monotherapy Ameliorate HF Through the AMPK Signalling Pathway
Astragaloside and astragalus polysaccharide are active ingredients derived from the dried root of Astragalus membranaceus, a traditional Chinese medicine. These components have various biological effects, including delaying cellular aging, inhibiting inflammatory mediators, and providing organ protection.90,91 Song et al92 discovered that astragalus polysaccharide activates the AMPK-related pathway, increases FAT/CD36 translocation and CPT1 expression, facilitates myocardial uptake and utilization of free fatty acids (FFA), and improves myocardial metabolism in chronic heart failure rats, thus ameliorating the condition. Similarly, Xinwei et al93 found that astragaloside effectively inhibits mitochondrial autophagy and cardiomyocyte apoptosis in rats with acute heart failure, with its regulatory mechanism involving the CaMkkβ/AMPK signaling pathway. Luo Han Guo flavonoids, derived from the ripe fruit of Luo Han Guo, have been shown by Weibin et al94,95 to enhance the activity of energy metabolism enzymes CK, LDH, and SDH, upregulate PPARα mRNA expression, and improve energy metabolism and myocardial tissue damage. Ginsenoside, the main active ingredient in ginseng, has been found to regulate ventricular remodeling and enhance fatty acid β-oxidation in heart failure rats through the activation of AMPK in a study on adriamycin-induced heart failure rats.96,97 Paeoniflorin, an active compound extracted from the dried root of Paeonia lactiflora, exerts cardioprotective effects by combating oxidative stress, inhibiting cardiomyocyte apoptosis, suppressing myocardial inflammation, and regulating autophagy.98 Yuqin et al99 demonstrated that paeoniflorin promotes AMPKα phosphorylation, reduces inflammatory factor levels in myocardial tissue, and protects myocardial endothelial cells. Tanshinone IIA, derived from dried tansy rhizomes, has multiple clinical effects, including cardiovascular protection, hepatoprotection, anti-inflammation, antioxidant, anti-tumor, and antifibrotic effects.100 Zhang et al101 discovered that tanshinone IIA administration to heart failure rats with ligated left anterior descending branches activates the AMPK/mTOR pathway, enhances autophagy (as observed through autophagy-associated factor proteins LC3, p62, and Beclin1), and improves cardiac function. Panax ginseng total saponin, the primary active component of Panax ginseng, exhibits pharmacological effects such as anti-inflammatory, lipid-lowering, and anti-tumor effects.102 Wang et al103 experimentally demonstrated that Panax ginseng total saponin protects the hearts of mice with left anterior descending ligation by enhancing glucose metabolism through phosphorylation of AMPK Thr172 and CaMKII Thr287 in cardiomyocytes under deprivation-induced autophagy. Additionally, berberine has been shown104 to inhibit cardiomyocyte hypertrophy through AMPK pathway-mediated promotion of autophagy, as evidenced by down-regulation of p62 expression and up-regulation of LC3II activity. Puerarin is extracted from the rhizome of Pueraria lobata. Zhou et al105 found that puerarin inhibits cardiomyocyte apoptosis and iron death by promoting AMPK phosphorylation, thereby alleviating inflammation and oxidative damage in myocardial tissues and exerting cardioprotective effects. Furthermore, Ma et al106 observed activation of AMPKα/mTOR and inhibition of endoplasmic reticulum stress in hypertrophied hearts treated with gardenia glycosides. They also found that knockdown of compound C (CpC) or AMPK-α limited the activation of mammalian target of rapamycin and ERK induced by hypertrophic stimuli, which suggests that gardenia jasminoides can inhibit endoplasmic reticulum stress through activation of the GLP-1 receptor/AMPK-α pathway, thus preventing cardiac hypertrophy. (See Table 1.)
 |
Table 1 Mechanism of Action of Chinese Herbal Monomers Modulating AMPK Signalling Pathway to Improve HF |
Chinese Herbal Compound Improves HF Through AMPK Signalling Pathway
Linggui Zhugan soup, which has the efficacy of warming heart yang, strengthening spleen qi, and resolving water-drinking, has been widely used in the treatment of CHF in clinics with precise and reliable efficacy.107,108 Juan et al109 experimentally found that Linggui Zhugan soup could alleviate oxidative stress and apoptotic injury of cardiomyocytes and improve mitochondrial function in HF rats, and its effect was related to mitochondrial fission-fusion and activation of Sirt3/AMPK signalling pathway. Yixintai is a commonly used clinical formula with the effect of benefiting qi, activating blood and inducing diuresis. After more than twenty years of clinical use of the drug has proved that it has an anti-ventricular remodelling effect, and it is safe, effective and without adverse reactions, which is worthy of clinical promotion and application.110–112 Yun et al111 experimentally found that Yixintai granules can promote the increase of AMPK protein expression, increase ATPase activity, inhibit fatty acid oxidation, reduce serum NT-pro BNP levels, and improve heart failure. Yixintai tablets have the efficacy of benefiting qi and nourishing yin, activating blood and inducing diuresis, and are effective in clinical practice.113 The results of Ren et al114 showed that Yangxin Kang Tablets had the effect of improving the ultrastructure of cardiomyocytes and regulating the level of cardiomyocyte autophagy in a rabbit model of heart failure after myocardial infarction, and the cardioprotective effect of Yangxin Kang Tablets was reduced after specific blockade of the Akt/AMPK-mTOR signalling pathway, which indicated that the effects of Yangxin Kang Tablets in improving ventricular remodeling were mediated by interfering with the Akt/AMPK-mTOR signaling pathway, suggesting that the improvement of ventricular remodeling by Yangxin Kang Tablets was achieved by interfering with the Akt/AMPK-mTOR signaling pathway and regulating the autophagy level of cardiomyocytes.
Xinshuai Ning Combination has the efficacy of benefiting qi and warming yang, promoting blood circulation and inducing diuresis, and has been used in clinical practice for a long time with remarkable efficacy and no obvious toxic side effects.115,116 Studies have shown that Xinshuai Ning Combination can improve cardiac function, regulate myocardial energy metabolism, intervene in ventricular remodelling, and anti-myocardial fibrosis in CHF rats.117,118 Yuanli et al119 found that Xinshuai Ning Combination can significantly reduce the degree of myocardial fibrosis in CHF rats, reduce the serum BNP level, reduce the serum and myocardial FFA level, increase the level of PPARα mRNA and protein expression, reduce the level of AMPK mRNA and protein expression, regulate the myocardial energy metabolism substrate of CHF rats, and delay the occurrence and development of CHF, and its mechanism is related to the regulation of myocardial energy metabolism. Occurrence and development, and its mechanism is related to the regulation of PPARα and AMPK expression, which in turn improves FFA levels. Chenghao et al120 found that Wenyang Yiqi Soup was able to improve myocardial function in post-infarction heart failure rats, and its mechanism of action was related to the inhibition of AMPK-mediated mitochondrial autophagy. The tonifying yang and returning five soup is a famous formula for the treatment of qi deficiency and blood stasis syndrome, which has the efficacy of benefiting qi, activating blood, and clearing the veins, and is widely used in the treatment of HF.121 Zhen et al122 found that Buyang Huanwu Soup could activate AMPK and up-regulate the expression of PPARα and PGC-1α, which could preliminarily confirm that Buyang Huanwu Soup could improve energy metabolism of the failing heart and delay the progression of HF by improving the function of cardiomyocytes’ mitochondria, promoting energy production of failing hearts and up-regulating the expression of AMPK/PPARα signalling pathway, promoting mitochondrial biogenesis, and thus improving energy metabolism of failing hearts. This will improve the energy metabolism of the failing heart and delay the progression of HF.
Shihai et al123 found that Shenkui Tongxin granules could regulate the AMPK-mt TFA-PINK1 signalling axis, promote mitochondrial autophagy in cardiomyocytes, enhance mitochondrial production, attenuate mitochondrial damage, and improve the disorders of energy metabolism, which could in turn alleviate the progression of HF. Fan et al124 found that Shenfu Qiangxin Soup may activate the AMPK-PGC-1α signalling pathway, increase the expression of p-AMPK and PGC-1α to regulate fatty acid oxidation metabolism and glucose transport, and thus improve the energy metabolism of the myocardium in heart failure rats, thus playing a therapeutic role in the treatment of heart failure. Wei et al125 found that Shenfu Xiongze Soup could increase the expression of AMPK and GLUT4 proteins and the content of serum CK and CK-MB in myocardial tissues, reduce the content of serum FFA, alleviate the pathological damage of myocardial tissues, and improve the effects of heart failure. Xianwei et al126 showed that ginseng injection could activate AMPK, reduce the content of IL-6 and TNF-α in myocardial tissues of rats with heart failure, inhibit inflammatory reactions, and delay ventricular remodelling and myocardial hypertrophy. Qiong et al127 experiments showed that Huanglian Jiandu soup could protect inflammation-injured endothelial cells by affecting AMPK and ICAM-1.(See Table 2)
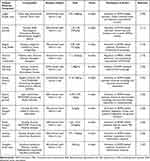 |
Table 2 Mechanism of Action of Chinese Herbal Medicine Combinations Regulating the AMPK Signalling Pathway to Improve Heart Failure |
Conclusion and Outlook
The pathogenesis of HF is complex, and the AMPK signalling pathway plays an important role in its pathogenesis. By regulating the AMPK pathway, HF can be alleviated by inhibiting inflammatory response, oxidative stress, autophagy, apoptosis and ferroptosis, etc. Currently, there is some research on the efficacy of Chinese herbal compounds, Chinese herbal active ingredients and Chinese herbal extracts in the treatment of HF, and the mechanism of action of the relevant drugs has been studied to some extent. Therefore, this paper reviewed the connection between the AMPK signalling pathway and the pathogenesis of HF, as well as the current progress of Chinese medicines in attenuating HF by interfering with the AMPK signalling pathway, with a view to better understanding the pathogenesis of HF and providing some theoretical basis for the treatment of HF with Chinese medicines. It is therefore concluded that traditional Chinese medicine can protect cardiomyocytes to attenuate HF by regulating the AMPK signalling pathway.
In summary, targeting the AMPK signalling pathway for the treatment of HF may be an effective means, and current research has achieved some results in modulating the AMPK signalling pathway in traditional Chinese medicine to alleviate HF. However, there are still shortcomings in the existing research, firstly, the current research on the AMPK signalling pathway of HF is mostly based on animal experiments, lack of relevant clinical research and a single research method, the future can be a combination of clinical research and experimental research to better elucidate its effectiveness and safety. Secondly, there are few studies that explore the complex relationship between the active ingredients of TCM and its compound and the mechanism of HF from the level of molecular mechanism. In the future, modern science and technology, such as network pharmacology and biological information, can be combined to conduct in-depth excavation, and further research can be carried out to explore the specific mechanisms in order to lay the foundation for the alleviation of HF.
Acknowledgments
Changxing Liu and Xinyi Guo are co-first authors for this study.
Disclosure
All the authors declare that they have no conflicts of interest.
References
1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi:10.1161/CIR.0000000000000757
2. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. doi:10.15420/cfr.2016:25:2
3. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American heart association [published correction appears in Circulation. 2022 Sep 6;146(10):e141]. Circulation. 2022;145(8):e153–e639. doi:10.1161/CIR.0000000000001052
4. Niskanen JE, Ohlsson Å, Ljungvall I, et al. Identification of novel genetic risk factors of dilated cardiomyopathy: from canine to human. Genome Med. 2023;15(1):73. doi:10.1186/s13073-023-01221-3
5. Zhou J, Luo Y, Kang X, Bian F, Liu D. The root extract of Scutellaria baicalensis Georgi promotes β cell function and protects from apoptosis by inducing autophagy. J Ethnopharmacol. 2022;284:114790. doi:10.1016/j.jep.2021.114790
6. Burchill LJ, Lee MGY, Nguyen VP, Stout KK. Heart failure in adult congenital heart disease. Cardiol Clin. 2020;38(3):457–469. doi:10.1016/j.ccl.2020.04.010
7. Goldstein D, Frishman WH. Diastolic heart failure: a review of current and future treatment options. Cardiol Rev. 2021;29(2):82–88. doi:10.1097/CRD.0000000000000303
8. Kim AH, Jang JE, Han J. Current status on the therapeutic strategies for heart failure and diabetic cardiomyopathy. Biomed Pharmacother. 2022;145:112463. doi:10.1016/j.biopha.2021.112463
9. MacDonald BJ, Virani SA, Zieroth S, Turgeon R. Heart failure management in 2023: a pharmacotherapy- and lifestyle-focused comparison of current international guidelines. CJC Open. 2023;5(8):629–640. doi:10.1016/j.cjco.2023.05.008
10. Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48(4):e224. doi:10.1038/emm.2016.16
11. Yan Y, Zhou XE, Xu HE, Melcher K. Structure and physiological regulation of AMPK. Int J Mol Sci. 2018;19(11):3534. doi:10.3390/ijms19113534
12. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi:10.1038/nrm.2017.95
13. Sanli T, Steinberg GR, Singh G, Tsakiridis T. AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther. 2014;15(2):156–169. doi:10.4161/cbt.26726
14. Xiao B, Sanders MJ, Underwood E, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472(7342):230–233. doi:10.1038/nature09932
15. Li X, Liu J, Lu Q, et al. AMPK: a therapeutic target of heart failure-not only metabolism regulation. Biosci Rep. 2019;39(1):BSR20181767. doi:10.1042/BSR20181767
16. Wu S, Zou MH. AMPK, mitochondrial function, and cardiovascular disease. Int J Mol Sci. 2020;21(14):4987. doi:10.3390/ijms21144987
17. Wen Y, Kunjian H, Li O, Xiaoyue C, Yingqiang Z. Mechanism of adenylate-activated protein kinase in the prevention and control of heart failure and progress of intervention in traditional Chinese medicine. Liaoning J Chin Med. 2023;50(04):212–216.
18. Yaqi H, Jianhe L. Progress of Chinese medicine in regulating AMPK signalling pathway for the treatment of myocardial ischemia-reperfusion injury. Chin J Exp Formulas. 2023;29(13):213–221.
19. Yu S, Qian H, Tian D, et al. Linggui Zhugan Decoction activates the SIRT1-AMPK-PGC1α signaling pathway to improve mitochondrial and oxidative damage in rats with chronic heart failure caused by myocardial infarction. Front Pharmacol. 2023;14:1074837. doi:10.3389/fphar.2023.1074837
20. Ge Y, Zhou M, Chen C, Wu X, Wang X. Role of AMPK mediated pathways in autophagy and aging. Biochimie. 2022;195:100–113. doi:10.1016/j.biochi.2021.11.008
21. Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26(3):190–201. doi:10.1016/j.tcb.2015.10.013
22. Hardie DG. AMPK--sensing energy while talking to other signaling pathways. Cell Metab. 2014;20(6):939–952. doi:10.1016/j.cmet.2014.09.013
23. Xiao B, Heath R, Saiu P, et al. Structural basis for AMP binding to mammalian AMP -activated protein kinase. Nature. 2007;449(7161):496–500. doi:10.1038/nature06161
24. Hardie DG, Carling D, Gamblin SJ. AMP-activated protein kinase: also regulated by ADP? Trends Biochem Sci. 2011;36(9):470–477. doi:10.1016/j.tibs.2011.06.004
25. Garcia D, Shaw RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017;66(6):789–800. doi:10.1016/j.molcel.2017.05.032
26. Timm KN, Tyler DJ. The role of AMPK activation for cardioprotection in doxorubicin-induced cardiotoxicity. Cardiovasc Drugs Ther. 2020;34(2):255–269. doi:10.1007/s10557-020-06941-x
27. Wang Q, Liu S, Zhai A, Zhang B, Tian G. AMPK-mediated regulation of lipid metabolism by phosphorylation. Biol Pharm Bull. 2018;41(7):985–993. doi:10.1248/bpb.b17-00724
28. Qiu Z, Li Y, Fu Y, Yang Y. Research progress of AMP-activated protein kinase and cardiac aging. Open Life Sci. 2023;18(1):20220710. doi:10.1515/biol-2022-0710
29. Wu ZZ, Rao M, Xu S, Hu HY, Tang QZ. Coumestrol ameliorates doxorubicin-induced cardiotoxicity via activating AMPKα. Free Radic Res. 2020;54(8–9):629–639. doi:10.1080/10715762.2020.1822525
30. Kurose H. Cardiac fibrosis and fibroblasts. Cells. 2021;10(7):1716. doi:10.3390/cells10071716
31. Díez J, De Boer RA. Management of cardiac fibrosis is the largest unmet medical need in heart failure. Cardiovasc Res. 2022;118(2):e20–e22. doi:10.1093/cvr/cvab228
32. Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51(4):600–606. doi:10.1016/j.yjmcc.2010.10.033
33. Desmouliere A, Geinoz A, Gabbiani F, et al. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–111. doi:10.1083/jcb.122.1.103
34. Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71(4):549–574. doi:10.1007/s00018-013-1349-6
35. Zhang YE. Non-Smad Signaling Pathways of the TGF-beta Family. Cold Spring Harb Perspect Biol. 2017;9(2):647–691. doi:10.1101/cshperspect.a022129
36. Chen R, Feng Y, Wu J, et al. Metformin attenuates angiotensin II-induced TGFbeta1 expression by targeting hepatocyte nuclear factor-4-alpha. Br J Pharmacol. 2018;175(8):1217–1229. doi:10.1111/bph.13753
37. Du J, Guan T, Zhang H, et al. Inhibitory crosstalk between ERK and AMPK in the growth and proliferation of cardiac fibroblasts. Biochem Biophys Res Commun. 2008;368(2):402–407. doi:10.1016/j.bbrc.2008.01.099
38. Chen X, Li X, Zhang W, et al. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-kappaB pathway. Metabolism. 2018;83(256):256–270. doi:10.1016/j.metabol.2018.03.004
39. Oh CM, Ryu D, Cho S, Jang Y. Mitochondrial quality control in the heart: new drug targets for cardiovascular disease. Korean Circ J. 2020;50(5):395–405. doi:10.4070/kcj.2019.0416
40. Gupta A, Chacko VP, Schär M, Akki A, Weiss RG. Impaired ATP kinetics in failing in vivo mouse heart. Circ Cardiovasc Imaging. 2011;4(1):42–50. doi:10.1161/CIRCIMAGING.110.959320
41. Gibbs CL, Loiselle DS. Cardiac basal metabolism. Jpn J Physiol. 2001;51(4):399–426. doi:10.2170/jjphysiol.51.399
42. Wasyluk W, Nowicka-Stążka P, Zwolak A. Heart metabolism in sepsis-induced cardiomyopathy-unusual metabolic dysfunction of the heart. Int J Environ Res Public Health. 2021;18(14):7598. doi:10.3390/ijerph18147598
43. Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004;555(Pt 1):1–13. doi:10.1113/jphysiol.2003.055095
44. Feng Y, Zhang Y, Xiao H. AMPK and cardiac remodelling. Sci China Life Sci. 2018;61(1):14–23. doi:10.1007/s11427-017-9197-5
45. Ng SM, Neubauer S, Rider OJ. Myocardial Metabolism in Heart Failure. Curr Heart Fail Rep. 2023;20(1):63–75. doi:10.1007/s11897-023-00589-y
46. Yang J, Holman GD. Insulin and contraction stimulate exocytosis, but increased AMP-activated protein kinase activity resulting from oxidative metabolism stress slows endocytosis of GLUT4 in cardiomyocytes. J Biol Chem. 2005;280(6):4070–4078. doi:10.1074/jbc.M410213200
47. Wolfgang MJ, Kurama T, Dai Y, et al. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc Natl Acad Sci U S A. 2006;103(19):7282–7287. doi:10.1073/pnas.0602205103
48. Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574(Pt 1):95–112. doi:10.1113/jphysiol.2006.109389
49. Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27(7):728–735. doi:10.1210/er.2006-0037
50. Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29(6):677–696. doi:10.1210/er.2008-0017
51. Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93(4):884S–90. doi:10.3945/ajcn.110.001917
52. Khan SU, Khan SU, Suleman M, et al. Natural allies for heart health: nrf2 activation and cardiovascular disease management [published online ahead of print, 2023 Sep 13]. Curr Probl Cardiol. 2023;49(1 Pt B):102084. doi:10.1016/j.cpcardiol.2023.102084
53. Guo S, Yao Q, Ke Z, Chen H, Wu J, Liu C. Resveratrol attenuates high glucose-induced oxidative stress and cardiomyocyte apoptosis through AMPK. Mol Cell Endocrinol. 2015;412:85–94. doi:10.1016/j.mce.2015.05.034
54. Xu L, Wang S, Li B, Sun A, Zou Y, Ge J. A protective role of ciglitazone in ox-LDL-induced rat microvascular endothelial cells via modulating PPARγ-dependent AMPK/eNOS pathway. J Cell Mol Med. 2015;19(1):92–102. doi:10.1111/jcmm.12463
55. Junlong W, Huiyu J, Zhihai F, et al. Research progress of Chinese medicine in regulating AMPK signalling pathway against obese type 2 diabetes[J/OL]. Chin J Exp Formulary. 2023:1–9. doi:10.1111/jcmm.12463
56. Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144(12):5179–5183. doi:10.1210/en.2003-0982
57. Barreto-Torres G, Parodi-Rullán R, Javadov S. The role of PPARα in metformin-induced attenuation of mitochondrial dysfunction in acute cardiac ischemia/reperfusion in rats. Int J Mol Sci. 2012;13(6):7694–7709. doi:10.3390/ijms13067694
58. Liu XD, Li YG, Wang GY, et al. Metformin protects high glucose-cultured cardiomyocytes from oxidative stress by promoting NDUFA13 expression and mitochondrial biogenesis via the AMPK signaling pathway. Mol Med Rep. 2020;22(6):5262–5270. doi:10.3892/mmr.2020.11599
59. Barreto-Torres G, Hernandez JS, Jang S, et al. The beneficial effects of AMP kinase activation against oxidative stress are associated with prevention of PPARα-cyclophilin D interaction in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2015;308(7):H749–H758. doi:10.1152/ajpheart.00414.2014
60. Cocco G, Jerie P, Amiet P, Pandolfi S. Inflammation in Heart Failure: known knowns and unknown unknowns. Expert Opin Pharmacother. 2017;18(12):1225–1233. doi:10.1080/14656566.2017.1351948
61. Bazoukis G, Stavrakis S, Armoundas AA. Vagus Nerve Stimulation and Inflammation in Cardiovascular Disease: a State-of-The-Art Review [published online ahead of print, 2023 Sep 18]. J Am Heart Assoc. 2023;12(19):e030539. doi:10.1161/JAHA.123.030539
62. Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: from Inflammation to Fibrosis. Circ Res. 2016;119(1):91–112. doi:10.1161/CIRCRESAHA.116.303577
63. Koyani CN, Plastira I, Sourij H, et al. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol Res. 2020;158:104870. doi:10.1016/j.phrs.2020.104870
64. Zhang Y, He Y, Liu S, et al. SGLT2 inhibitors in aging-related cardiovascular disease: a review of potential mechanisms [published online ahead of print, 2023 Aug 24]. Am J Cardiovasc Drugs. 2023. doi:10.1007/s40256-023-00602-8
65. Meng Z, Liu X, Li T, et al. The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int Immunopharmacol. 2021;94:107492. doi:10.1016/j.intimp.2021.107492
66. Zhang L, Tian J, Diao S, Zhang G, Xiao M, Chang D. GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1β-induced metabolic disturbance and mitochondrial dysfunction. Chem Biol Interact. 2020;332:109252. doi:10.1016/j.cbi.2020.109252
67. Pei C, Zhang Y, Wang P, et al. Berberine alleviates oxidized low-density lipoprotein-induced macrophage activation by downregulating galectin-3 via the NF-κB and AMPK signaling pathways. Phytother Res. 2019;33(2):294–308. doi:10.1002/ptr.6217
68. Packer M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc Diabetol. 2020;19(1):62. doi:10.1186/s12933-020-01041-4
69. Kubli DA, Gustafsson AB. Cardiomyocyte health: adapting to metabolic changes through autophagy. Trends Endocrinol Metab. 2014;25(3):156–164. doi:10.1016/j.tem.2013.11.004
70. Gurusamy N, Lekli I, Mukherjee S, et al. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res. 2010;86(1):103–112. doi:10.1093/cvr/cvp384
71. Li Y, Chen C, Yao F, et al. AMPK inhibits cardiac hypertrophy by promoting autophagy via mTORC1. Arch Biochem Biophys. 2014;558(79):79–86. doi:10.1016/j.abb.2014.06.023
72. Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi:10.1016/j.molcel.2008.03.003
73. Zheng Q, Zhao K, Han X, et al. Inhibition of AMPK accentuates prolonged caloric restriction-induced change in cardiac contractile function through disruption of compensatory autophagy. Biochim Biophys Acta. 2015;1852(2):332–342. doi:10.1016/j.bbadis.2014.04.023
74. Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi:10.1038/ncb2152
75. Liu L, Wang C, Lin Y, et al. Suppression of calcium-sensing receptor ameliorates cardiac hypertrophy through inhibition of autophagy. Mol Med Rep. 2016;14(1):111–120. doi:10.3892/mmr.2016.5279
76. Schips TG, Wietelmann A, Hohn K, et al. FoxO3 induces reversible cardiac atrophy and autophagy in a transgenic mouse model. Cardiovasc Res. 2011;91(4):587–597. doi:10.1093/cvr/cvr144
77. Wang S, Binder P, Fang Q, et al. Endoplasmic reticulum stress in the heart: insights into mechanisms and drug targets. Br J Pharmacol. 2018;175(8):1293–1304. doi:10.1111/bph.13888
78. Wei J, Fang D. Endoplasmic reticulum stress signaling and the pathogenesis of hepatocarcinoma. Int J Mol Sci. 2021;22(4):1799. doi:10.3390/ijms22041799
79. Huang J, Pan H, Wang J, et al. Unfolded protein response in colorectal cancer. Cell Biosci. 2021;11(1):26. doi:10.1186/s13578-021-00538-z
80. Okada K, Minamino T, Tsukamoto Y, et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110(6):705–712. doi:10.1161/01.CIR.0000137836.95625.D4
81. Zhuo XZ, Wu Y, Ni YJ, et al. Isoproterenol instigates cardiomyocyte apoptosis and heart failure via AMPK inactivation-mediated endoplasmic reticulum stress. Apoptosis. 2013;18(7):800–810. doi:10.1007/s10495-013-0843-5
82. Xu L, Cai Y, Wang Y, Xu C. Meteorin-Like (METRNL) Attenuates Myocardial Ischemia/Reperfusion Injury-Induced Cardiomyocytes Apoptosis by Alleviating Endoplasmic Reticulum Stress via Activation of AMPK-PAK2 Signaling in H9C2 Cells. Med Sci Monit. 2020;26:e924564. doi:10.12659/MSM.924564
83. Park HW, Park H, Ro SH, et al. Hepatoprotective role of Sestrin2 against chronic ER stress. Nat Commun. 2014;5:4233. doi:10.1038/ncomms5233
84. Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11(7):3052–3059. doi:10.7150/thno.54113
85. Lillo-Moya J, Rojas-Solé C, Muñoz-Salamanca D, Panieri E, Saso L, Rodrigo R. Targeting Ferroptosis against Ischemia/Reperfusion Cardiac Injury. Antioxidants (Basel). 2021;10(5):667. doi:10.3390/antiox10050667
86. Park TJ, Park JH, Lee GS, et al. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019;10(11):835. doi:10.1038/s41419-019-2061-8
87. Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018;29(17):1727–1745. doi:10.1089/ars.2017.7342
88. Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38(4):769–789. doi:10.1080/03602530600971974
89. Lu H, Xiao H, Dai M, et al. Britanin relieves ferroptosis-mediated myocardial ischaemia/reperfusion damage by upregulating GPX4 through activation of AMPK/GSK3β/Nrf2 signalling. Pharm Biol. 2022;60(1):38–45. doi:10.1080/13880209.2021.2007269
90. Wu Y, Fan Z, Chen Z, et al. Astragaloside IV protects human cardiomyocytes from hypoxia/reoxygenation injury by regulating miR-101a. Mol Cell Biochem. 2020;470(1–2):41–51. doi:10.1007/s11010-020-03743-5
91. Xia ML, Xie XH, Ding JH, et al. Astragaloside IV inhibits astrocyte senescence: implication in Parkinson’s disease. J Neuroinflammation. 2020;17(1):105. doi:10.1186/s12974-020-01791-8
92. Song J, Yangqian H, Jian L, et al. Effects of astragalus polysaccharide on myocardial AMPK activity and FFA metabolism in rats with chronic heart failure. Chin J Pathophysiol. 2015;31(01):28–32.
93. Xinwei R, Weiguo Z, Yuzhen N. Mechanism of action of astragaloside on mitochondrial autophagy in rat cardiomyocytes with acute heart failure via CaMkkβ/AMPK pathway. J Med Forum. 2023;44(09):41–46.
94. Hao-yu L, Xing-jun X, Xue-han L, et al. Effects of total flavonoids of Luo Han Guo on antioxidant capacity and inflammatory response in mice with chronic sleep deprivation. J Animal Nutr. 2023;35(07):4668–4677.
95. Weibin M, Mingming G, Ting L, et al. Effects of Rosmarinus officinalis flavonoids on myocardial energy metabolising enzymes and PPARα mRNA expression in exercising rats. Chin J Exp Formulas. 2013;19(14):203–208.
96. Luoqi Z, Sen L, Ruyi C, et al. Interaction of ginsenosides with MAPK signalling pathway based on SPRi technology and molecular docking. Chin Patent Med. 2023;45(09):3123–3127.
97. Kong HL, Hou AJ, Liu NN, Chen BH, Dai SN, Huang HT. The effects of ginsenoside Rb1 on fatty acid β-oxidation, mediated by AMPK, in the failing heart. Iran J Basic Med Sci. 2018;21(7):731–737. doi:10.22038/IJBMS.2018.24002.6016
98. Qingrui S, Xiaojun Z, Weide Z, Houang L, Jianzhe L. Progress of cardioprotective effect and mechanism of action of paeoniflorin. Zhongnan Pharmacol. 2023;21(09):2386–2391.
99. Yuqin G, Jiping H, Guoping Z. Protective effect of AMPKα activation by paeoniflorin pretreatment on myocardial ischaemia-reperfusion injury in in vivo rats. Hainan Med. 2015;26(15):2185–2189.
100. Keran F, Weixia L, Xiaoyan W, et al. Predictive analyses of chemical constituents, pharmacological effects and their quality markers (Q-Marker) of Salvia miltiorrhiza. Chin Herbal Med. 2022;53(02):609–618.
101. Zhang X, Wang Q, Wang X, et al. Tanshinone IIA protects against heart failure post-myocardial infarction via AMPKs/mTOR-dependent autophagy pathway. Biomed Pharmacother. 2019;112:108599. doi:10.1016/j.biopha.2019.108599
102. Xiaolian L, Jianlian L, Wenli L, et al. Progress of pharmacological effects and clinical application of total saponins of Panax ginseng. Hubei Agri Sci. 2021;60(06):15–19.
103. Wang D, Lv L, Xu Y, et al. Cardioprotection of Panax Notoginseng saponins against acute myocardial infarction and heart failure through inducing autophagy. Biomed Pharmacother. 2021;136:111287. doi:10.1016/j.biopha.2021.111287
104. Zhi-Cong Z, Feng-Hsia L, Yuan-Gui Z, et al. Mechanism study of berberine-mediated LncRNA-MIAT regulation of autophagy to inhibit cardiomyocyte hypertrophy. World Sci Technol Modern Trad Chin Med. 2019;21(10):2113–2120.
105. Zhou B, Zhang J, Chen Y, et al. Puerarin protects against sepsis-induced myocardial injury through AMPK-mediated ferroptosis signaling. Aging (Albany NY). 2022;14(8):3617–3632. doi:10.18632/aging.204033
106. Ma ZG, Dai J, Zhang WB, et al. Protection against cardiac hypertrophy by geniposide involves the GLP-1 receptor / AMPKα signalling pathway. Br J Pharmacol. 2016;173(9):1502–1516. doi:10.1111/bph.13449
107. De-You J, Wan-Qiu Z, Jie-Ru H. Research progress of Ling Gui Zhu Gan Tang. J Chin Med. 2021;36(12):2562–2567.
108. Peng Z, Jinling J. Progress of clinical application and pharmacological effects of Ling Gui Zhu Gan Tang in the prevention and treatment of chronic heart failure. Shizhen Guojian Guojian. 2018;29(09):2231–2233.
109. Juan Y, Rui D, Xiangyang L, et al. Effects of Ling Gui Zhu Gan Tang on mitochondrial fission-fusion and Sirt3/AMPK signalling pathway in post-infarction chronic heart failure rats. Chin J Exp Formulas. 1–9. doi:10.13422/j.cnki.syfjx.20231004
110. Jiaming W, Yuying L, Jialing L, et al. Effects of effective components of Yixintai on CaN in myocardial tissue of rabbits with chronic heart failure. Chin Pharmacol Bull. 2021;37(10):1457–1463.
111. Yun T, Yang L. Effects of Yixintai granules on myocardial AMPK protein expression in rats with chronic heart failure with cardiac qi deficiency and blood stasis and water stagnation. Chin Med Introd. 2021;27(03):19–22.
112. Yun T, Zhi-Hua G, Tong-Yu Z, et al. Effects of Yixintai alcoholic extract on angiotensin II-induced Bax and Bcl-2 protein expression in rabbit cardiac fibroblasts. China J Trad Chin Med Infor. 2021;28(08):83–86.
113. Xian SX, Yang ZQ, Ren PH, et al. Effect of yangxinkang tablets on chronic heart failure: a multi-center randomized double-blind placebo-controlled trial. Chin J Integr Med. 2015;21(10):733–742. doi:10.1007/s11655-015-2170-x
114. Ren PH, Zhang ZM, Wang P, Zhu HP, Li ZQ. Yangxinkang tablet protects against cardiac dysfunction and remodelling after myocardial infarction in rats through inhibition of AMPK/mTOR-mediated autophagy. Pharm Biol. 2020;58(1):321–327. doi:10.1080/13880209.2020.1748662
115. Gang S, Jianling Z, Zhiyi W, et al. Clinical study of heart failure ning for treating chronic heart failure of cardiac and renal yang deficiency type. Shizhen Guomao Guomao. 2017;28(11):2686–2687.
116. Jinhong W, Gang S. Observation on the efficacy of heart failure ning on chronic moderate and severe heart failure. Liaoning J Chin Med. 2014;41(12):2613–2614.
117. Yuanli H, Zhenxiang A, Ruilin Y, et al. Effects of heart failure granules on transforming growth factor β1 in myocardial tissue of rats with chronic heart failure. J Guiyang Coll Trad Chin Med. 2016;38(05):23–27.
118. Zhen Z, Yongping Z. Effects of different dosage forms of Heart Failure Ning on haemodynamics. Chin J Exp Formulas. 2014;20(13):169–171.
119. Yuanli H, Zhenxiang A, Ruilin Y, Lei G. Effects of Heart Failure Ning Combination on myocardial AMPK and PPARα in rats with chronic heart failure. New Chin Med Clin Pharmacol. 2020;31(03):287–293.
120. Chenghao C, Lihua H, Huichao Z. Pharmacological mechanism of Wen Yang Yi Qi formula for improving post-infarction heart failure in rats based on AMPK-mediated mitochondrial autophagy analysis. Chin J Comp Med. 2019;29(12):39–44.
121. Xiaomin H, Zhengxu F, Meiying H. Clinical observation on the treatment of chronic heart failure with tonifying yang and returning five soups. Guangming Trad Chin Med. 2021;36(15):2551–2553.
122. Zhen W, Jiebai L, Xin D, Xiaoxu S. Effects of tonifying Yang and restoring Wu Tang on myocardial mitochondrial energy metabolism and AMPK/PPARα signalling pathway in rats with diastolic heart failure. Chin J Exp Formulas. 2019;25(09):12–17.
123. Shihai Y, Huihua F, Lei T, Qiyi L. Exploring the mechanism of improving myocardial mitochondrial damage in chronic heart failure by shenkui tongwei granules based on AMPK-mt TFA-PINK1 signalling. New Chin Med Clin Pharmacol. 2018;29(06):738–743.
124. Fan Z, Wei F, Guangli Z. Effects of ginseng and cardiotonic combination on AMPK-PGC-1α pathway of myocardial energy metabolism in heart failure rats. Zhejiang J Integr Chin West Med. 2018;28(10):834–837.
125. Wei Z, Yuanwang Y, Shuzhen Z, et al. Effects of ginseng and ligusticum formula on myocardial energy metabolism in rats with heart failure model after myocardial infarction. J Changchun Univ Trad Chin Med. 2018;34(03):415–418.
126. Xianwei W, Wenfeng S, Xiaoxiao Y, et al. Effects of Huanglian detoxification soup on adenylate-activated protein kinase and intercellular adhesion molecule-1 in inflammation-injured endothelial cells. J Xinxiang Med Coll. 2014;31(07):513–516.
127. Qiong H, Shanning Y, Lijun J. Experimental study on the protective effect of ginseng and sorrel injection on chronic heart failure in rats. Mod Clin Med. 2012;38(03):173–175.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
