Back to Journals » International Journal of General Medicine » Volume 16
Altered Spontaneous Brain Activity and Its Predictive Role in Patients with Central Retinal Artery Occlusion Using fMRI and Machine Learning
Received 23 May 2023
Accepted for publication 31 July 2023
Published 18 August 2023 Volume 2023:16 Pages 3593—3601
DOI https://doi.org/10.2147/IJGM.S421215
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yan Tong,1 Xin Huang2
1Department of Ophthalmology and Visual Sciences, the Chinese University of Hong Kong, Hong Kong, People’s Republic of China; 2Department of Ophthalmology, Jiangxi Provincial People’s Hospital, the First Affiliated Hospital of Nanchang Medical College, Nanchang, People’s Republic of China
Correspondence: Xin Huang, Department of Ophthalmology, Jiangxi Provincial People’s Hospital, the First Affiliated Hospital of Nanchang Medical College, No. 152, Ai Guo Road, Dong Hu District, Nanchang, Jiangxi, 330006, People’s Republic of China, Email [email protected]
Objective: To investigate spontaneous neuronal activity changes in patients with central retinal artery occlusion (CRAO) using the resting-state functional magnetic resonance imaging (fMRI) and detect whether these brain functional alterations can represent an objective biomarker of clinical response using a machine learning algorithm.
Methods: Eighteen patients with CRAO and eighteen healthy controls (HCs) were recruited. The regional homogeneity (ReHo) method of resting-state fMRI was conducted to evaluate the synchronous brain activity alterations between two groups. Differences of ReHo values between two groups were compared using the independent two-sample t-test. The support vector machine algorithm was to distinguish patients of CRAO from HCs based on the two groups’ whole-brain ReHo patterns. The accuracy, sensitivity, and specificity for the classification were calculated. The classification performance was evaluated using the non-parametric permutation test.
Results: Compared to HCs, individuals with CRAO showed significantly lower ReHo in the right cerebellum and precuneus. Meanwhile, significant higher ReHo values were observed in the left superior temporal gyrus, postcentral gyrus, and precentral gyrus in the CRAO group compared to HCs. Furthermore, our results suggested that 77.78% individuals with CRAO could be successfully distinguished from HCs by the machine learning, with a sensitivity of 72.22% and a specificity of 83.33%, respectively. The area of receiver operating characteristic curve was calculated to be 0.85.
Conclusion: This study uncovered individuals with CRAO exhibited disturbed synchronous neuronal activities in multiple brain areas using neuroimaging techniques. The ReHo variability could distinguish individuals with CRAO from HCs with high accuracy.
Keywords: central retinal artery occlusion, functional magnetic resonance imaging, regional homogeneity, machine learning, support vector machine
Introduction
Central retinal artery occlusion (CRAO) is an ophthalmic emergency which is analogous to an acute stroke of the eye.1 It typically presents as severe, painless and sudden visual loss in the affected eye. Typical early-phase characteristics include retinal swelling, a cherry red spot in the macula, edema and whitening of retinal inner layers. Later phase observations include thinning and atrophy of the inner retinal layers, further vessel attenuation and flattening of the foveal depression.2 The incidence of CRAO has been estimated at 1 to 2/100,000 people per year.3 Because CRAO is common in the elderly and the prevalence of CRAO is likely to increase with an aging global population, proper management of incident CRAO is also crucial in terms of the public health burden.2
Neuroimaging studies have increasingly showed that the optic nerve atrophy and damage would lead to transsynaptic neurodegenerative changes the visual pathway, including functions in the visual cortex.4 Anatomically, the central retinal artery arises from the ophthalmic artery, the first intracranial branch of internal carotid artery which provides the main blood supply to the brain.5 Thus, the brain and retina share the same arterial supply. Moreover, CRAO and stroke have multiple overlapping systemic risk factors, such as hyperlipidemia, diabetes mellitus, and hypertension.6 Several studies have reported an association between retinal artery occlusion and stroke and found that retinal artery occlusion increases the risk for subsequent stroke.7 CRAO is regarded as an early warning for subsequent stroke. Nowadays, the principle of CRAO treatment is based on quick reperfusion of the retina and restoration of oxygen delivery, with an effective treatment window of within four to six hours. However, the final visual acuity of 90% of non-arteritic CRAO patients without cilioretinal artery sparing is 20/400 or even worse;8 and only one-third of ophthalmologists transfer patients with incident CRAO to an emergency department for immediate evaluation.9
Functional magnetic resonance imaging (fMRI) methods have been applied to explore the alterations in spontaneous brain activity in human brain.10 Regional homogeneity (ReHo) analysis can be conducted to explore the homogeneity of brain activity by calculating the synchronization or functional coherence of a specific voxel with its nearest voxels. This method has discovered alterations in homogeneity of neural activity in multiple vision-related diseases.11,12 Compared to other techniques commonly used in fMRI (such as amplitude of low-frequency fluctuation, independent component analysis-derived network-based functional connectivity and seed-based functional connectivity), ReHo has been shown to have advantages of high test–retest stability and can be used as a stable indicator for measuring resting brain activity even under the random noise interference.13 Furthermore, ReHo offers the possibility to search for abnormalities in the whole brain functional connectome without predefining region of interests. Biousse et al reported that acute cerebral infarctions were found in 27% to 76.4% of CRAO patients and emphasized the need to assess patients with isolated acute retinal ischemia urgently with brain imaging.14 Nevertheless, the underlying mechanism of CRAO and changes of spontaneous brain activity remain unknown.
Recently, an increasing number of studies have applied multivariate machine learning techniques to neuroimaging datasets to predict and characterize diseases.15 In our previous study, we also successfully applied this method to classify patients with iridocyclitis from HCs utilizing the functional connectivity of primary visual cortex fMRI data.16 In the current study, we aimed to utilize machine learning method to classify patients with CRAO from HCs on the basis of ReHo brain abnormalities.
Based on previous studies and the apparent-impaired visual function in CRAO, we hypothesized that patients with CRAO might have spontaneous neuronal activity abnormalities in specific brain regions such as visual information processing and cognition-related areas. We attempted to uncover how CRAO affects brain function using the ReHo method and to obtain a better understanding of neurobiology of CRAO at the level of the brain network. Furthermore, we aim to explore the utility of ReHo in the classification of individuals diagnosed with CRAO and examine the predictive power to understand the predictive range of this technique.
Materials and Methods
Participants
Eighteen patients with CRAO and 18 HCs matched with age, sex, and educational level were recruited in the current study. This study was performed in accordance with Declaration of Helsinki. All subjects were informed with the whole study procedures and provided with the written informed consent.
The inclusion criteria for patients with CRAO were: (1) subjects with a painless, sudden monocular loss of visual acuity and peripheral vision; (2) slow segmental blood in distal arteries on fluorescein fundus angiography; (3) the diffuse retinal whitening in the infarcted edematous retina on the typical funduscopic; (4) right-handed. The exclusion criteria for individuals with CRAO were as below: (1) history of psychiatric diseases or psychotropic drug use; (2) history of ocular trauma or vitreoretinal surgery; (3) presence of other ophthalmic diseases; (4) presence of systemic diseases (including hypertension, diabetes, and heart disease).
The inclusion criteria for HCs were as below: (1) no other ocular diseases; (2) no history of medications or alcohol abuse; (3) right-handed; (4) no abnormalities in brain structures on magnetic resonance imaging; (5) no magnetic resonance imaging contraindications; (6) binocular visual acuity ≥1.0.
Ethical Statement
The research protocol adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Jiangxi Provincial People’s Hospital. All subjects provided written informed consent to participate in the study. All research procedures in the guidelines outlined in the Declaration of Helsinki were followed.
Ophthalmic Examinations
All subjects in the current study underwent systemic ophthalmic assessments and other related clinical measurements including fundus photographs, fundus fluorescein angiography and optical coherence tomography. Data including gender, age, best-corrected visual acuity, intraocular pressure treatment strategies and comorbidities were recorded from the medical charts. The LogMAR values of the best-corrected visual acuity of both eyes were measured in two groups using the LogMAR table.
MRI Parameters
Both T1-weighted and whole-brain functional magnetic resonance imaging scanning were performed on a 3.0 T GE MR 750W scanner (GE Healthcare) with a standard head coil. All subjects were instructed to stay awake with their eyes closed until the entire scanning was over. The entire scanning time was about 15 minutes. The three-dimensional spoiled gradient-recalled echo sequence was set to obtain the anatomical T1-weighted images with following parameters: repetition time/echo time, 8.5 ms/3.3 ms; field of view, 240×240 mm2; gap, 0 mm; thickness, 1.0 mm; acquisition matrix, 256 x 256; and flip angle, 12°. In addition, the gradient-recalled echo-planar imaging sequence was set to acquire the whole-brain fMRI data with following parameters: repetition time/echo time, 2000 ms/25 ms; field of view, 240×240 mm2; gap, 1.2 mm; thickness, 3.0 mm; acquisition matrix, 256 x 256; and flip angle, 90°.
fMRI Data Preprocessing
The rest preprocessing steps were conducted as following steps. [1] Convert the original DICOM files to the NIFTI format. [2] Exclude the first ten volumes of each functional time series. The rest 230 volumes of fMRI data were modified by taking the slicing time effects, head-motion corrected and realigned. fMRI data from individuals whose head-motion having head rotation over 1.5 or maximum displacement in any direction over 2.0 mm would be discarded. [3] Registered subjects’ T1-weighted images to the mean fMRI data.17 All data had been normalized to the standard Montreal Neurological Institute (MNI) space. Then, the time courses detrend and linear regression analysis were conducted. The latter step was to exclude nuisance covariates. Finally, the FMRI data were ban pass-filtered (0.01–0.08Hz).
ReHo Calculation
In the present study, we calculated the ReHo values for subjects’ functional data by using the REST toolbox. ReHo values represent the local synchronization between the spontaneous activity of a specific voxel and its nearest neighboring voxels.
Classification Machine Learning Analysis
The individual subject mean ReHo maps would serve to be inputs for the multivariate classification machine learning analyses. The support vector machine (SVM) algorithm for binary classification implemented on the Pattern Recognition for Neuroimaging Toolbox was performed to measure the diagnostic prediction in individuals with CRAO.18 During the training process, the leave-one-out cross-validation procedure was used to perform validation to examine the model’s estimation power. Statistical significance of the accuracy calculations was determined by permutation testing. The total accuracy, sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) were calculated to evaluate the algorithm’s performance for classifying the patients with CRAO and HCs.
Statistical Analysis
Clinical data collected from subjects were analyzed by the SPSS 20.0 software (P < 0.05 indicates statistical significance). The independent two-sample t-test and Chi-square test were applied to evaluate the differences in the baseline age and sex between two groups. The one-sample t-test and two-sample independent t-test were conducted to analyze ReHo maps within and between groups using the SPM 12 software. The statistical threshold of multiple comparison correction for significance was set by using the Gaussian Random Field (GRF) correction (voxel-level: P < 0.01, GRF correction, cluster-level: P < 0.05).
Results
Demographics and Visual Measurements
The statistic results of demographic and clinical measurements are exhibited in Table 1. There are no significant differences between age, sex, or educational level between the CRAO group and HCs group. By contrast, significant statistically differences were detected in the best-corrected visual acuity between these two groups (P < 0.01).
 |
Table 1 Demographic and Clinical Measurement in CRAO and HCs Groups |
Comparisons of ReHo Values Between the CRAO Group and HCs Group
Figure 1 displays the intra-group comparisons of ReHo values within the CRAO (Figure 1A) and HCs groups (Figure 1B) respectively, which shows the spatial distribution of ReHo signal values of patients with CRAO and HCs. Compared to HCs, individuals with CRAO showed significantly lower ReHo value in the right cerebellum and precuneus. Meanwhile, significant higher ReHo values were observed in the left superior temporal gyrus, postcentral gyrus, and precentral gyrus in the CRAO group compared to HCs (voxel-level: P < 0.01, GRF correction, cluster-level: P < 0.05) (Figure 2 and Table 2). The altered mean ReHo values changes between two groups is shown in Figure 3. However, there was no significance between the mean ReHo values of distinct brain areas and the current clinical data (P > 0.05).
 |
Table 2 Brain Regions with Significant Differences in ReHo Values Between CRAO and HCs Groups |
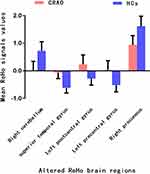 |
Figure 3 The mean ReHo signal values between the CRAO and HCs group. Data are presented as mean± standard deviation. |
Support Vector Machine Classification Results
The machine learning classification results based on ReHo are exhibited in Figure 4. The confusion matrix (Figure 4A) revealed the specific assignments of different predictions for each subject and showed a diagonal pattern of classification. The accuracy is the total number of correctly classified test samples from every leave-one-out cross-validation set divided by the total number of test samples. For the whole-brain ReHo map total classification accuracy was 77.78% with a sensitivity of 72.22% and a specificity of 83.33% (p < 0.01). A receiver operating characteristic curve of the binary classifiers was generated to evaluate the system’s performance on distinguishing individuals with CRAO from HCs (Figure 4B) and the AUC was 0.85. The SVM finds what is known as the maximum margin decision boundary, which is the hyperplane that is furthest from the least discriminating features of the discriminated categories. Normalized decision function values are plotted for CRAO and HCs participants (Figure 4C). Furthermore, in order to assign classification power to specific locations in the brain, PRoNTo takes the linear SVM models and recovers model weights and transforms the weights vector into a map in the original image space. Thus, the weight map exhibited the contribution of each voxel in the image for the linear predictive function (Figure 4D).
Discussion
To the best of our knowledge, this is the first study that combined ReHo technique and machine learning have been applied to evaluate the spontaneous neuronal activities and investigate its predictive validity in patients with CRAO.
Interestingly, our results showed that patients with CRAO exhibited significantly decreased ReHo in the right cerebellum. The cerebellum is known to interact functionally with the frontal eye fields and take part in visuomotor, memory, and higher cognitive function. It is also reported that the cerebellum showed connections with premotor, motor, and posterior parietal cortices. Previous studies have uncovered that dysfunction of the cerebellum is associated with bipolar disorder, depression, and mode disorders. CRAO serves a harbinger of cardiovascular and cerebrovascular events, and more recent studies using fMRI have identified changes in cerebellum function that correlate with ischemia stroke.19 However, very few studies have used fMRI as an outcome measure for CRAO. Thus, we speculated that individuals with CRAO might lead to the cognitive dysfunction and visuomotor coordination impairment.
Compared with HCs, patients with CRAO exhibited higher ReHo in the left precentral gyrus and postcentral gyrus. The precentral gyrus is the motor region which manages the opposite side half body to move at will and controls the movement frequency and quantity.20 The post central gyrus serves as the primary somatosensory cortex, which takes an important role in various sensory perceptions.21 Increased ReHo suggests that the functional neuronal activity and its adjacent neurons are more temporally synchronous in CRAO patients and indicates the adaptive alterations of these neurons to achieve a specific function.
Previous studies have observed that there are multiple brain regions display higher activity during the resting state than task performance, which consist of the default-mode network (DMN).22 In this study, we demonstrated that the ReHo value was significantly reduced in the CRAO group. Decreased synchrony within this brain area may be associated with impairment in the processes of self-evaluation and emotion. In addition, precuneus is a crucial component of the parieto-occipital pathway and an important part of the dorsal visual stream. It is also associated with visuospatial processing.23 A previous study discovered that an increase volume in the precuneus in a group with primary open-angle glaucoma.24 Therefore, our results suggested the dysfunction of visuospatial processing occurs in patients with CRAO.
Notably, we present the first machine learning study to predict the diagnosis of CRAO and HCs using the neuroimaging data modalities. The multivariate machine learning analysis uncovered that ReHo features were able to classify the CRAO group with AUC of 0.85, which suggest that distinct patterns of ReHo maps may be helpful in classifying individual patient populations with CRAO versus HCs.
However, there are some limitations in this study. First, the sample size of the study is relatively small due to the stringent inclusion criteria. Further work with expanding number of subjects is needed to validate current results and obtain more reliable findings. Second, physiological indicators including respiratory rate, individual blood oxygen levels, and heart rate could be potential confounding factors on spontaneous neuronal activities that were not excluded. Third, the study was performed with a single time frame and did not investigate longitude alterations. Thus, a longitudinal study with multi-modal fMRI analytic approaches should be conducted to explore the dynamic changes within local neuronal activity over time in the future research.
In conclusion, we present the first study to explore the alterations of spontaneous brain activity within patients with CRAO and HCs and utilize machine learning analyses to predict accurately the classification of CRAO. The ReHo variability could classify patients with CRAO from HCs with substantial accuracy. These findings might provide helpful information to understand the underlying mechanisms of CRAO and indicated that ReHo could be applied as a biomarker for identifying CRAO.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available. Further inquiries can be directed to the corresponding author.
Acknowledgments
We acknowledge the assistance provided by the Natural Science Foundation of Jiangxi Province (20212BAB216058), Jiangxi Provincial Health Technology Project (202210012 and 202310114), and Jiangxi Provincial traditional Chinese Technology Project (2022B840).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye. 2013;27(6):688–697. doi:10.1038/eye.2013.25
2. Park SJ, Choi N-K, Yang BR, et al. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmology. 2015;122(11):2336–2343.e2332. doi:10.1016/j.ophtha.2015.07.018
3. Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol. 2011;152(5):820–823.e822. doi:10.1016/j.ajo.2011.05.005
4. Mock VL, Luke KL, Hembrook-Short JR, Briggs F. Dynamic communication of attention signals between the LGN and V1. J Neurophysiol. 2018;120(4):1625–1639. doi:10.1152/jn.00224.2018
5. Hayreh SS. Orbital vascular anatomy. Eye. 2006;20(10):1130–1144. doi:10.1038/sj.eye.6702377
6. Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24(4):493–519. doi:10.1016/j.preteyeres.2004.12.001
7. Chang Y-S, Jan R-L, Weng S-F, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol. 2012;154(4):645–652.e641. doi:10.1016/j.ajo.2012.03.046
8. Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140(3):
9. Atkins EJ, Bruce BB, Newman NJ, Biousse V. Translation of clinical studies to clinical practice: survey on the treatment of central retinal artery occlusion. Am J Ophthalmol. 2009;148(1):172–173. doi:10.1016/j.ajo.2009.03.020
10. Lang S, Duncan N, Northoff G. Resting-State functional magnetic resonance imaging. Neurosurgery. 2014;74(5):453–465. doi:10.1227/NEU.0000000000000307
11. Bhattacharya S, Song Y, Mu K, et al. Altered spontaneous brain activity in primary open angle glaucoma: a resting-state functional magnetic resonance imaging study. PLoS One. 2014;9(2):e89493.
12. Tang L-Y, H-J L, Huang X, et al. Assessment of synchronous neural activities revealed by regional homogeneity in individuals with acute eye pain: a resting-state functional magnetic resonance imaging study. J Pain Res. 2018;11:843–850. doi:10.2147/JPR.S156634
13. Li Z, Kadivar A, Pluta J, Dunlop J, Wang Z. Test-retest stability analysis of resting brain activity revealed by blood oxygen level-dependent functional MRI. J Magnetic Resonance Imaging. 2012;36(2):344–354. doi:10.1002/jmri.23670
14. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia. Ophthalmology. 2018;125(10):1597–1607. doi:10.1016/j.ophtha.2018.03.054
15. Nicholson AA, Densmore M, McKinnon MC, et al. Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: a multimodal neuroimaging approach. Psychol Med. 2018;49(12):2049–2059. doi:10.1017/S0033291718002866
16. Tong Y, Huang X, Qi C-X, Shen Y. Altered Functional Connectivity of the Primary Visual Cortex in Patients With Iridocyclitis and Assessment of Its Predictive Value Using Machine Learning. Front Immunol. 2021;12.
17. Goto M, Abe O, Aoki S, et al. Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra provides reduced effect of scanner for cortex volumetry with atlas-based method in healthy subjects. Neuroradiology. 2013;55(7):869–875. doi:10.1007/s00234-013-1193-2
18. Chang -C-C, Lin C-J. Libsvm. ACM Transactions Intelligent Sys Technol. 2011;2(3):1–27. doi:10.1145/1961189.1961199
19. Crofts A, Kelly ME, Gibson CL. Imaging Functional Recovery Following Ischemic Stroke: clinical and Preclinical fMRI Studies. J Neuroimaging. 2019;30(1):5–14. doi:10.1111/jon.12668
20. Kim JA, Eliassen JC, Sanes JN. Movement quantity and frequency coding in human motor areas. J Neurophysiol. 2005;94(4):2504–2511. doi:10.1152/jn.01047.2004
21. Wrigley PJ, Press SR, Gustin SM, et al. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141(1):52–59. doi:10.1016/j.pain.2008.10.007
22. Chang Y-T, Huang C-W, Chang Y-H, et al. Amyloid burden in the hippocampus and default mode network. Medicine. 2015;94(16):e89493.
23. Mahayana IT, Tcheang L, Chen C-Y, Juan C-H, Muggleton NG. The precuneus and visuospatial attention in near and far space: a transcranial magnetic stimulation study. Brain Stimul. 2014;7(5):673–679. doi:10.1016/j.brs.2014.06.012
24. Jiang -M-M, Zhou Q, Liu X-Y, Shi C-Z, Chen J, Huang X-H. Structural and functional brain changes in early- and mid-stage primary open-angle glaucoma using voxel-based morphometry and functional magnetic resonance imaging. Medicine. 2017;96(9). doi:10.1097/MD.0000000000006139
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.