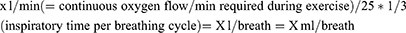Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Ambulatory Long-Term Oxygen Therapy in Patients with Severe COPD: A Randomized Crossover Trial to Compare Constant-Minute-Volume and Constant-Bolus Systems
Authors Majorski DS , Khan SG, Stanzel SB , Wollsching-Strobel M, Kroppen D, Mathes T, Zimmermann M , Windisch W , Magnet FS
Received 20 June 2023
Accepted for publication 1 October 2023
Published 11 November 2023 Volume 2023:18 Pages 2543—2553
DOI https://doi.org/10.2147/COPD.S426749
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Daniel Sebastian Majorski,1 Saba Gul Khan,1 Sarah Bettina Stanzel,1 Maximilian Wollsching-Strobel,1 Doreen Kroppen,1 Tim Mathes,2 Maximilian Zimmermann,1 Wolfram Windisch,1 Friederike Sophie Magnet1
1Department of Pneumology, Cologne Merheim Hospital, Kliniken der Stadt Köln gGmbH, Witten/Herdecke University, Cologne, Germany; 2Institute for Medical Statistics, University Medical Center Göttingen, Göttingen, Germany
Correspondence: Wolfram Windisch, Department of Pneumology, Cologne Merheim Hospital; Kliniken der Stadt Köln gGmbH, Witten/Herdecke University, Ostmerheimer Strasse 200, Köln, D-51109, Germany, Tel +49 221/8907-18929, Fax +49 221/8907-8305, Email [email protected]
Background and Methods: Constant-minute-volume and constant-bolus devices serve as two different means of portable oxygen conservation. A prospective randomised crossover study was conducted in COPD GOLD IV patients to investigate the effect of these two devices on dyspnea, oxygenation and 6-minute walking test (6MWT) distance. The primary endpoint was the final operating level required (operating level range 1– 5 for both devices) by either device to meet the success criteria for mobile oxygen therapy, as outlined in the British Thoracic Society guidelines (SpO2 ≥ 90% throughout 6MWT; ≥ 10% increase in walking distance from baseline; improvement in BORG of at least 1 point from baseline).
Results: Twenty-five patients were enrolled in the study and randomly assigned to one of two sequences involving the use of each type of portable oxygen conservation device. 14 female, 67.9 years (± 7.8); FEV1: 27.3%pred. (± 8.4); PaO2 at rest without oxygen: 50.3mmHg (± 5.9). For both systems, 24/25 patients (96%) were successfully recruited. The mean operating-level difference when success criteria were met was − 0.58 in favor of the constant bolus device (95% CI: − 0.88 to − 0.28, P < 0.001). Secondary endpoints (walking distance, respiratory rate and BORG dyspnea) showed no statistically significant or clinically relevant differences. An algorithm created especially for this study showed a high success rate in terms of titration for the required operating level.
Conclusion: Both portable oxygen-conserving devices met the success criteria in 96% of patients in the 6MWT when they were titrated to the correct level. The constant-bolus device required a significantly lower operating level to achieve the success criteria, hereby reducing energy consumption. Individual titration of the respective device is recommended, which can be facilitated by the novel titration algorithm described here.
Keywords: COPD, LTOT, ambulatory oxygen therapy, portable oxygen conserving devices, 6MWT
Introduction
Long-term oxygen therapy is understood as the administration of oxygen in patients with chronic respiratory insufficiency type 1 either administered for a minimum of 15h/day (16h/day) as well as the administration of oxygen during physical activity.1–3 Mobile oxygen therapy can generally be administered via the use of three different systems: mobile oxygen cylinders, mobile concentrators and mobile liquid oxygen (LOX).4–6 Nowadays, there is a particularly high demand for mobile concentrators. They are relatively inexpensive and, unlike the LOX systems, do not require regular oxygen-refilling by the provider. Another advantage of mobile concentrators is that they come in the form of small and light devices, and can even be powered by a car battery, which further promotes patient mobility. The disadvantages of this system are the constant operating noise, the need for regular filter changes and a lower maximum oxygen application than LOX systems.7
In order to save oxygen for mobile use and thus extend the running time of the system, so-called oxygen-conserving devices (OCDs, alternatively: demand systems) have been developed, which apply oxygen exclusively during inspiration via a nasal cannula.4 Many mobile oxygen concentrators are only designed as OCDs and cannot deliver a constant flow of oxygen. In addition, not every patient is able to trigger the OCD, especially during periods of increased breathing activity or exertion, because inspiration via the mouth becomes dominant and can then lead to insufficient nasal inspiratory flow.7
Moreover, different concentrators may deliver different amounts of oxygen at specific, pre-defined levels. Furthermore, it is a common misconception, even among health care practitioners, that the operating level of such an OCD is equivalent to liters/per minute in a LOX system. Another technical difference between OCDs is the amount of oxygen delivered per breath. Some devices deliver a fixed amount of oxygen per breath, while others deliver a fixed amount per minute.8 For example, in the constant-bolus device, the average inspiratory oxygen fraction (FiO2) per breath largely remains constant, even if the respiratory rate changes, while in the constant-minute-volume device, the volume of oxygen applied upon each breath decreases as soon as the respiratory rate increases. In turn, FiO2 increases as the patient reduces his/her respiratory rate, since the minute volume of delivered oxygen remains constant.8
Given the considerable technical differences between these devices, patients for whom an OCD is planned must be tested on an individual basis to identify the appropriate device, and the level (= running rate) to which it should be set needs to be determined through titration.
Study Hypothesis
It was hypothesized that by design the constant-bolus device could be operated at a lower flow-rate (= setting), compared to that of a constant-minute-volume system. As a result, the unit could then be operated at a lower level.
Materials and Methods
This trial was designed as a crossover randomized trial. The study protocol was approved by the Ethics Committee at Witten/Herdecke University, Witten, Germany, and was undertaken at the Department of Pneumology, Lung Clinic, Cologne Merheim Hospital, Witten/Herdecke University, Germany, as well as the Department of Pneumology, Augustinerinnen Hospital, Cologne, Germany. The study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki. The study was registered at the German Clinical Trials Register (DRKS00017000). Informed written consent was obtained from all subjects.
Patients
COPD patients with an established diagnosis of COPD GOLD IV (FEV1 <30%pred., FEV1/IVC <70%) and/or pre-existing chronic respiratory insufficiency type I (defined by a PaO2 ≤ 60 mmHg at rest) were enrolled in the study. At the time of inclusion, patients were undergoing optimal inhalation therapy, to which no further changes were made during the trial. All patients were required to be free of exacerbation, which was defined as an acute worsening of respiratory symptoms that led to a change in medication for four weeks prior to study inclusion.9 Furthermore, patients with signs of cardiorespiratory instability at rest (SpO2 <80% despite supplemental oxygen therapy, heart rate >140/min, breathing frequency >25/min) were excluded.10–12
Devices
Two certified OCDs were compared:
- Inogen One® G3 (Fa. Inogen, Goleta, USA), a constant-minute-volume system
- Zen-O lite™ (Fa. GCE Ltd, United Kingdom) a constant-bolus system
Both are portable oxygen-conserving devices and can be adjusted to operating levels ranging from 1 to 5.
Study Design and Measurements
Patients were included in the study protocol after declaring their willingness to participate and providing written consent. Upon provision of written consent, basic diagnostics, arterial blood gases12 (ABL 90, Radiometer GmbH, Willich, Germany) and a 6-minute walking test (6MWT) without supplemental oxygen was performed whilst constantly monitoring SpO2 (MightySat© Rx Masimo Health Irvine, California, USA) and optically measuring breathing frequency. Demographic data was documented. For the crossover trial, patients were randomly assigned to one of two sequences: “Period 1: 6MWT with constant-bolus device; Period 2: 6MWT with constant-minute-volume system” or “Period 1: 6MWT with constant-minute-volume system; Period 2: 6MWT with constant-bolus device”. Success criteria for mobile oxygen therapy were chosen according to prerequisites stated in the BTS guidelines for LTOT, and were used as a basis for successful titration of the OCD-operating level for each patient.3
Success Criteria for Ambulatory Oxygen Therapy
A specifically designed titration algorithm was used to determine the correct operating level required for each individual patient.
Titration of oxygen flow was performed according to BTS recommendations,3 which are based on maximum desaturation during an endurance shuttle walking test (ESWT). Although the ESWT is advantageous for titration in ambulatory oxygen therapy (AOT),13 the 6MWT is more commonly used both nationally and internationally for titration in AOT; both walking tests are recommended in the UK as well as in German guidelines.3,14 It has been shown that the ESWT is significantly more demanding than the 6MWT, and that peripheral saturation is lower at the end of an ESWT.13 Therefore, the recommended oxygen flow rate during the 6MWT in the present study was titrated and calculated to be 1-l/min lower than that stated in the corresponding BTS recommendation.
Since these recommendations apply to a constant flow of oxygen, conversion to the required flow-rate per breath (or per minute) was made using the following approximation: Assuming (i) that oxygen is only delivered during inspiration in oxygen conserving devices, (ii) that inspiration during exertion is typically 33% of the breathing cycle (in patients with severe COPD), and (iii) an average breathing rate of 25/min during exertion,15 the likely required rate for OCDs can be approximated using the following formula:
For example:
1 L/min/25 * 1/3= 0.133 L/breath= 13.33 mL/breath
2 L/min/25 * 1/3= 0.2666 L/breath= 26.67 mL/breath
3 L/min/25 * 1/3= 0.04 L/breath= 40 mL/breath
Accordingly, the following titration algorithm was established and tested, based on benchmark testing of the two devices (see Table 1).
 |
Table 1 Titration Algorithm for Establishing the Operating Level of Each Oxygen-Conserving Device (OCD) |
Given the design of the two devices under investigation, this led to a lower setting for the constant-bolus system and formed the basis for our study hypothesis. All patients who took part in this trial fulfilled the following success criteria that are laid down in the BTS guidelines for long-term oxygen therapy:3
“2 out of 3 of the following markers are required to show that the patient benefits from ambulatory oxygen
- SpO2 ≥90% throughout 6MWT
- ≥10% increase in walking distance from baseline
- Improvement in BORG of at least 1 point from baseline”
If patients did not fulfill the success criteria, the 6MWT was repeated and the operating level of the respective device was increased by 1. If patients did fulfill all success criteria and SpO2 during exercise was ≥93%, the operating level was reduced by 1. In order to prevent exhaustion through over-motivation, a maximum of five 6MWTs per patient/per day was predefined. To detect false triggering in either of the OCDs, the investigator performing the 6MWT listened out for acoustic signals in correlation with breathing frequency throughout the entire period, in order to detect absent oxygen triggers. At the end of the trial, patients were asked to state their personal device preference via a standardized questionnaire.
Analysis
The primary aim of this trial was to compare the two underlying devices for ambulatory oxygen therapy. The primary endpoint was the final (ideal) operating level of either device at exercise necessary to reach success criteria. The null hypothesis, that there is no difference in final (ideal) operating level, which was tested at a two-sided alpha level of 0.05 against the alternative hypothesis that there is a difference. There was no formal sample size calculation.
Secondary outcomes were the ability to reach success criteria for mobile oxygen therapy for either device as well as differences in exercise capability (SpO2, walking distance, BORG dyspnea scale).
The effects of the intervention on all endpoints were evaluated using linear mixed models. The factors intervention, period and randomization sequence were included as fixed effects, and the patient within the randomized sequence was included as a random effect to account for the multiple measures. Tests for a period effect and carryover effects were performed in these models. No alpha adjustment was performed for the multiple testing of the secondary endpoints. Thus, the p-values of the statistical tests of effects on the secondary endpoints should be interpreted as exploratory analyses.
IBM SPSS 28.0 was used for all analyses.
Results
Of the 47 patients with COPD and chronic respiratory failure who were screened, 32 met the inclusion criteria at rest. Twenty-five patients met all criteria and were consecutively enrolled and randomly assigned to one of the two sequences (Figure 1).
 |
Figure 1 Study flow chart according to CONSORT for crossover randomized trials. |
Lung function data, blood gases and demographic data are presented in Table 2.
 |
Table 2 Patient Characteristics at Baseline |
Regarding the primary endpoint, the mean ideal operating levels were 3.64 (±1.1) for the constant-bolus device and 4.24 (±0.9) for the constant-minute-volume system, leading to a difference of −0.58 (95% CI: −0.88 – −0.29; p= <0.01) (Figure 2a).
Figure 2 Continued.
The respective results for walking distance in 6MWT, breathing frequency and BDS did not significantly differ between devices (Figures 2b–e).
The use of the constant minute volume system led to an overall higher mean oxygen saturation of 90% (±3.6%) after exercise testing compared to the constant bolus device (88.4% ± 3.8%), leading to a mean difference of 1.6% (95% CI: −3.3 – −0.01; p= 0.049) (Figure 2e).
In twelve cases the 6-MWT needed to be readjusted to a higher operating level (six for the constant-bolus device, with an increase of 1 in the operating level; five for the constant-minute-volume device, with an increase of 1 in the operating level; one for the constant-minute-volume device, with an increase of 2 in the operating level in each group in order to find the correct titration level), leading to an algorithm success rate of 76% in this cohort.
The success criteria for oxygen titration (2/3 criteria met) were fulfilled in 24/25 cases (96%) with the constant-minute-volume system, as well as in 24/25 cases (96%) with the constant-bolus device (Figure 3). In 9/25 patients, all criteria for successful oxygen titration were met with the constant-minute-volume system (36%) and in 6/25 (24%) with the constant-bolus device. The patient questionnaire findings revealed that most patients (72%) preferred the constant-minute-volume system, whereas 20% of stated they had no preference between the two devices and 8% preferred the constant-bolus device. When asked to describe their subjective feelings in terms of receiving enough oxygen, 64% of patients favored the constant-minute-volume system, while 36% remarked that they did not notice a difference between the two devices. Twenty-three patients (92%) subsequently asked for a prescription for a portable oxygen conserving device in preference to their pre-existing mobile systems.
 |
Figure 3 The two devices under investigation (constant-bolus- system and constant-minute-volume system) and their ability to reach success criteria. |
Discussion
This is the first randomized crossover trial to investigate two “demand” oxygen concentrators with differing technical designs, namely, a constant-minute-volume device and a constant-bolus device. The three major findings of this trial are as follows: Firstly, the constant-bolus device was capable of achieving successful oxygen titration at a significantly lower operational setting; despite these lower settings, treatment success with the constant-bolus device – as estimated by walking distance, exercise-related dyspnea and the breathing frequency – was comparable to that of the constant-minute-volume device. Secondly, both portable oxygen-conserving devices were capable of promoting successful oxygen titration in 96% of cases within a cohort of severely impaired COPD patients.
Thirdly, the specifically designed titration algorithm, which was used to find an approximation of the correct level, showed first-round titration success in 76% of cases, thereby rendering it capable of facilitating everyday practice in finding the correct operating level of such OCDs.
These major findings have important clinical implications, the most prominent of which is that they were able to confirm that oxygen-conserving devices are efficient and safe, irrespective of the specific technique used (ie constant minute volume or constant bolus). This confirms previous findings, where liquid oxygen systems were mostly investigated and shown to achieve similar effects to those observed in continuous flow systems in COPD patients.5,7,16
Data comparisons between LOX and oxygen concentrators are scarce. Two trials concluded that oxygen concentrators are comparably effective, although it should be noted that one of these found LOX systems to be especially beneficial in terms of usage time and social outings.17,18
Given the advantages of oxygen-conserving devices, particularly regarding the fact that oxygen-conserving devices are lighter than oxygen-delivery devices without “demand” settings,1,9 these devices may become the preferred mode for mobile oxygen therapy.
The identification of further advantages associated with constant-bolus devices compared to constant-minute-volume devices is also of particular clinical interest, whereby the use of a lower operational setting is suggested to protract battery running time, although this would need to be further validated in a clinical trial setting. The reasons for this clinical finding remain unclear, and supporting evidence from the literature does not yet exist. Furthermore, the present study clearly shows that when constant-bolus devices are used with lower operational settings, overall clinical treatment success is not inferior to that observed with the use of constant-minute-volume devices. However, the use of the constant-minute-volume system was associated with a generally higher oxygen saturation level, where the difference of 1.6% lies within the range of accepted measurement error for pulse oximetry.10 Despite this, the tendency in favor of constant-minute-volume devices was statistically significant. Therefore, it cannot be excluded with certainty that individual patients may experience a clinically significant difference in oxygen saturation with the constant bolus device running at lower operational settings compared to that measured in association with use of constant-minute-volume devices. Hence, individual titration is still recommended.
Finally, this is the first study to provide titration algorithms necessary to identify the required operating level for oxygen-conserving concentrators with a demand setting, which fits the balance of physiological needs and helps to avoid oversupply that can have a subsequent negative impact on battery running time. Although previous research on this topic is lacking, it should be noted that if the patient becomes dyspneic during an exacerbation of his/her primary condition, it may be necessary to temporarily increase oxygen flow in order to relieve dyspnea. However, in some cases the maximum setting might not deliver enough oxygen to compensate for this (eg, in situations where the maximum setting is needed to ensure oxygenation during rest).
One limitation of this trial is that a self-paced 6MWT was chosen for exercise testing. The disadvantage of this test procedure is that the patient’s walking performance cannot be standardized and outcome measures at the end of the 6MWT may not be comparable due to different workloads. On the other hand, it could be argued that this better reflects real-life situations, in contrast to an endurance shuttle walking test. Furthermore, it is reasonable to assume that patients generally focus more on their breathing pattern during the test than they do during everyday activities, which can potentially lead to an artificially low false-trigger likelihood for the demand devices.
It is of particular interest that a significant number of patients (23/25) in the current trial showed a subjective preference for one of the two demand devices over the continuous flow device already prescribed to them. As technical developments continue to advance, the potential to further improve health-related quality of life in a severely impaired patient collective is considerably increased. Future technological developments need to incorporate the need for keeping the weight of the portable oxygen devices to a minimum, since previous trials were able to show that patients often experienced decreased mobility due to the heavy weight of their portable oxygen cylinders; this resulted in decreased autonomy and further isolation, leading to an overall decreased quality of life.19 Finally, since the present study was able to show that different oxygen-conserving devices require different operating levels for treatment success, it is of utmost importance to titrate each device to the correct level.
Conclusion
The constant-bolus and constant-minute-volume devices that were investigated in the present study yielded similar results in the 6MWTs, meeting the international success criteria for mobile oxygen titration in nearly all study patients, and the newly developed titration algorithm is a reliable means of predicting the appropriate operating level of the device and thus facilitating daily clinical practice. Portable oxygen-conserving devices can therefore serve to promote mobility in patients with severe COPD and chronic respiratory failure, warranting further studies in a clinical-trial setting.
Data Sharing Statement
The authors do not intend to share individual deidentified participant data. The authors do only, without exception, share anonymized data with written permission given by each participant. The authors intend to publish an original contribution and will not share any other information concerning this trial on other platforms. The data will be accessible through the corresponding author Prof. Dr. Wolfram Windisch at [email protected] permanently.
Acknowledgments
We acknowledge all participants for the effort they devoted to this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
A research grant was provided by GCE Healthcare (Malmö, Sweden). The authors state that neither the study design, results, interpretation of the findings, nor any other subject discussed in the submitted manuscript was dependent on financial support.
Disclosure
The Cologne study group (F.S.M., D.K., M.Z., S.B.S., D.S.M., M.WS and W.W.) received an open research grant from Weinmann/Germany, Vivisol/Germany, Heinen und Löwenstein/Germany, GCE Ltd. UK and VitalAire/Germany Philips Respironics/Netherlands and Löwenstein Medical/Germany. F.S.M., and S.B.S. received personal travel grants from companies dealing with LTOT. W.W. received speaking fees from companies dealing with LTOT. MZ reports personal fees from Resmed, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93(3):391–398. doi:10.7326/0003-4819-93-3-391
2. Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1(8222):681–686.
3. Hardinge M, Annandale J, Bourne S, et al. British Thoracic Society guidelines for home oxygen use in adults: accredited by NICE. Thorax. 2015;70(Suppl 1):i1–i43. doi:10.1136/thoraxjnl-2015-206865
4. Magnet FS, Storre JH, Windisch W. Home oxygen therapy: evidence versus reality. Expert Rev Respir Med. 2017;11(6):425–441. doi:10.1080/17476348.2017.1325323
5. Braun SR, Spratt G, Scott GC, et al. Comparison of six oxygen delivery systems for COPD patients at rest and during exercise. Chest. 1992;102(3):694–698. doi:10.1378/chest.102.3.694
6. Fuhrman C, Chouaid C, Herigault R, et al. Comparison of four demand oxygen delivery systems at rest and during exercise for chronic obstructive pulmonary disease. Respir Med. 2004;98(10):938–944. doi:10.1016/j.rmed.2004.03.010
7. Gloeckl R, Osadnik C, Bies L, et al. Comparison of continuous flow versus demand oxygen delivery systems in patients with COPD: a systematic review and meta-analysis. Respirology. 2019;24(4):329–337. doi:10.1111/resp.13457
8. Chatburn RL, Williams TJ. Performance comparison of 4 portable oxygen concentrators. Respir Care. 2010;55(4):433–442.
9. Vogelmeier C, Buhl R, Burghuber O, et al. Leitlinie zur Diagnostik und Therapie von Patienten mit chronisch obstruktiver Bronchitis und Lungenemphysem (COPD) [Guideline for the diagnosis and treatment of COPD patients - issued by the German Respiratory Society and the German Atemwegsliga in Cooperation with the Austrian Society of Pneumology]. Pneumologie. 2018;72(4):253–308. German. doi:10.1055/s-0043-125031
10. American Association for Respiratory Care. AARC clinical practice guideline. Sampling for arterial blood gas analysis. Respir Care. 1992;37(8):913–917.
11. Theodore AC. UpToDate. Arterial blood gases. Available from: http://www.uptodate.com/contents/arterial-blood-gases.
12. Magnet FS, Majorski DS, Callegari J, et al. Capillary PO2 does not adequately reflect arterial PO2 in hypoxemic COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2647–2653. doi:10.2147/COPD.S140843
13. Revill SM, Noor MZ, Butcher G, et al. The endurance shuttle walk test: an alternative to the six-minute walk test for the assessment of ambulatory oxygen. Chron Respir Dis. 2010;7(4):239–245. doi:10.1177/1479972310378311
14. Haidl P, Jany B, Geiseler J, et al. Leitlinie zur Langzeit-Sauerstofftherapie - S2k-Leitlinie herausgegeben von der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin e. V. (DGP) [Guideline for long-term oxygen therapy - S2k-guideline published by the German Respiratory Society]. Pneumologie. 2020;74(12):813–841. German. doi:10.1055/a-1252-1492
15. Gloeckl R, Teschler S, Jarosch I, et al. Comparison of two- and six-minute walk tests in detecting oxygen desaturation in patients with severe chronic obstructive pulmonary disease - A randomized crossover trial. Chron Respir Dis. 2016;13(3):256–263. doi:10.1177/1479972316636991
16. Marti S, Pajares V, Morante F, et al. Are oxygen-conserving devices effective for correcting exercise hypoxemia? Respir Care. 2013;58(10):1606–1613. doi:10.4187/respcare.02260
17. Nasilowski J, Przybylowski T, Zielinski J, et al. Comparing supplementary oxygen benefits from a portable oxygen concentrator and a liquid oxygen portable device during a walk test in COPD patients on long-term oxygen therapy. Respir Med. 2008;102(7):1021–1025. doi:10.1016/j.rmed.2008.02.005
18. Su CL, Lee CN, Chen HC, et al. Comparison of domiciliary oxygen using liquid oxygen and concentrator in northern Taiwan. J Formos Med Assoc. 2014;113(1):23–32. doi:10.1016/j.jfma.2012.03.013
19. AlMutairi HJ, Mussa CC, Lambert CT, et al. Perspectives from COPD subjects on portable long-term oxygen therapy devices. Respir Care. 2018;63(11):1321–1330. doi:10.4187/respcare.05916
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.